High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
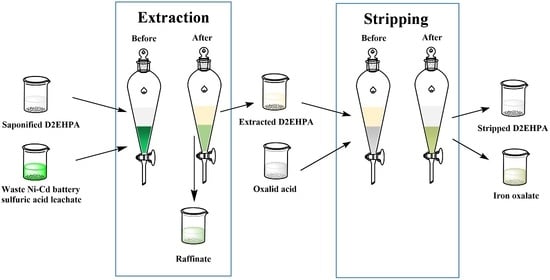
2.2. Experimental Procedure
2.3. Analytical Method
3. Results and Discussion
3.1. Extraction Performance of Fe3+
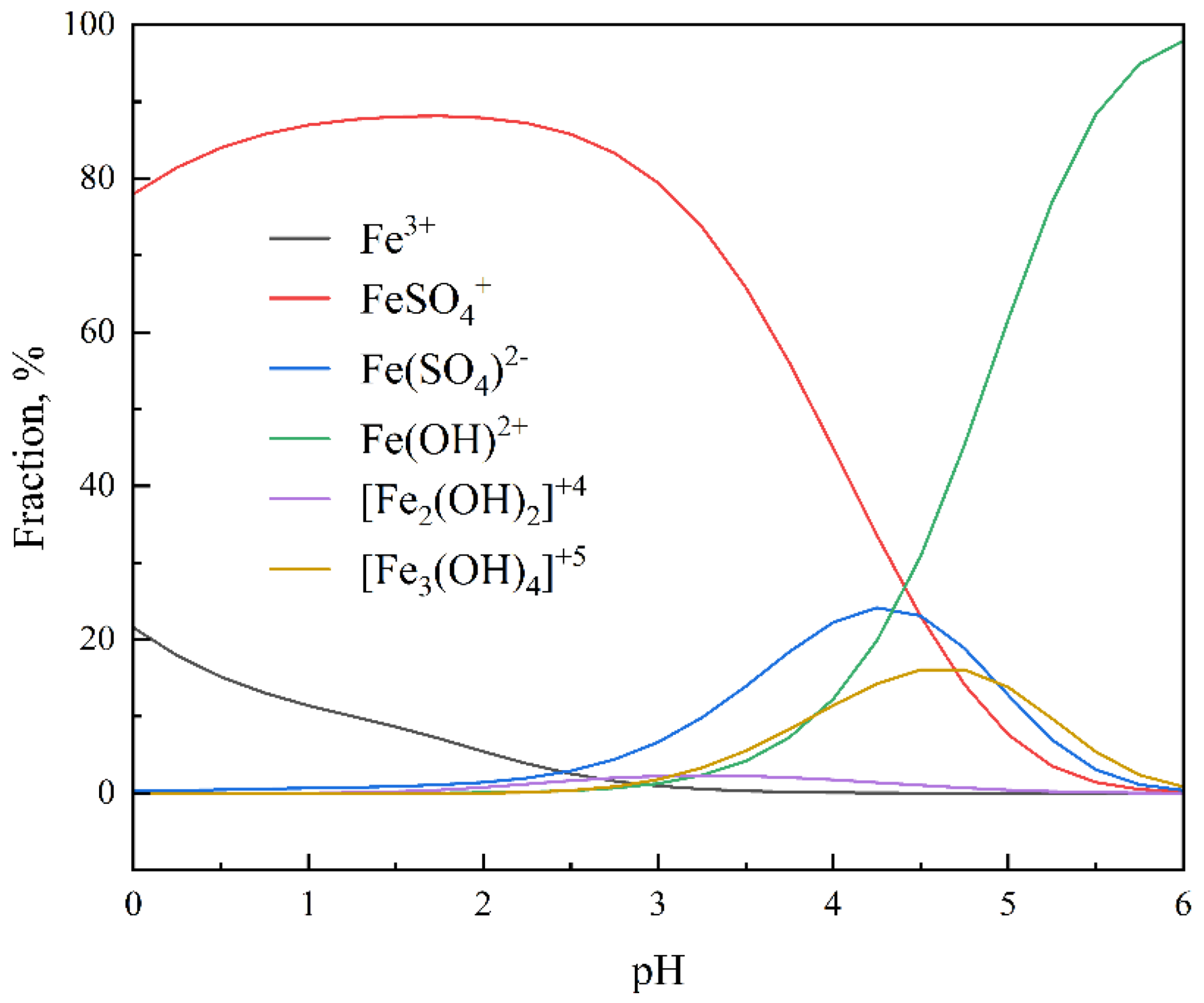
3.1.1. Extraction of Fe3+ from the Waste Ni-Cd Battery Sulfuric Acid Leachate
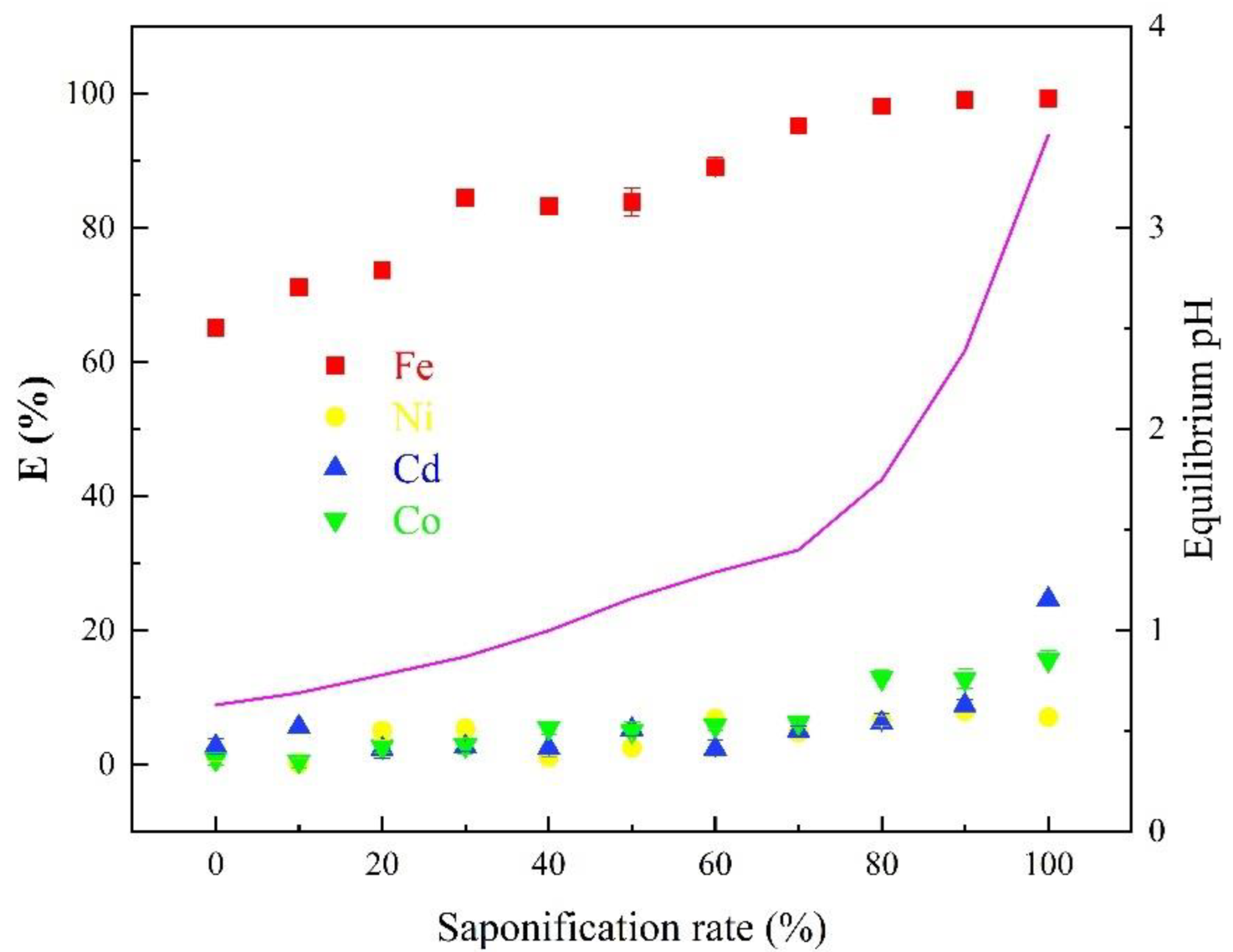
3.1.2. Effects of Saponification Rate of D2EHPA
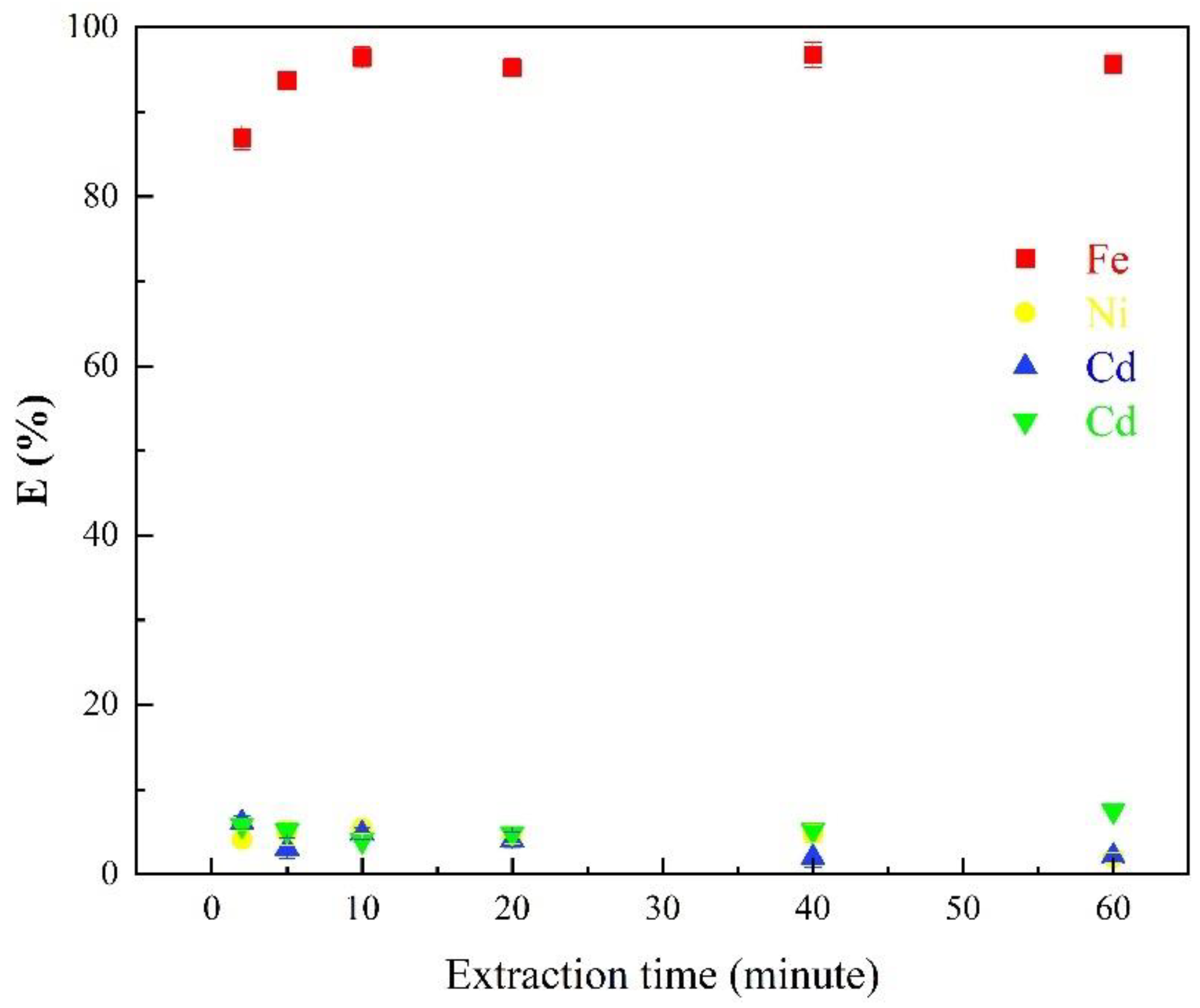
3.1.3. Effects of Extraction Time
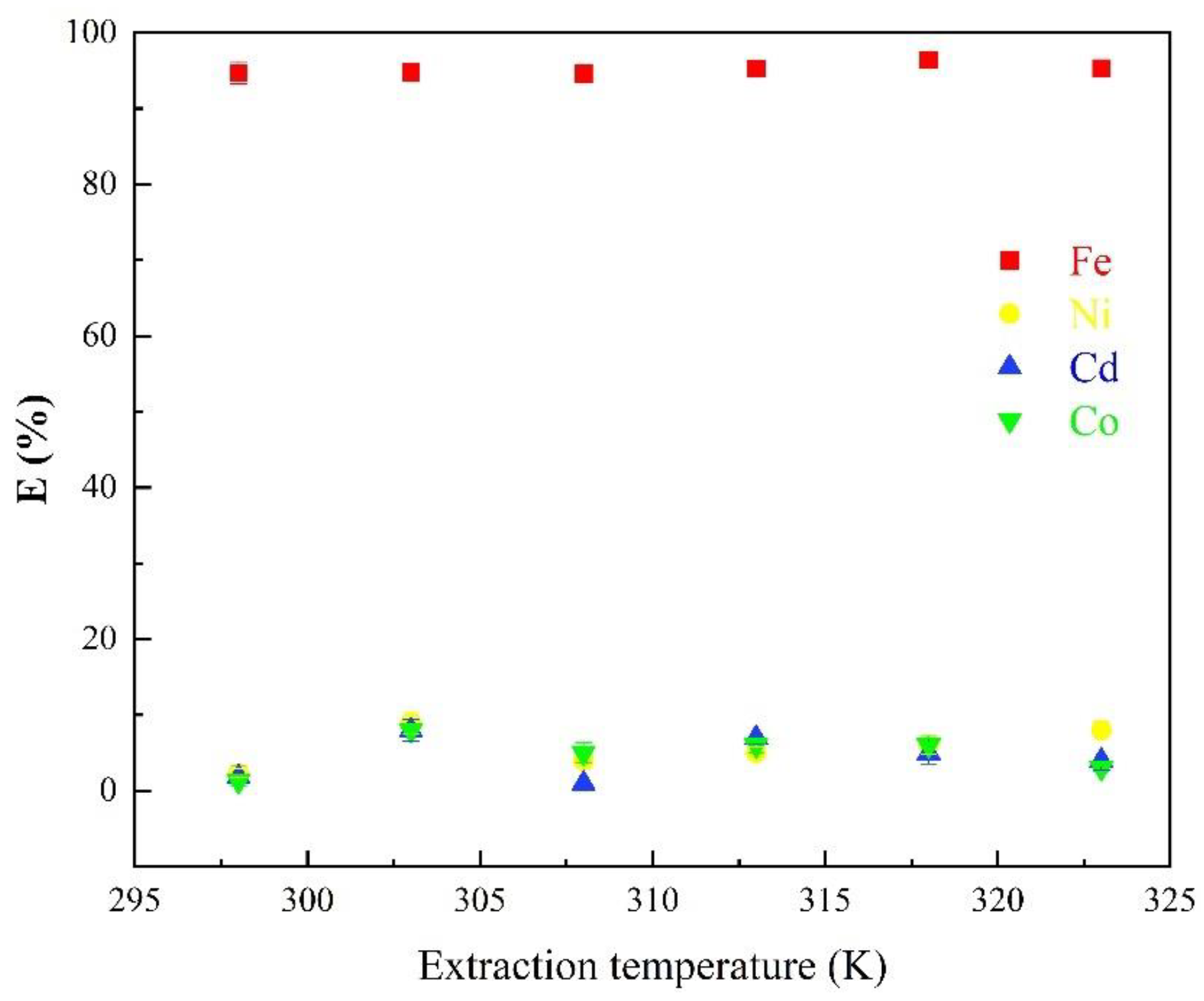
3.1.4. Effects of Temperature on the Extraction
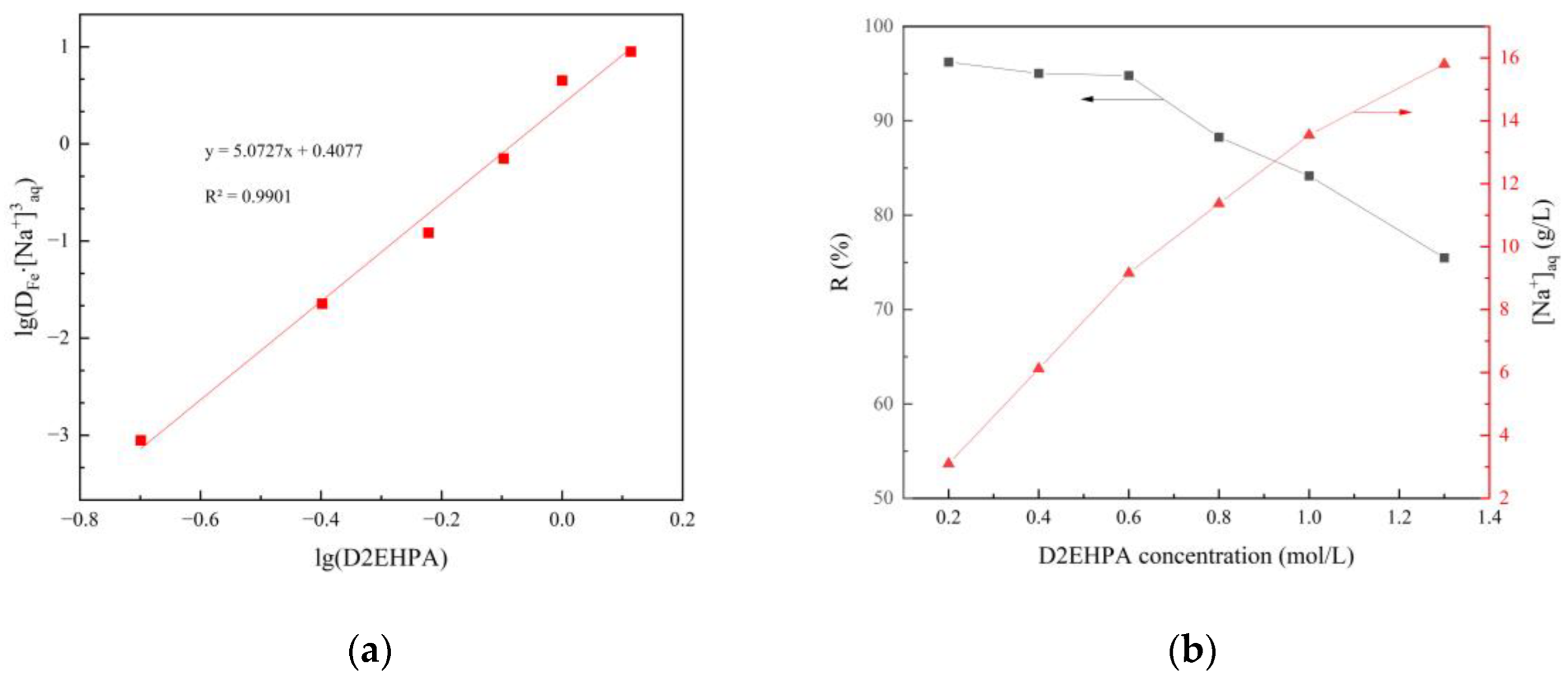
3.1.5. Effects of D2EHPA Concentration on the Extraction
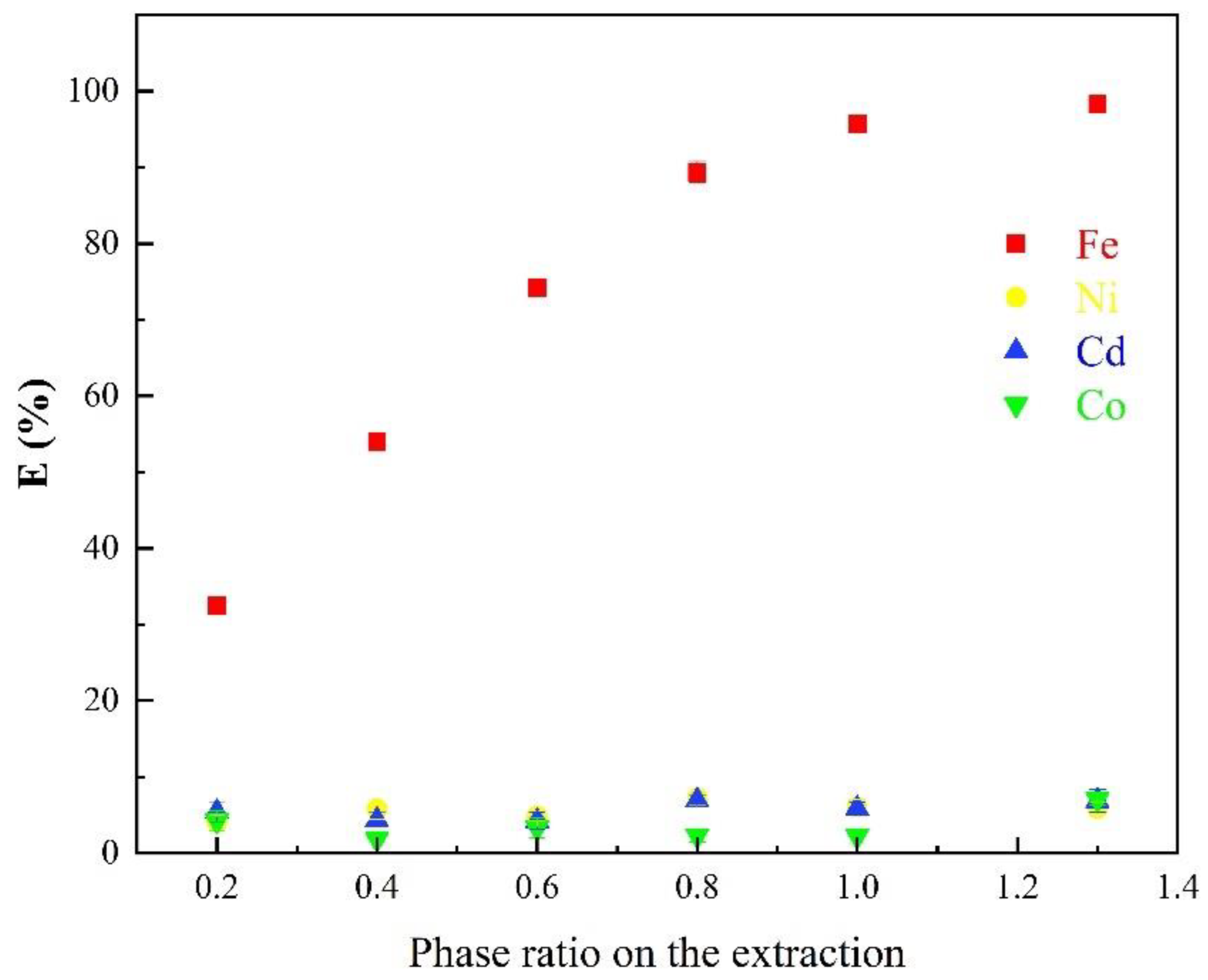
3.1.6. Effects of the Phase Ratio on the Extraction
3.2. Extraction Mechanism
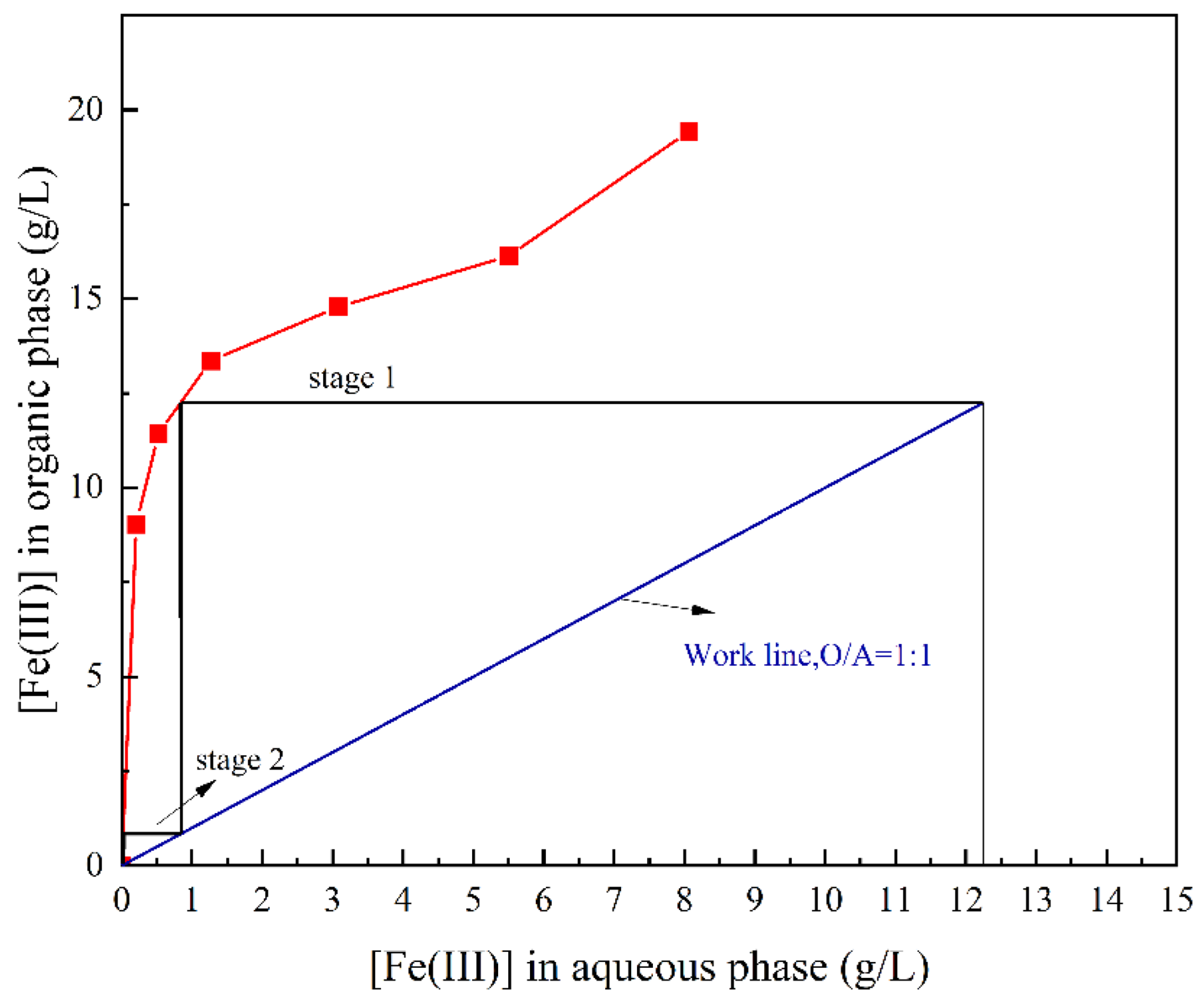
3.2.1. Slope Analysis of the Extraction
