Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Experimental Method
3. Results
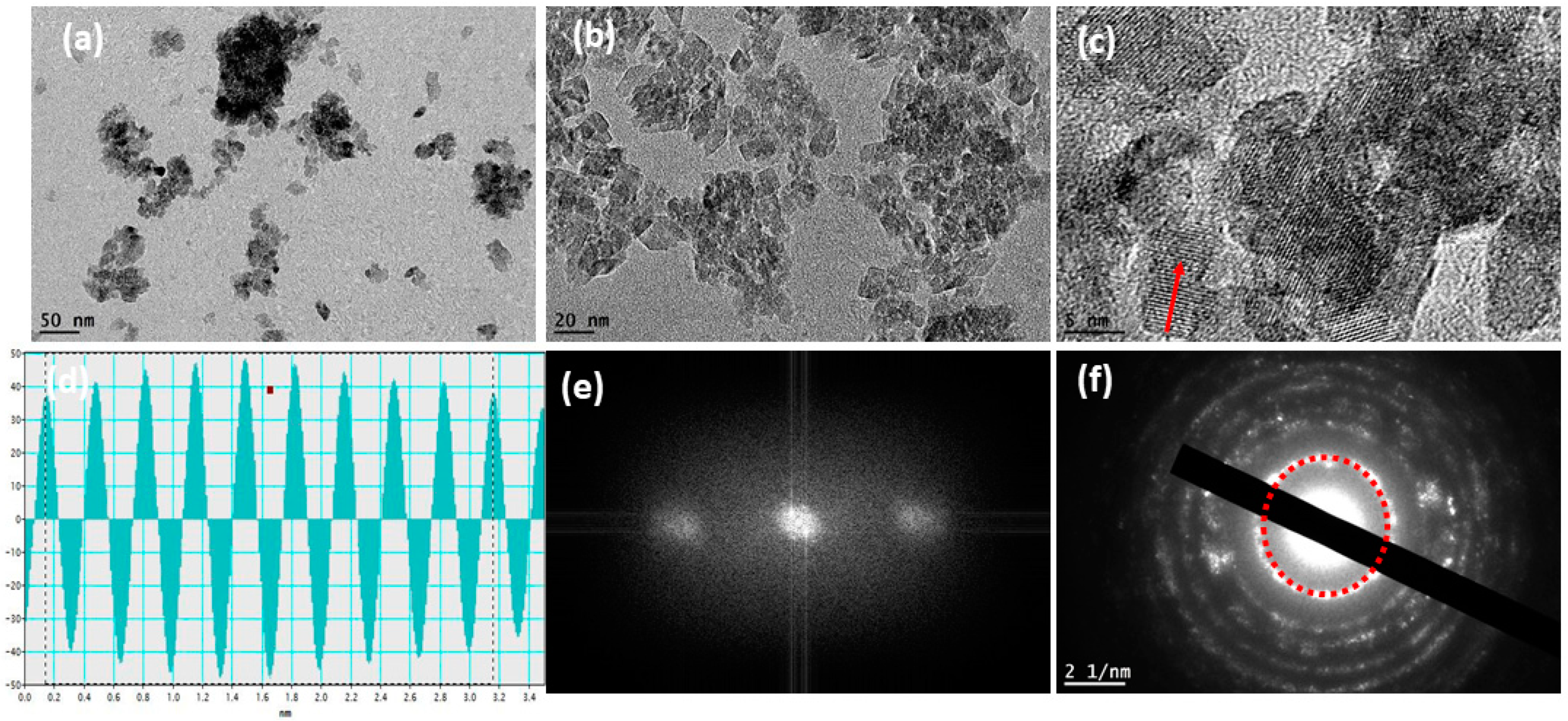
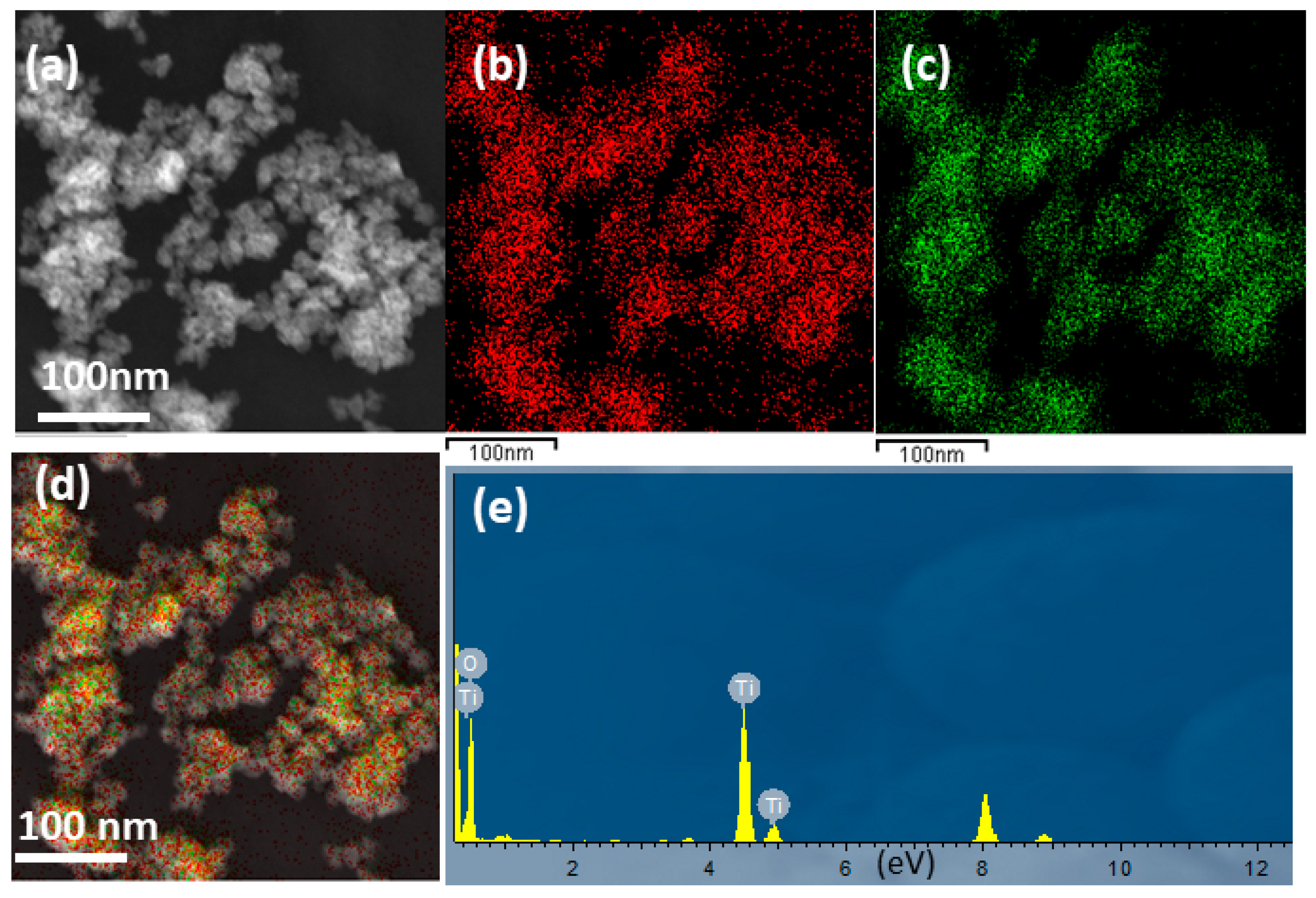
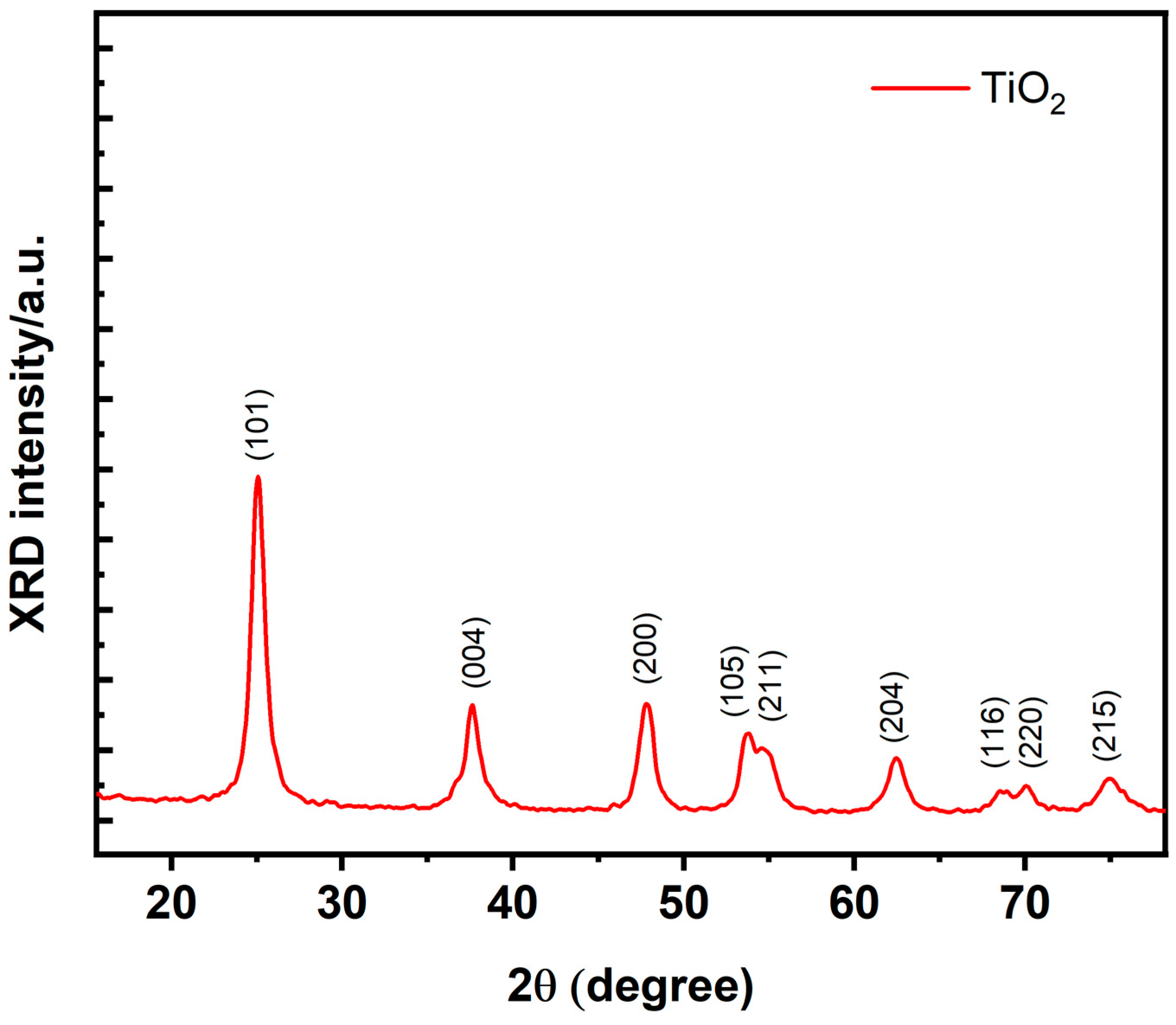
3.1. Structural Study
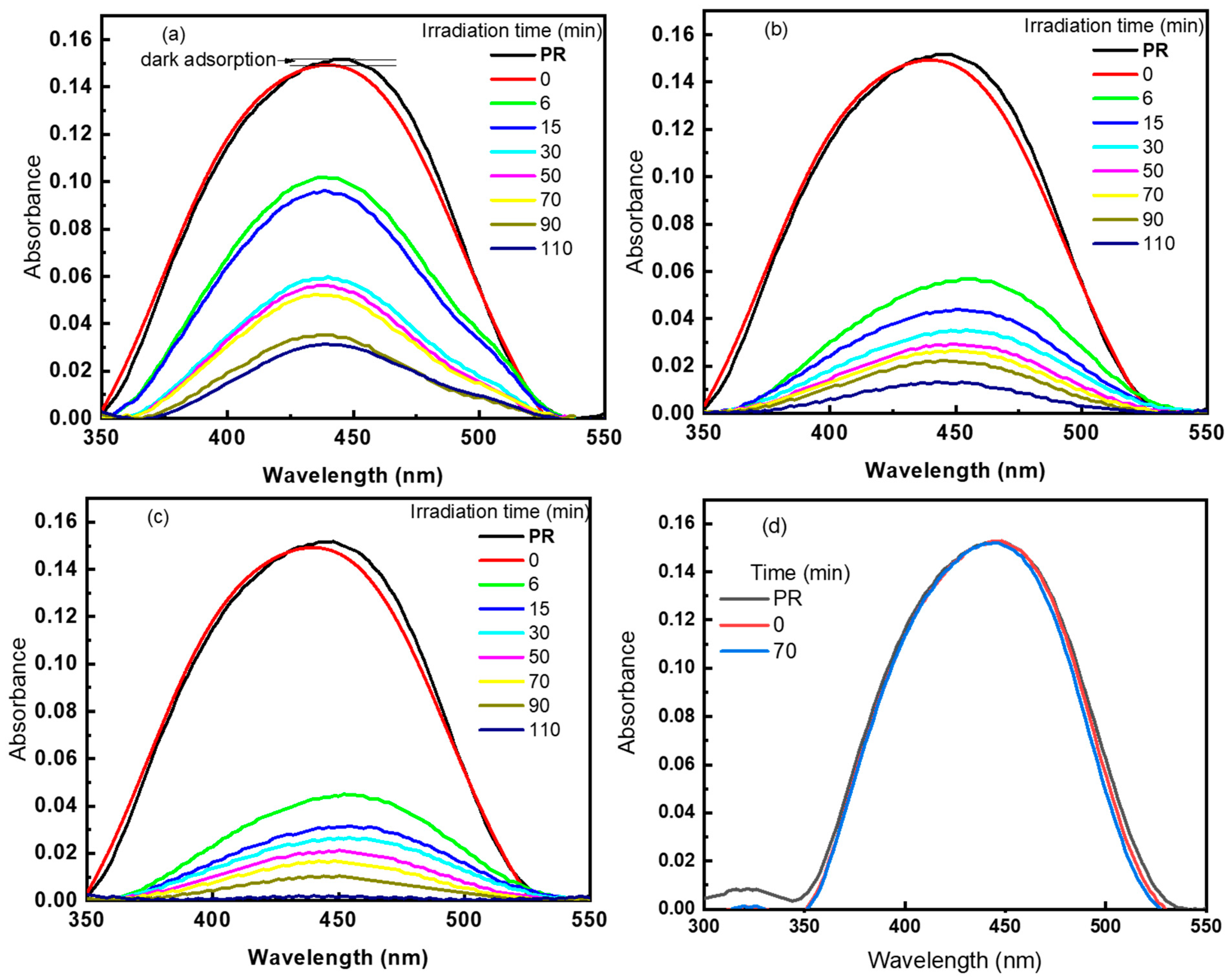
3.2. Phenol Red Photodegradation
3.3. Photocatalysis Reactions and KBrO3 Mechanism
3.4. Analysis of Photodecomposition Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.; Cartine, J.; Jönander, C.; Karlsson, M.; Langlois, J.; Lindström, M.; Lundin, J.; Melander, N.; Pesqueda, A.; Rahmqvist, I.; et al. Assessing the effects of textile leachates in fish using multiple testing methods: From gene expression to behavior. Ecotoxicol. Environ. Saf. 2021, 207, 111523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Pollution and Treatment of Dye Waste-Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 52001. [Google Scholar] [CrossRef]
- Gautam, A.; Rawat, S.; Verma, L.; Singh, J.; Sikarwar, S.; Yadav, B.C.; Kalamdhad, A.S. Green synthesis of iron nanoparticle from extract of waste tea: An application for phenol red removal from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2018, 10, 377–387. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Madhusudan Reddy, K.; Manorama, S.V.; Ramachandra Reddy, A. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO 2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Dharmale, N.; Chaudhury, S.; Mahamune, R.; Dash, D. Comparative study on structural, electronic, optical and mechanical properties of normal and high pressure phases titanium dioxide using {DFT}. Mater. Res. Express 2020, 7, 54004. [Google Scholar] [CrossRef]
- Ahmed, F.; Kanoun, M.B.; Awada, C.; Jonin, C.; Brevet, P.-F. An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals. Crystals 2021, 11, 1488. [Google Scholar] [CrossRef]
- Dues, C.; Schmidt, W.G.; Sanna, S. Water Splitting Reaction at Polar Lithium Niobate Surfaces. ACS Omega 2019, 4, 3850–3859. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Rho, Y.; Allen, F.I.; Minor, A.M.; Ko, S.H.; Grigoropoulos, C.P. Synthesis of hierarchical TiO2 nanowires with densely-packed and omnidirectional branches. Nanoscale 2013, 5, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Saranya, K.S.; Vellora Thekkae Padil, V.; Senan, C.; Pilankatta, R.; Saranya, K.; George, B.; Wacławek, S.; Černík, M. Green Synthesis of High Temperature Stable Anatase Titanium Dioxide Nanoparticles Using Gum Kondagogu: Characterization and Solar Driven Photocatalytic Degradation of Organic Dye. Nanomaterials 2018, 8, 1002. [Google Scholar] [CrossRef]
