ZIF-8 Assisted Polyacrylamide Functionalized Silica Core-Shell Stationary Phase for Hydrophilic Interaction Liquid Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Preparation of the ZIF-8
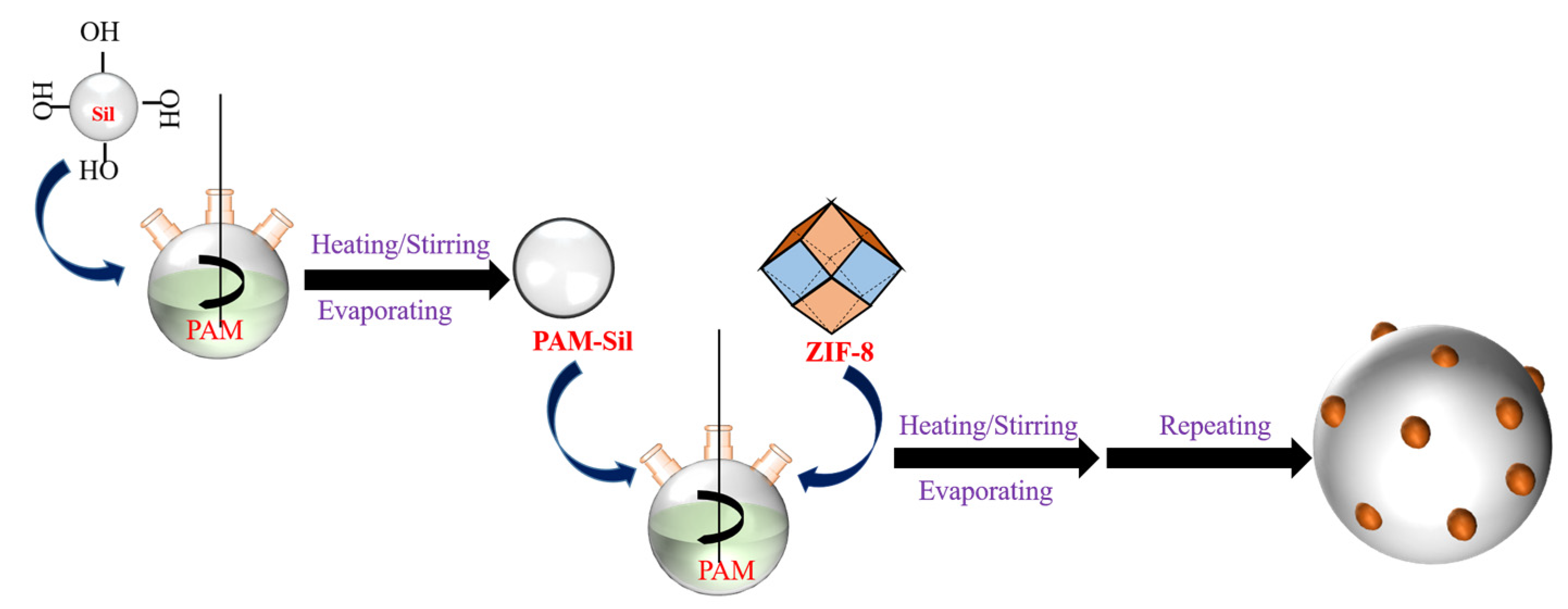
2.3. Preparation of the ZIF-8 and PAM Co-Functionalized Silica Stationary Phase and Column Packing
3. Results and Discussion
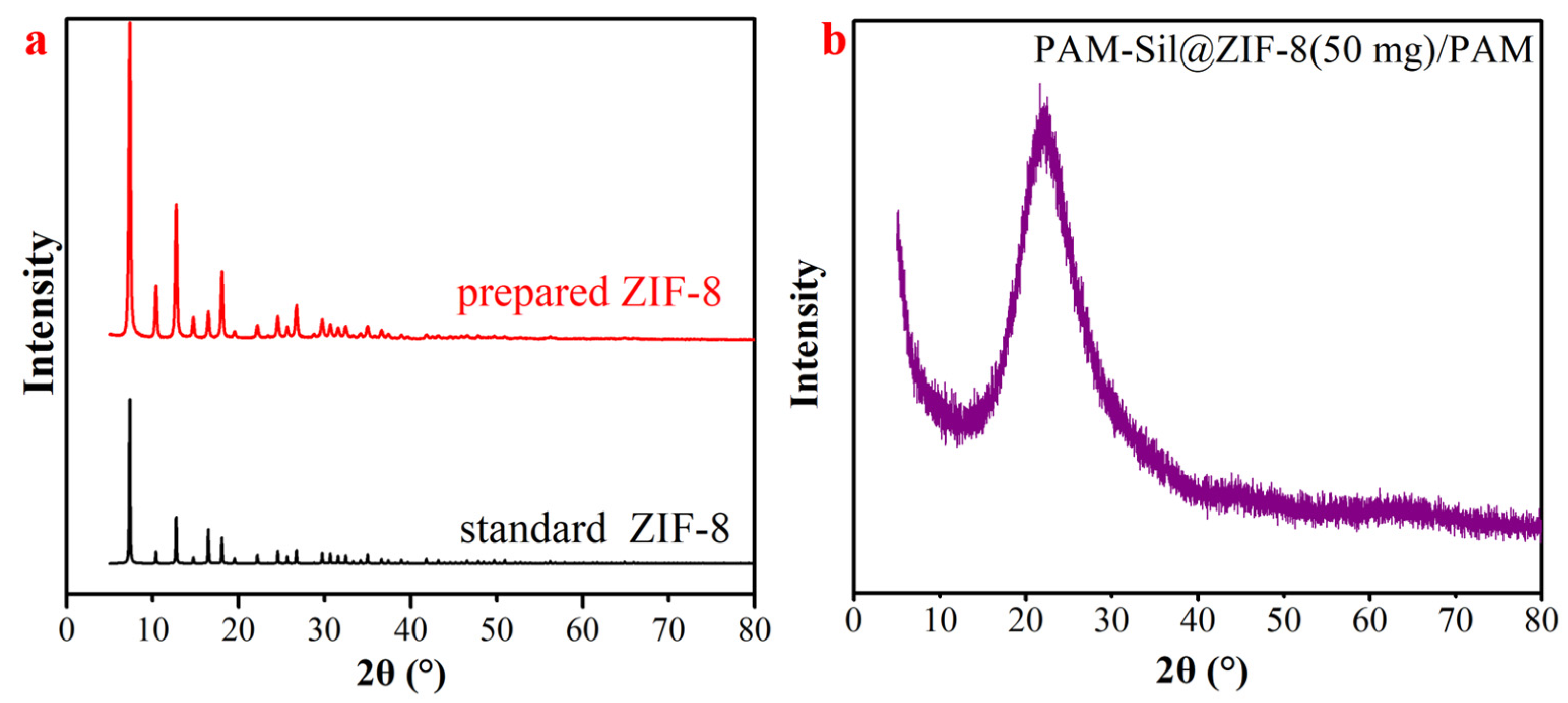
3.1. Characterization of the Prepared Materials
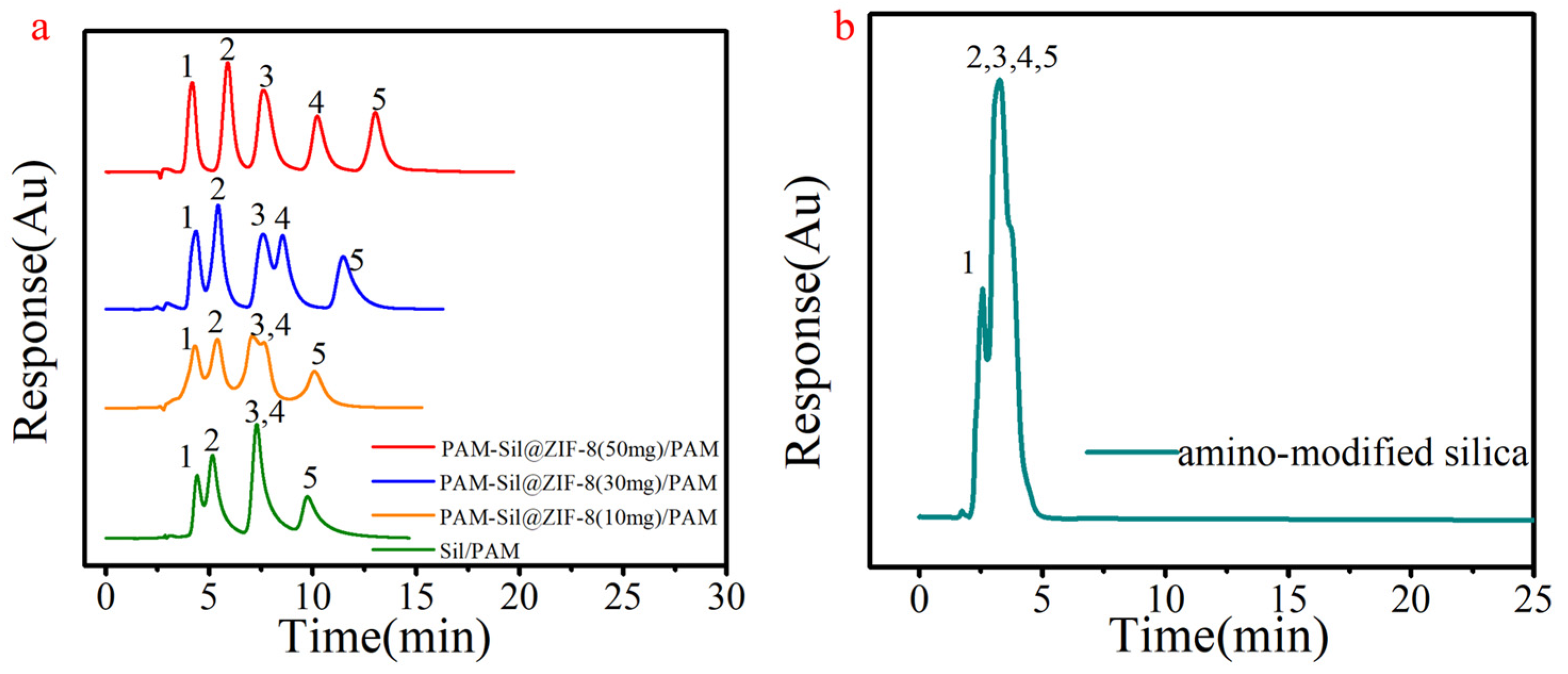
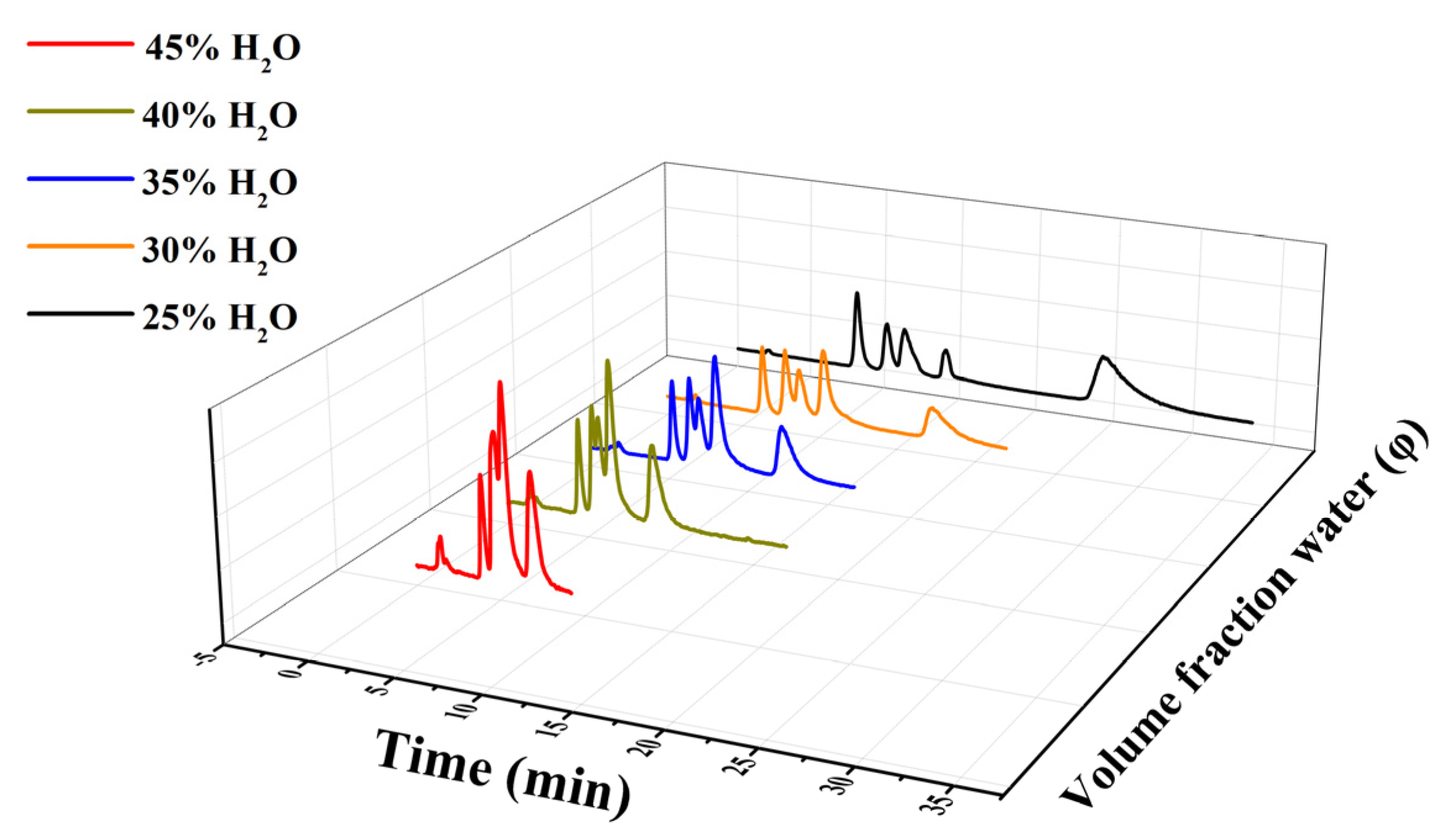
3.2. Effect of ZIF-8 on Chromatographic Separation
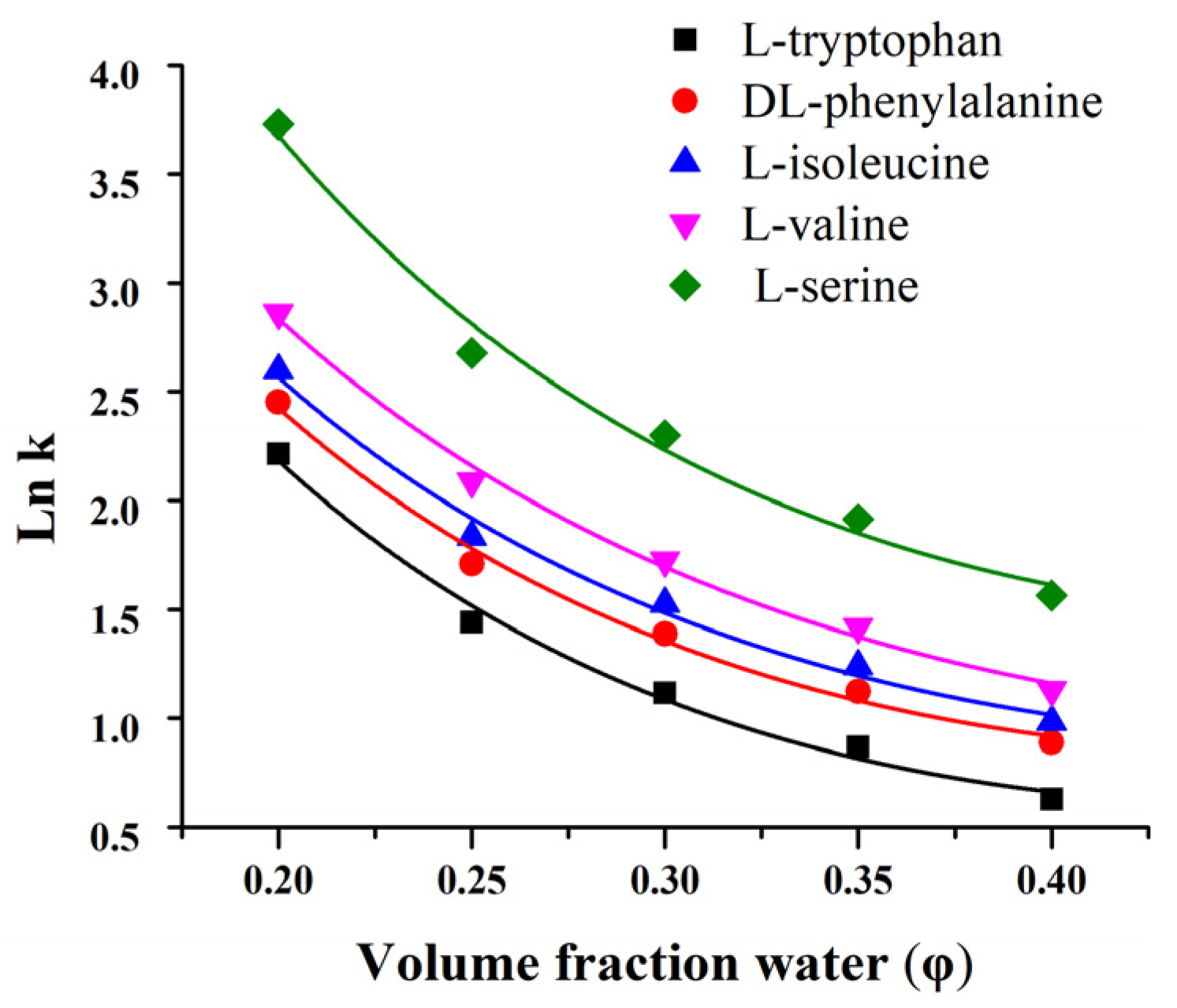
3.3. Adherence to a Quantitative HILIC Retention Model
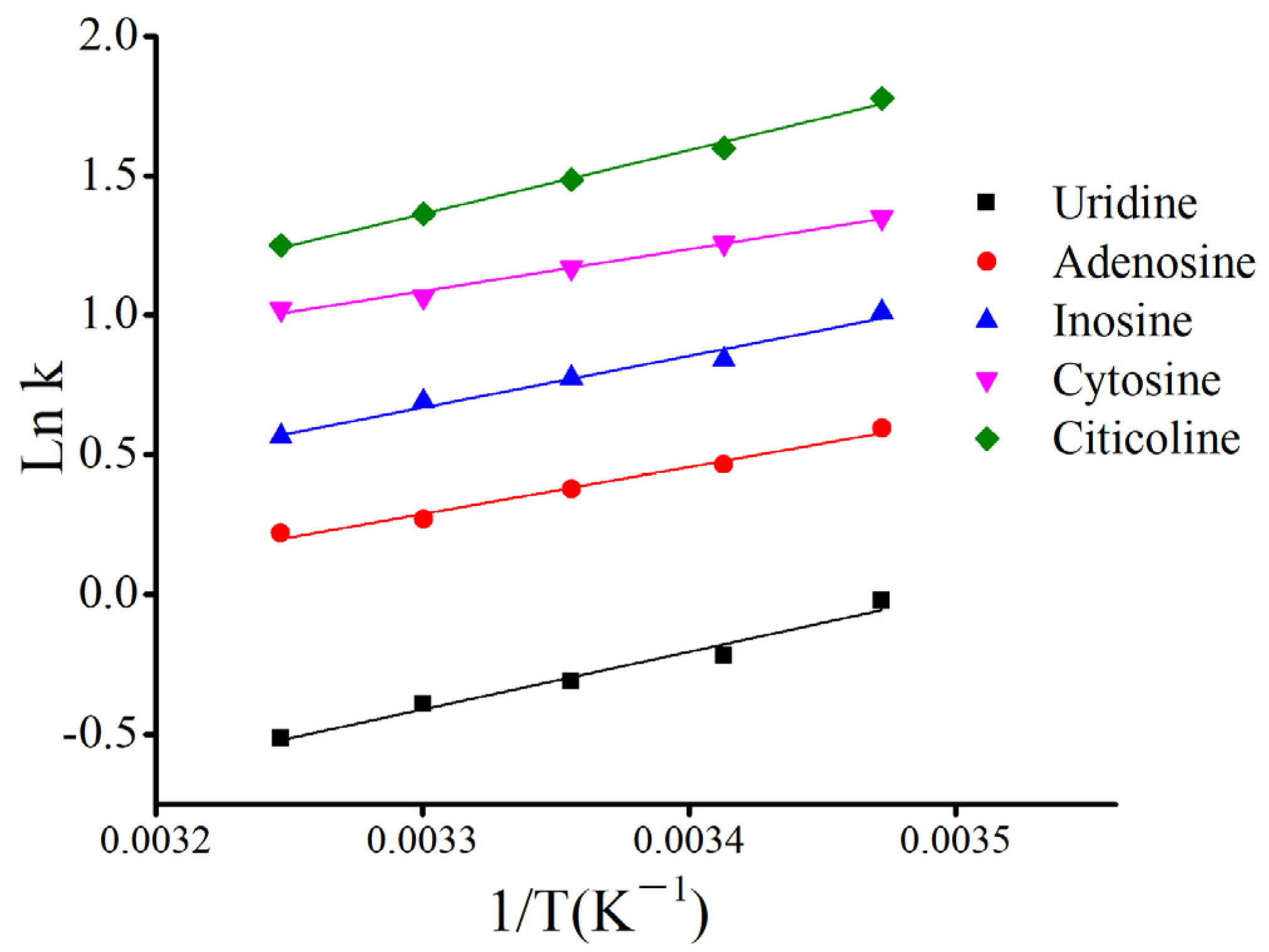
3.4. Effect of Column Temperature
3.5. Separation Performance of Alkaloids
3.6. Separation Performance of Amino Acids and Saccharides
3.7. Stability and Reproducibility of PAM-Sil@ZIF-8(50 mg)/PAM for Separation