3.2.2. FT-IR Analysis of the D2EHPA after Extraction
3.3. Stripping of Loaded D2EHPA
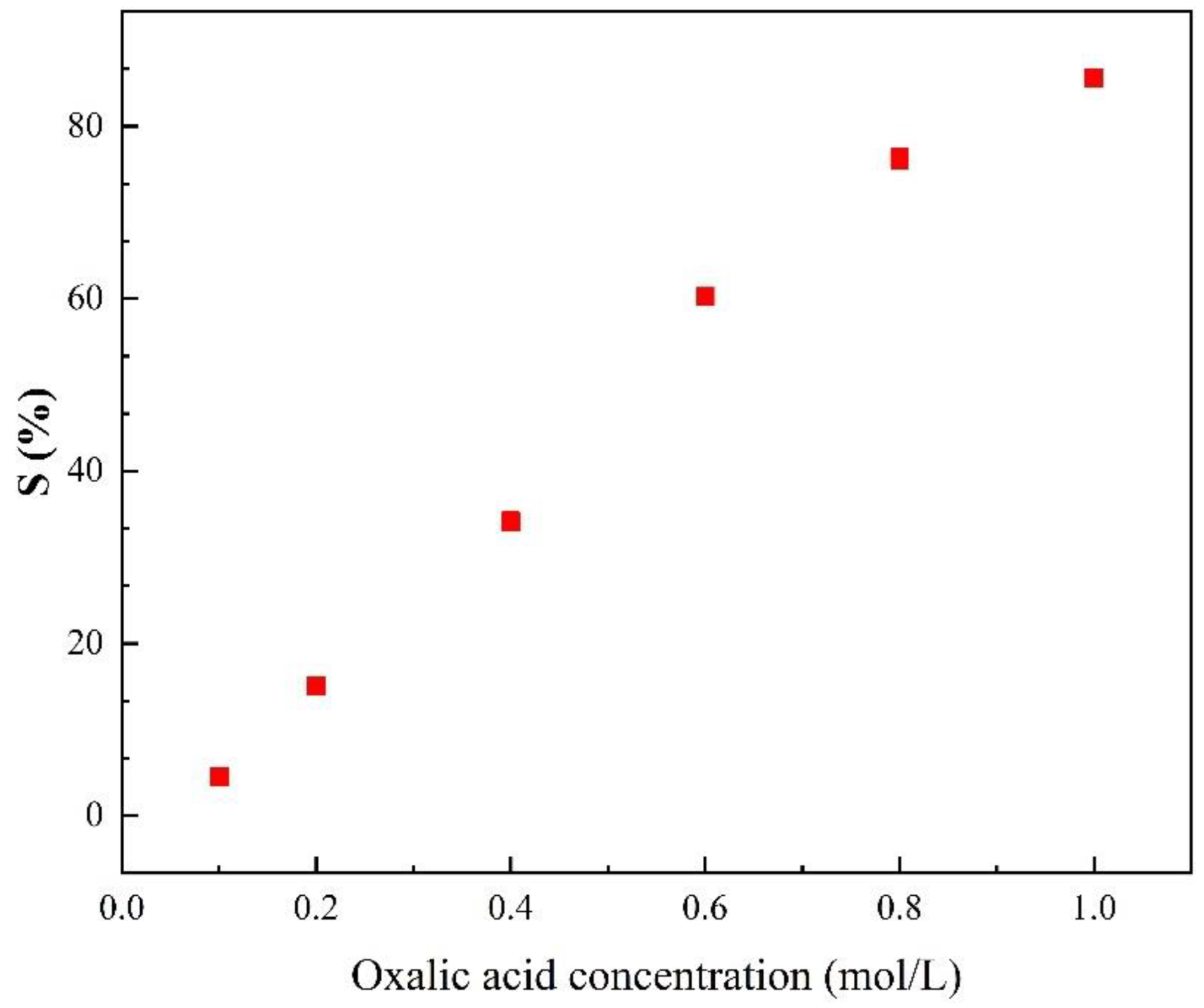
3.3.1. Effects of Oxalic Acid Concentration on the Stripping
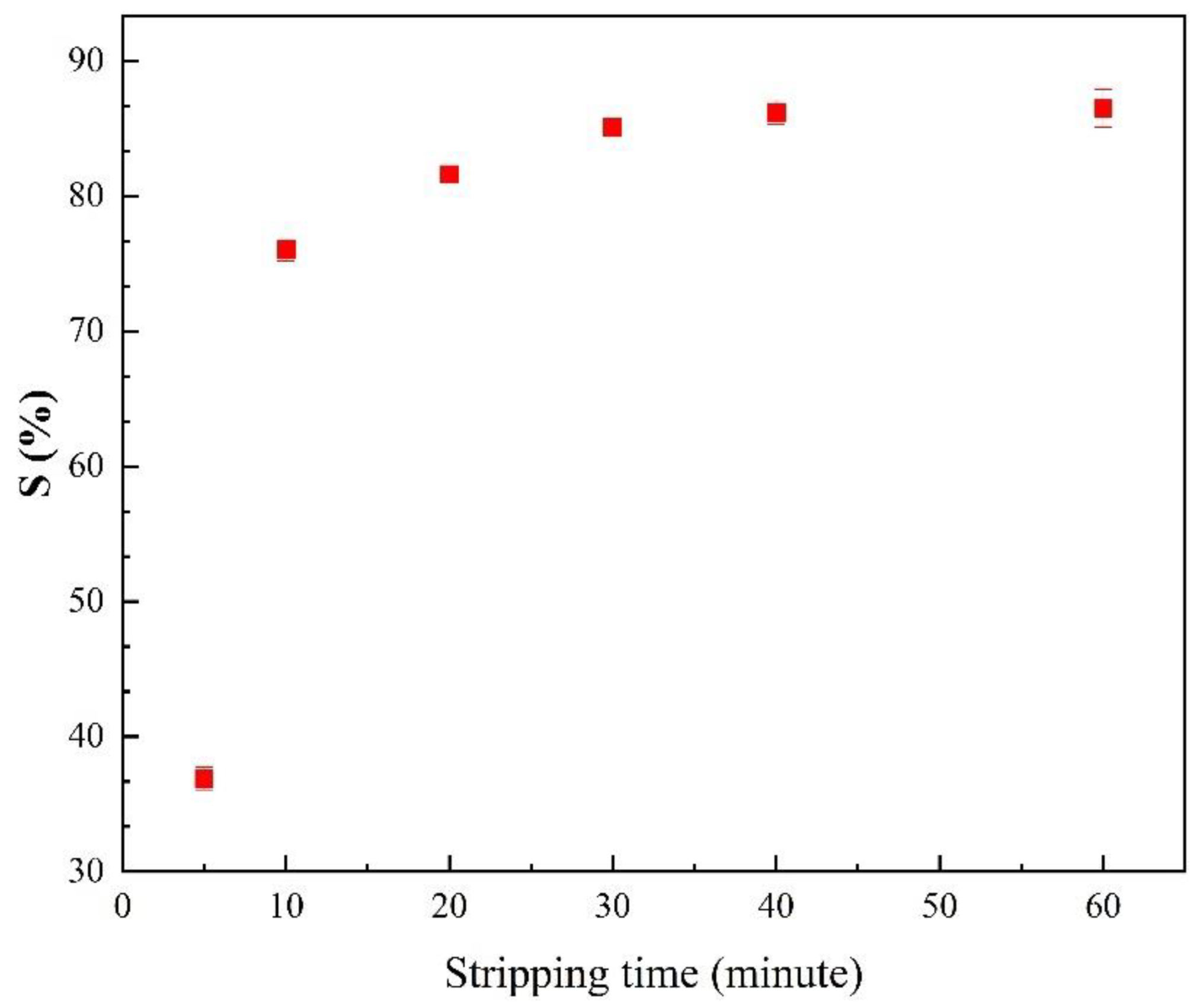
3.3.2. Effects of Stripping Time
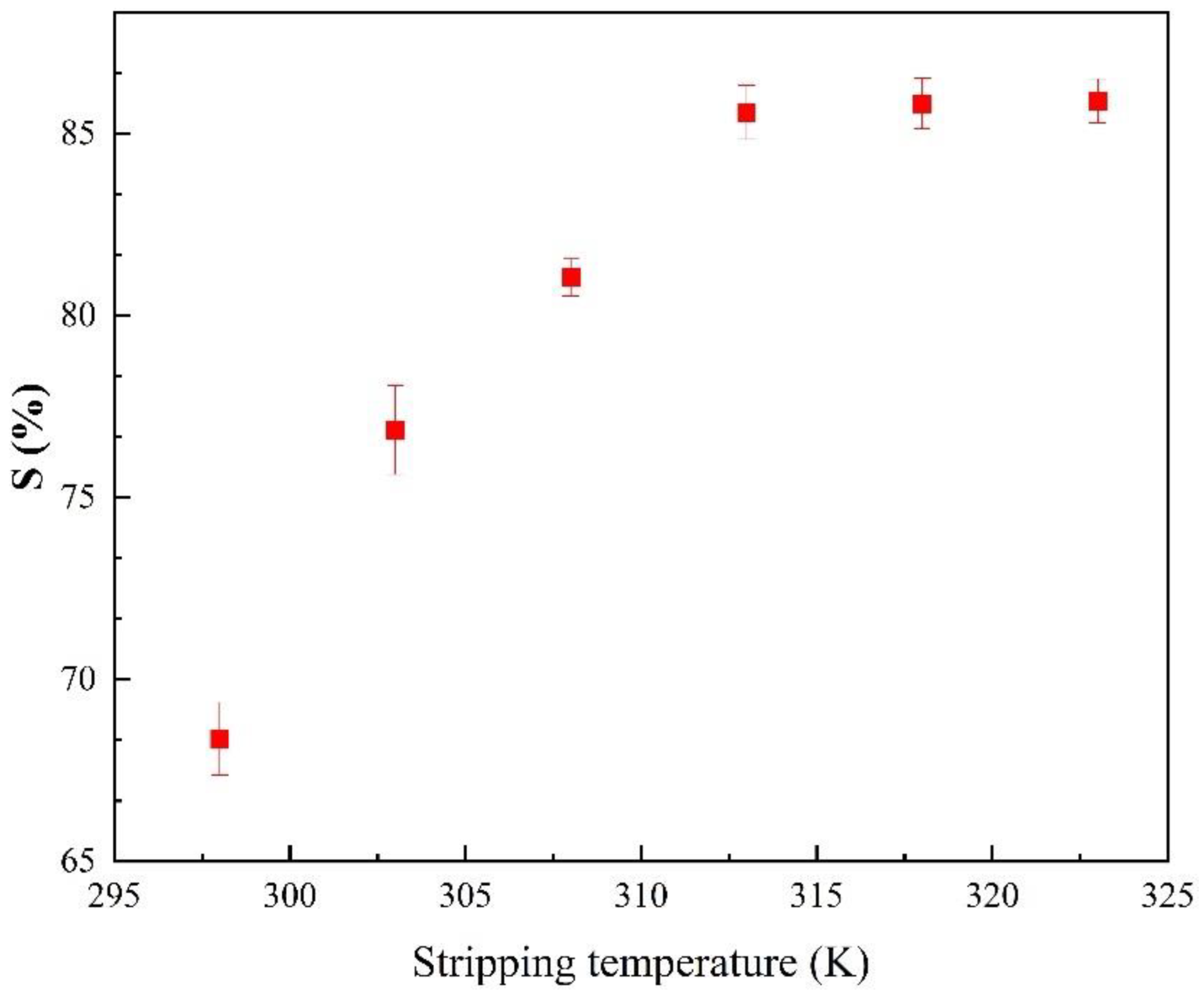
3.3.3. Effects of Stripping Temperature
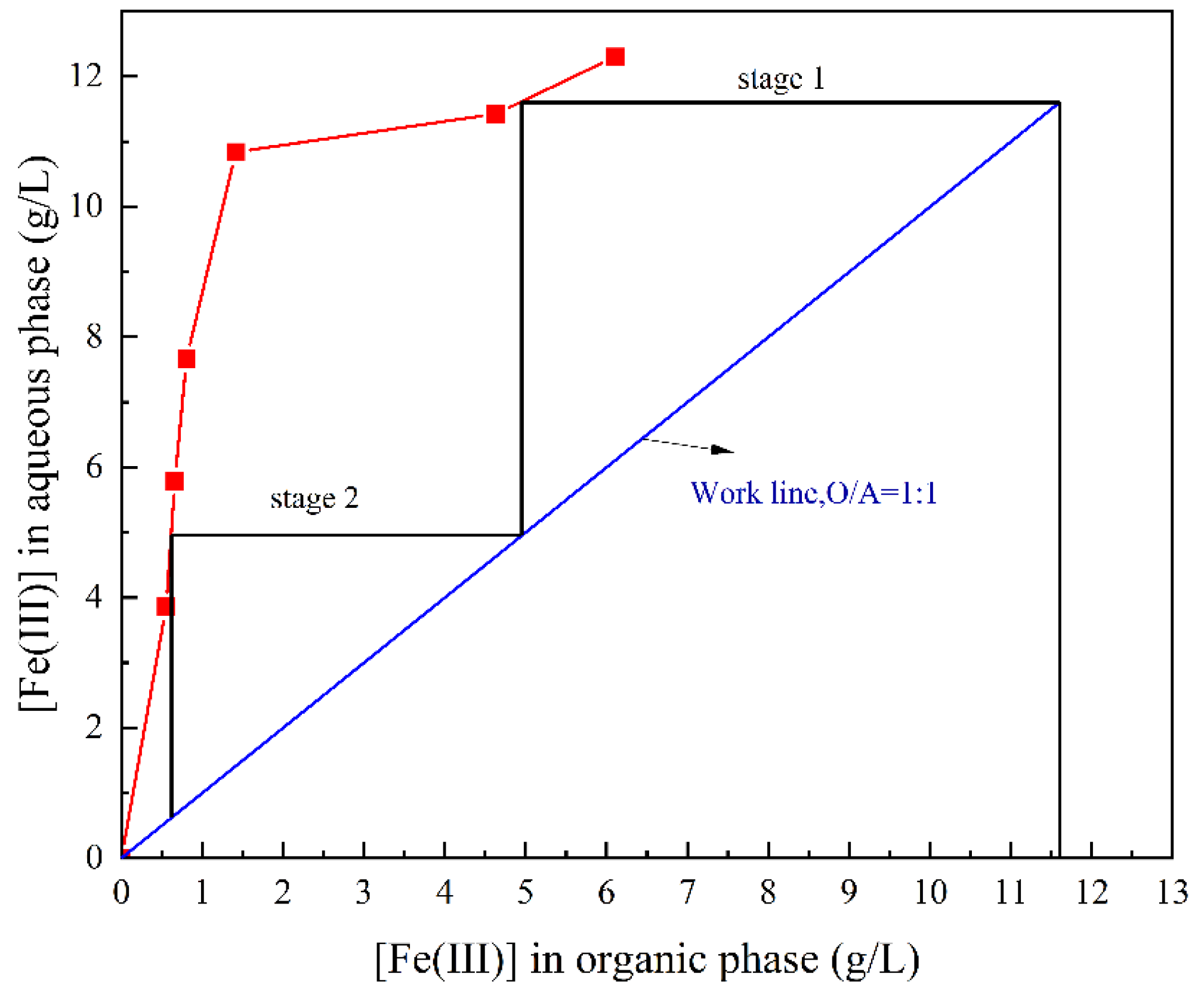
3.3.4. Effects of the Phase Ratio on the Stripping
3.3.5. Stripping Experiment under the Optimal Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium Recovery from Spent Ni-Cd Batteries: A Brief Review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- Guo, X.; Song, Y.; Nan, J. Flow evaluation of the leaching hazardous materials from spent nickel-cadmium batteries discarded in different water surroundings. Environ. Sci. Pollut. Res. Int. 2018, 25, 5514–5520. [Google Scholar] [CrossRef] [PubMed]