- Wei, Z.; Li, R.; Huang, T.; Yu, A. Fabrication of morphology controllable rutile TiO2 nanowire arrays by solvothermal route for dye-sensitized solar cells. Electrochim. Acta 2011, 56, 7696–7702. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Qi, H.-P.; Wang, H.-L.; Zhao, D.-Y.; Jiang, W.-F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Chandanshive, V.; Kadam, S.; Rane, N.; Jeon, B.-H.; Jadhav, J.; Govindwar, S. In situ textile wastewater treatment in high rate transpiration system furrows planted with aquatic macrophytes and floating phytobeds. Chemosphere 2020, 252, 126513. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Zeng, X.; Li, M. Effects of Electron Donors on the TiO2 Photocatalytic Reduction of Heavy Metal Ions under Visible Light. Energy Procedia 2012, 17, 422–428. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Manchala, S.; Danquah, M.K. Wastewater Treatment by Photocatalytic Biosynthesized Nanoparticles. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3135–3157. ISBN 978-3-030-36268-3. [Google Scholar]
- Almohammed, S.; Fularz, A.; Kanoun, M.B.; Goumri-Said, S.; Aljaafari, A.; Rodriguez, B.J.; Rice, J.H. Structural Transition-Induced Raman Enhancement in Bioinspired Diphenylalanine Peptide Nanotubes. ACS Appl. Mater. Interfaces 2022, 14, 12504–12514. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide—Solar process. Arab. J. Chem. 2016, 9, S1858–S1868. [Google Scholar] [CrossRef]
- Wahab, H.S.; Hussain, A.A. Photocatalytic oxidation of phenol red onto nanocrystalline TiO2 particles. J. Nanostruct. Chem. 2016, 6, 261–274. [Google Scholar] [CrossRef]
- Asiri, A.M.; Al-Amoudi, M.S.; Al-Talhi, T.A.; Al-Talhi, A.D. Photodegradation of Rhodamine 6G and phenol red by nanosized TiO2 under solar irradiation. J. Saudi Chem. Soc. 2011, 15, 121–128. [Google Scholar] [CrossRef]
- Belattar, S.; Debbache, N.; Ghoul, I.; Sehili, T.; Abdessemed, A. Photodegradation of phenol red in the presence of oxyhydroxide of Fe(III) (Goethite) under artificial and a natural light. Water Environ. J. 2018, 32, 358–365. [Google Scholar] [CrossRef]
- Dhanalakshmi, R.; Muneeswaran, M.; Shalini, K.; Giridharan, N. V Enhanced photocatalytic activity of La-substituted BiFeO3 nanostructures on the degradation of phenol red. Mater. Lett. 2016, 165, 205–209. [Google Scholar] [CrossRef]