3.8. Comparison with Other Stationary Phases in Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides nucleic acids and other polar compounds. J. Chromatogr. 1990, 499, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Janas, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Jing, J.; Zhang, F.; Zhang, F.; Yang, B. A polar stationary phase obtained by surface-initiated polymerization of hyperbranched polyglycerol onto silica. Talanta 2020, 209, 120525. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, Q.; Yang, B.; Zhang, F.; Chu, C.; Liang, X. Silica based click amino stationary phase for ion chromatography and hydrophilic interaction liquid chromatography. Analyst 2012, 137, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, F.; Yang, B.; Chu, C.; Liang, X. A novel amide stationary phase for hydrophilic interaction liquid chromatography and ion chromatography. Talanta 2013, 115, 129–132. [Google Scholar] [CrossRef]
- Qian, K.; Peng, Y.; Zhang, F.; Yang, B.; Liang, X. Preparation of a low bleeding polar stationary phase for hydrophilic interaction liquid chromatography. Talanta 2018, 182, 500–504. [Google Scholar] [CrossRef]
- Fan, F.; Lu, X.; Wang, L.; Liang, X.; Guo, Y. Hydrogel Coating with Temperature Response Retention Behavior and Its Application in Selective Separation of Liquid Chromatography. Anal. Chem. 2021, 93, 16017–16024. [Google Scholar] [CrossRef]
- Ji, S.; Zheng, Y.; Zhang, F.; Liang, X.; Yang, B. A polyvinyl alcohol-coated silica gel stationary phase for hydrophilic interaction chromatography. Analyst 2015, 140, 6250–6253. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Tang, L.; Goh, T.W.; Qi, S.; Volkov, A.; Pei, Y.; Qi, Z.; Tsung, C.K.; Stanley, L.; et al. Cooperative Multifunctional Catalysts for Nitrone Synthesis: Platinum Nanoclusters in Amine-Functionalized Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 16371–16375. [Google Scholar] [CrossRef]
- Suh, B.L.; Lee, S.; Kim, J. Size-Matching Ligand Insertion in MOF-74 for Enhanced CO2 Capture under Humid Conditions. J. Phys. Chem. C 2017, 121, 24444–24451. [Google Scholar] [CrossRef]
- Yang, C.X.; Yan, X.P. Metal-organic framework MIL-101(Cr) for high-performance liquid chromatographic separation of substituted aromatics. Anal. Chem. 2011, 83, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Yang, L.; Zeng, J.; Yan, X.; Wang, Q. Orderly MOF-Assembled Hybrid Monolithic Stationary Phases for Nano-Flow HPLC. Anal. Chem. 2020, 92, 15757–15765. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Liu, M.; Zhou, Y.; Zhang, L.; Qiao, X. Core-Shell Metal-Organic Frameworks as the Mixed-Mode Stationary Phase for Hydrophilic Interaction/Reversed-Phase Chromatography. ACS Appl. Mater. Interfaces 2019, 11, 10320–10327. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Ma, J.; Lu, X.; Wang, L.; Liang, X.; Wang, S. Core–Shell Metal–Organic Frameworks as the Stationary Phase for Hydrophilic Interaction Liquid Chromatography. ACS Appl. Nano Mater. 2020, 3, 351–356. [Google Scholar] [CrossRef]
- Si, T.; Liang, X.; Lu, X.; Wang, L.; Wang, S.; Guo, Y. 2D metal-organic framework nanosheets-assembled core-shell composite material as stationary phase for hydrophilic interaction liquid chromatography. Talanta 2021, 222, 121603. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Lu, X.; Wang, S.; Wang, L.; Liang, X.; Guo, Y. Mesoporous nanomaterial-assisted hydrogel double network composite for mixed-mode liquid chromatography. Mikrochim. Acta 2021, 188, 433. [Google Scholar] [CrossRef]
- Fan, F.; Lu, X.; Liang, X.; Wang, L.; Guo, Y. Preparation of hydrogel nanocomposite functionalized silica microspheres and its application in mixed-mode liquid chromatography. J. Chromatogr. A 2022, 1662, 462745. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Yang, C.X.; Yan, X.P. Fabrication of ZIF-8@SiO2 core-shell microspheres as the stationary phase for high-performance liquid chromatography. Chem. Eur. J. 2013, 19, 13484–13491. [Google Scholar] [CrossRef]
- Wei, Q.; Lian, C.; Su, H.; Gao, D.; Wang, S. Composite of ZIF-8 and totally porous silica gel for HPLC stationary phase. Microporous Mesoporous Mater. 2019, 288, 109574. [Google Scholar] [CrossRef]
- Lan, X.; Xue, K.; Wang, T. Combined synergetic and steric effects for highly selective hydrogenation of unsaturated aldehyde. J. Catal. 2019, 372, 49–60. [Google Scholar] [CrossRef]