- Abidli, A.; Huang, Y.; Ben Rejeb, Z.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yu, L.; Cui, K.; Fu, T.; Mao, H. Effective separation and recovery of valuable metals from waste Ni-based batteries: A comprehensive review. Chem. Eng. J. 2022, 439, 135767. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Fan, E.; Xue, Q.; Bian, Y.; Wu, F.; Chen, R. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 2018, 47, 7239–7302. [Google Scholar] [CrossRef]
- Randhawa, N.S.; Gharami, K.; Kumar, M. Leaching kinetics of spent nickel–cadmium battery in sulphuric acid. Hydrometallurgy 2016, 165, 191–198. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J. Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Sahu, K.K. Iron and Copper Recovery/Removal from Industrial Wastes: A Review. Ind. Eng. Chem. Res. 2009, 48, 6145–6161. [Google Scholar] [CrossRef]
- El-Asmy, A.A.; Serag, H.M.; Mahdy, M.A.; Amin, M.I. Purification of phosphoric acid by minimizing iron, copper, cadmium and fluoride. Sep. Purif. Technol. 2008, 61, 287–292. [Google Scholar] [CrossRef]
- Vinco, J.H.; Botelho Junior, A.B.; Duarte, H.A.; Espinosa, D.C.R.; Tenório, J.A.S. Purification of an iron contaminated vanadium solution through ion exchange resins. Miner. Eng. 2022, 176, 107337. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Zhang, M.; Meng, X.; Peng, X.; Zeb, S.; Lu, Y.; Qiao, D.; Cui, Y.; Sun, G. Removal of Fe(III) from sulfuric acid leaching solution of phosphate ores with bisphosphonic acids. Hydrometallurgy 2022, 208, 105799. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Y.; Chen, D.; Wang, Y.; Qi, T.; Wang, L. Selective removal of iron(III) from highly salted chloride acidic solutions by solvent extraction using di(2-ethylhexyl) phosphate. Front. Chem. Sci. Eng. 2020, 15, 528–537. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Cui, L.; Gao, J.; Guo, Y.; Cheng, F. A sustainable approach for advanced removal of iron from CFA sulfuric acid leach liquor by solvent extraction with P507. Sep. Purif. Technol. 2020, 251, 117371. [Google Scholar] [CrossRef]
- Ma, H.-R.; Li, H.; Wu, W.; Qiao, X.-R. Separation of Fe(III) and Cr(III) from tannery sludge bioleachate using organophosphorus acid extractants. Res. Chem. Intermed. 2016, 43, 2333–2350. [Google Scholar] [CrossRef]
- Yi, X.; Huo, G.; Tang, W. Removal of Fe(III) from Ni-Co-Fe chloride solutions using solvent extraction with TBP. Hydrometallurgy 2020, 192, 105265. [Google Scholar] [CrossRef]
- Pavón, S.; Haneklaus, N.; Meerbach, K.; Bertau, M. Iron(III) removal and rare earth element recovery from a synthetic wet phosphoric acid solution using solvent extraction. Miner. Eng. 2022, 182, 107569. [Google Scholar] [CrossRef]
- Lupi, C.; Pilone, D. Effectiveness of saponified D2EHPA in Zn(II) selective extraction from concentrated sulphuric solutions. Miner. Eng. 2020, 150, 106278. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z.; He, L. Lithium recovery from Li3PO4 leaching liquor: Solvent extraction mechanism of saponified D2EHPA system. Sep. Purif. Technol. 2020, 249, 117161. [Google Scholar] [CrossRef]
- Song, Y.; He, L.; Zhao, Z.; Liu, X. Separation and recovery of lithium from Li3PO4 leaching liquor using solvent extraction with saponified D2EHPA. Sep. Purif. Technol. 2019, 229, 115823. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Delmas, F. New flowsheet for the recovery of cadmium, cobalt and nickel from spent Ni–Cd batteries by solvent extraction. Hydrometallurgy 1999, 52, 267–287. [Google Scholar] [CrossRef]