- Ahlawat, A.; Dhiman, T.K.; Solanki, P.R.; Rana, P.S. Enhanced Photocatalytic Degradation of p-Nitrophenol and Phenol Red Through Synergistic Effects of a CeO2-TiO2 Nanocomposite. Catal. Res. 2022, 2, 39. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Guo, H.; Yang, H.; Zhang, G.; Ji, S.; Zeng, T. Low-temperature synthesis of anatase TiO2 nanoparticles with tunable surface charges for enhancing photocatalytic activity. PLoS ONE 2014, 9, e114638. [Google Scholar] [CrossRef]
- Victoria Dimas, B.; Hernández Pérez, I.; Garibay Febles, V.; Díaz Barriga Arceo, L.; Suárez Parra, R.; Rivera Olvera, J.N.; Luna Paz, R.; Melo Máximo, D.V.; González Reyes, L. Atomic-Scale Investigation on the Evolution of Tio(2)-Anatase Prepared by a Sonochemical Route and Treated with NaOH. Materials 2020, 13, 685. [Google Scholar] [CrossRef]
- Orendorz, A.; Brodyanski, A.; Lösch, J.; Bai, L.H.; Chen, Z.H.; Le, Y.K.; Ziegler, C.; Gnaser, H. Phase transformation and particle growth in nanocrystalline anatase TiO2 films analyzed by X-ray diffraction and Raman spectroscopy. Surf. Sci. 2007, 601, 4390–4394. [Google Scholar] [CrossRef]