- Jin, G.; Guo, Z.; Zhang, F.; Xue, X.; Jin, Y.; Liang, X. Study on the retention equation in hydrophilic interaction liquid chromatography. Talanta 2008, 76, 522–527. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, F.; Liang, X.; Yang, B.; Liu, X.; Dasgupta, P.K. Poly(vinyl alcohol) Modified Porous Graphitic Carbon Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Anal. Chem. 2016, 88, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Wang, S.; Zhang, H.; Lu, X.; Wang, L.; Liang, X.; Guo, Y. An alternative strategy to construct uniform MOFs-Grafted silica core-shell composites as mixed-mode stationary phase for chromatography separation. Anal. Chim. Acta 2021, 1183, 338942. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Lu, X.; Wang, S.; Liang, X.; Wang, L.; Guo, Y. Non-conjugated flexible network for the functional design of silica-base stationary phase for mixed-mode liquid chromatography. Talanta 2021, 233, 122548. [Google Scholar] [CrossRef]
- Si, M.; Li, X.; Cheng, T.; Yu, Q. Preparation and characterization of perhydroxyl-cucurbit[6]uril bonded silica stationary phase for hydrophilic-interaction chromatography. Talanta 2004, 64, 929–934. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Li, Y.; Lu, X.; Guo, Y.; Wang, L.; Liang, X. ZIF-8 Assisted Polyacrylamide Functionalized Silica Core-Shell Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Separations 2023, 10, 219. https://doi.org/10.3390/separations10030219
Zhang T, Li Y, Lu X, Guo Y, Wang L, Liang X. ZIF-8 Assisted Polyacrylamide Functionalized Silica Core-Shell Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Separations. 2023; 10(3):219. https://doi.org/10.3390/separations10030219
Chicago/Turabian StyleZhang, Tong, Yijing Li, Xiaofeng Lu, Yong Guo, Licheng Wang, and Xiaojing Liang. 2023. "ZIF-8 Assisted Polyacrylamide Functionalized Silica Core-Shell Stationary Phase for Hydrophilic Interaction Liquid Chromatography" Separations 10, no. 3: 219. https://doi.org/10.3390/separations10030219
APA StyleZhang, T., Li, Y., Lu, X., Guo, Y., Wang, L., & Liang, X. (2023). ZIF-8 Assisted Polyacrylamide Functionalized Silica Core-Shell Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Separations, 10(3), 219. https://doi.org/10.3390/separations10030219