- Kim, H.-I.; Moon, G.; Choi, I.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical process development for the extraction, separation and recovery of vanadium from spent desulfurization catalyst bio-leach liquors. J. Clean. Prod. 2018, 187, 449–458. [Google Scholar] [CrossRef]
- Jin, Y.; Ma, Y.; Weng, Y.; Jia, X.; Li, J. Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene. J. Ind. Eng. Chem. 2014, 20, 3446–3452. [Google Scholar] [CrossRef]
- Belhadj, N.; Benabdallah, T.; Coll, M.T.; Fortuny, A.; Hadj Youcef, M.; Sastre, A.M. Counter-current separation of cobalt(II)–nickel(II) from aqueous sulphate media with a mixture of Primene JMT-Versatic 10 diluted in kerosene. Sep. Sci. Technol. 2019, 55, 513–522. [Google Scholar] [CrossRef]




| Elements | Content (g/L) |
|---|---|
| Fe | 12.25 |
| Ni | 56.30 |
| Cd | 23.28 |
| Co | 0.52 |
| Elements | Metal Concentration in the Stripping Aqueous (g/L) | Metal Stripping Rate |
|---|---|---|
| Fe | 9.951 | 85.51% |
| Ni | 0.011 | 0.85% |
| Cd | 0.018 | 3.53% |
| Co | 0.006 | 28.93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhang, Y.; Zhang, L.; Wu, X.; Jiang, R.; Wang, L. High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA. Separations 2023, 10, 251. https://doi.org/10.3390/separations10040251
Zhou L, Zhang Y, Zhang L, Wu X, Jiang R, Wang L. High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA. Separations. 2023; 10(4):251. https://doi.org/10.3390/separations10040251
Chicago/Turabian StyleZhou, Lei, Yongqing Zhang, Lijin Zhang, Xuefeng Wu, Ran Jiang, and Lu Wang. 2023. "High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA" Separations 10, no. 4: 251. https://doi.org/10.3390/separations10040251
APA StyleZhou, L., Zhang, Y., Zhang, L., Wu, X., Jiang, R., & Wang, L. (2023). High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA. Separations, 10(4), 251. https://doi.org/10.3390/separations10040251