- Cheng, G.; Akhtar, M.S.; Yang, O.-B.; Stadler, F.J. Structure modification of anatase TiO2 nanomaterials-based photoanodes for efficient dye-sensitized solar cells. Electrochim. Acta 2013, 113, 527–535. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Radhakrishna, S.; Karguppikar, A.M. Raman Spectra of Irradiated Alkali Bromate Crystals. J. Phys. Soc. Jpn. 1973, 35, 578–581. [Google Scholar] [CrossRef]
- Bates, J.B.; Stidham, H.D. Isotope effects in Raman spectra of crystalline alkali-metal chlorates and bromates. J. Phys. Chem. Solids 1976, 37, 183–188. [Google Scholar] [CrossRef]
- Yan, H.; Hou, J.; Fu, Z.; Yang, B.; Yang, P.; Liu, K.; Wen, M.; Chen, Y.; Fu, S.; Li, F. Growth and photocatalytic properties of one-dimensional ZnO nanostructures prepared by thermal evaporation. Mater. Res. Bull. 2009, 44, 1954–1958. [Google Scholar] [CrossRef]
- Rashad, M.; Shaalan, N.M.; Abd-Elnaiem, A.M. Degradation enhancement of methylene blue on ZnO nanocombs synthesized by thermal evaporation technique. Desalin. Water Treat. 2016, 57, 26267–26273. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Almulhem, N.K.; Awada, C.; Alnaim, N.M.; Al Taisan, N.; Alshoaibi, A.A.; Shaalan, N.M. Synergistic Effect of the KBrO3 Electron Acceptor on the Photocatalytic Performance of the Nb-TiO2 Nanocomposite for Polluted Phenol Red Wastewater Treatment. Crystals 2022, 12, 1758. [Google Scholar] [CrossRef]
- Ben Mabrouk, K.; Kauffmann, T.H.; Aroui, H.; Fontana, M.D. Raman study of cation effect on sulfate vibration modes in solid state and in aqueous solutions. J. Raman Spectrosc. 2013, 44, 1603–1608. [Google Scholar] [CrossRef]
- Du, Z.; Chen, J.; Ye, W.; Guo, J.; Zhang, X.; Zheng, R. Investigation of two novel approaches for detection of sulfate ion and methane dissolved in sediment pore water using Raman spectroscopy. Sensors 2015, 15, 12377–12388. [Google Scholar] [CrossRef]

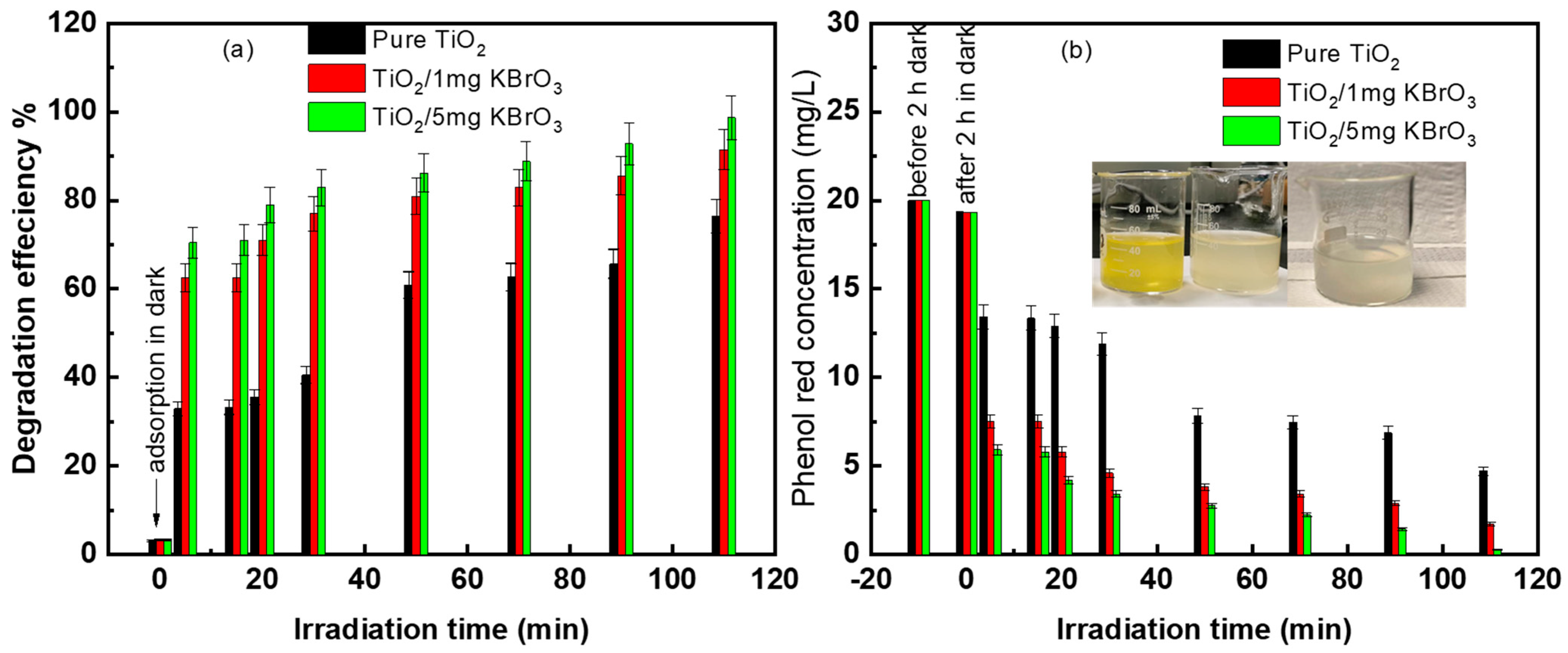
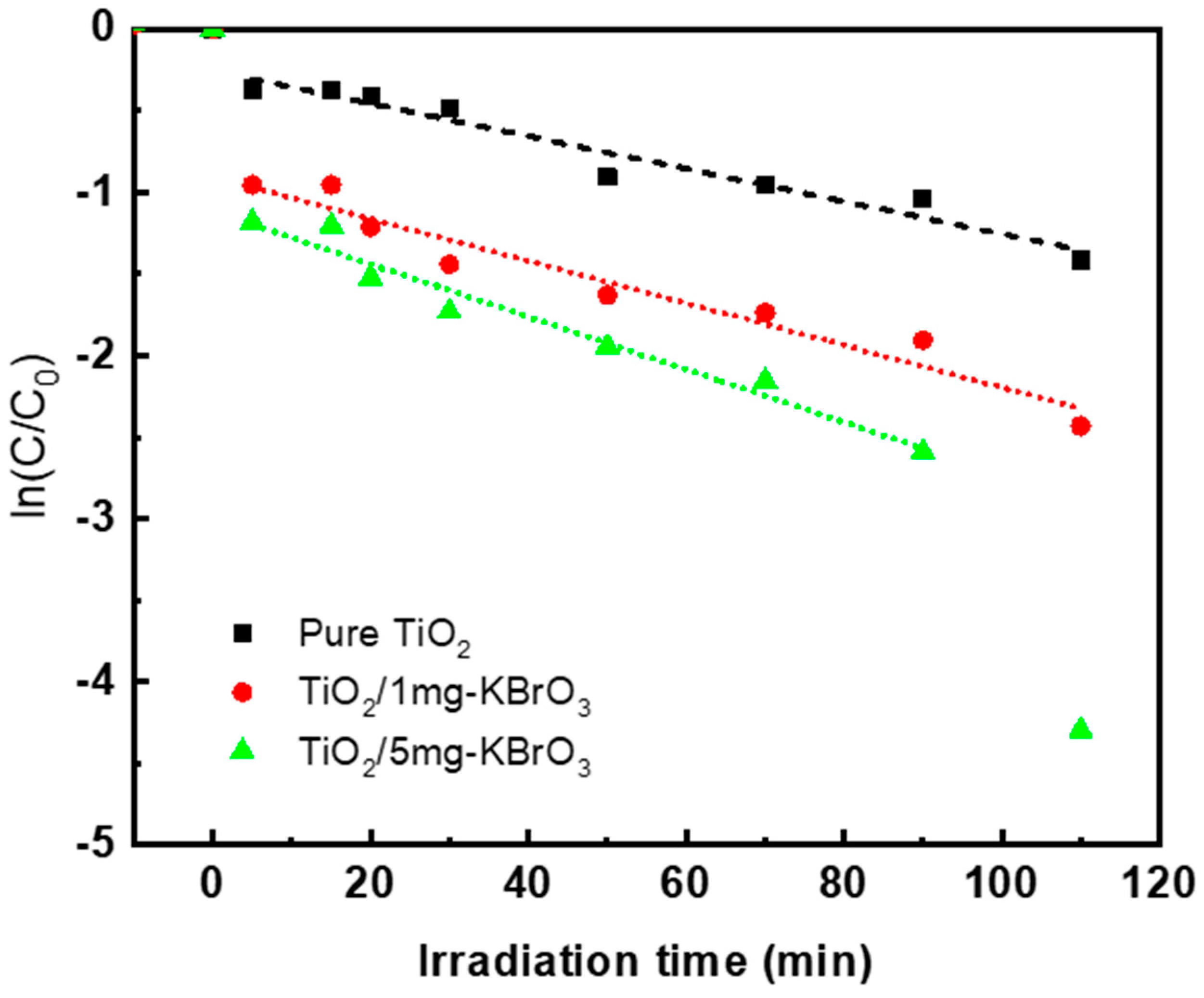


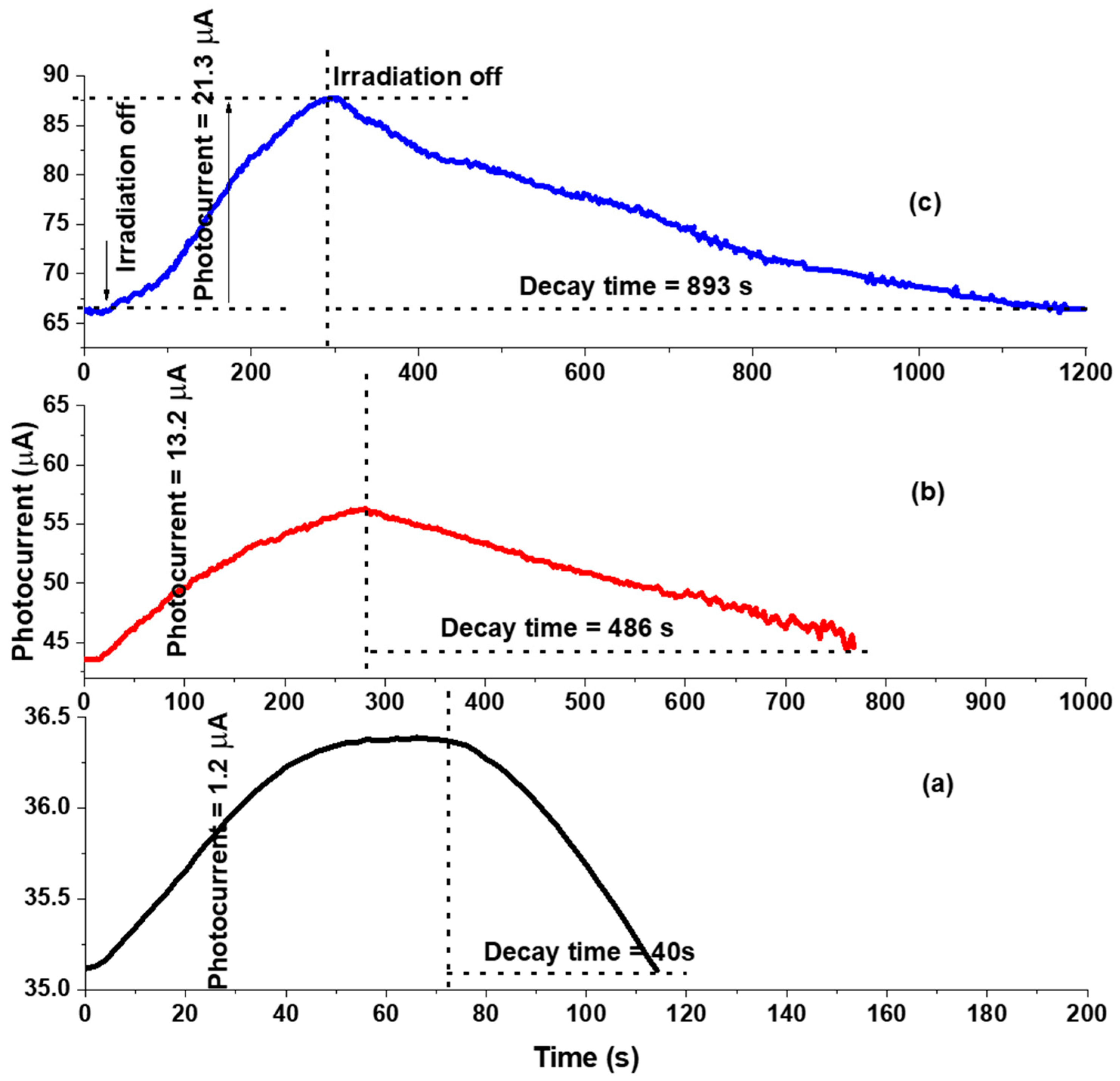
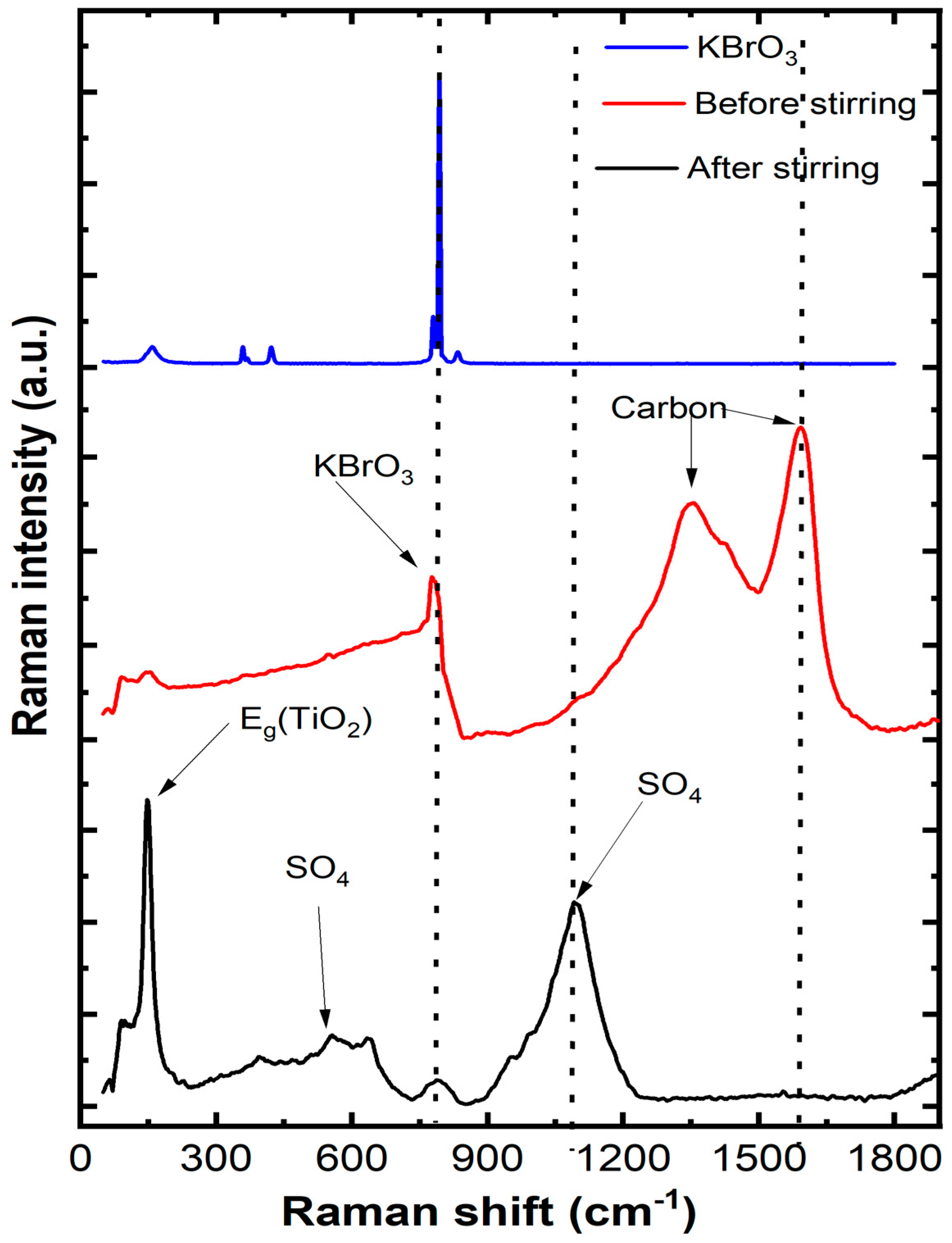
| Sample | k | K (s−1) | R2 |
|---|---|---|---|
| TiO2 | 0.75 | 0.013 | 0.95 |
| TiO2/1 mg-KBrO3 | 0.27 | 0.046 | 0.94 |
| TiO2/5 mg KBrO3 | 0.28 | 0.057 | 0.96 |
| Photocatalysts | Catalyst (gL−1) | PR Con. (M) | Acceptors/Donors Con. (M) | Degradation (%)/Duration (Min) | Refs |
|---|---|---|---|---|---|
| TiO2 | 0.5 | 2.8 × 10−5 | H2O2 (3 × 10−3) | 94/300 | [23] |
| n-TiO2 | 0.6 | 3.76 × 10−5 | no | 95/100 | [24] |
| Goethite | 1.0 | 1.0 ×10−5 | H2O2 (5 × 10−3) | 80/100 | [25] |
| Bi1-xLaxFeO3 | 1.0 | 1.0 ×10−5 | no | 90/102 | [26] |
| CeO2-TiO2 | 1.0 | 2.8 × 10−5 | No | 99/80 | [27] |
| Nb-TiO2 | 0.1 | 5.6 × 10−5 | KBrO3(10−3) | 95/110 | [38] |
| TiO2 | 0.1 | 5.6 × 10−5 | KBrO3(10−3) | 98/110 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almulhem, N.K.; Awada, C.; Shaalan, N.M. Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment. Separations 2023, 10, 162. https://doi.org/10.3390/separations10030162
Almulhem NK, Awada C, Shaalan NM. Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment. Separations. 2023; 10(3):162. https://doi.org/10.3390/separations10030162
Chicago/Turabian StyleAlmulhem, Najla Khaled, Chawki Awada, and Nagih M. Shaalan. 2023. "Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment" Separations 10, no. 3: 162. https://doi.org/10.3390/separations10030162
APA StyleAlmulhem, N. K., Awada, C., & Shaalan, N. M. (2023). Study of Phenol Red Photocatalytic Decomposition on KBrO3-Supported TiO2 Nanoparticles for Wastewater Treatment. Separations, 10(3), 162. https://doi.org/10.3390/separations10030162











