Recent Advancements in the Treatment of Emerging Contaminants Using Activated Persulfate Oxidation Process
Abstract
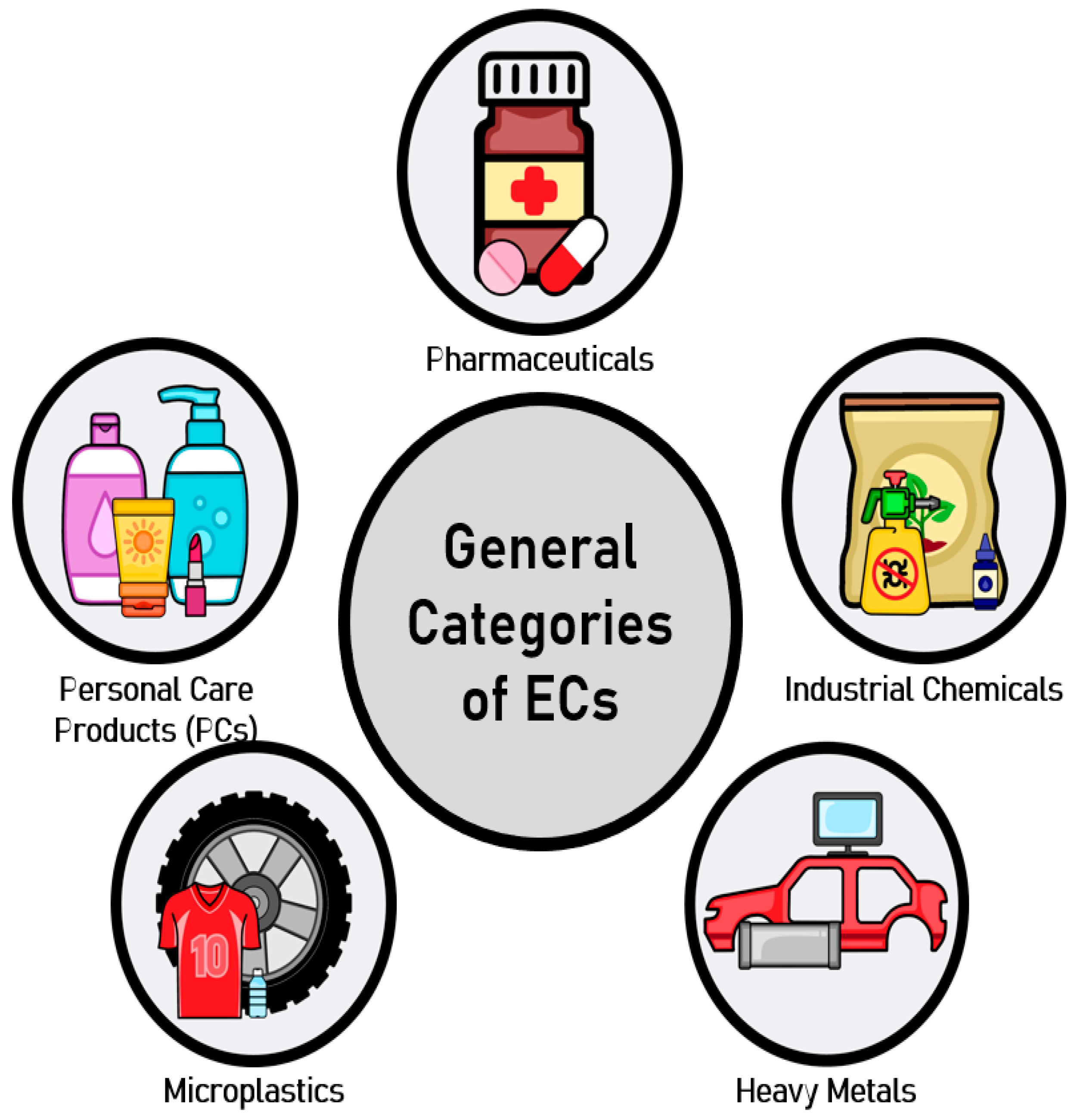
1. Introduction
2. ECs Treatment Technologies
3. Persulfate Oxidation Process
3.1. Electrochemically Activated Persulfate
3.2. Thermally Activated Persulfate
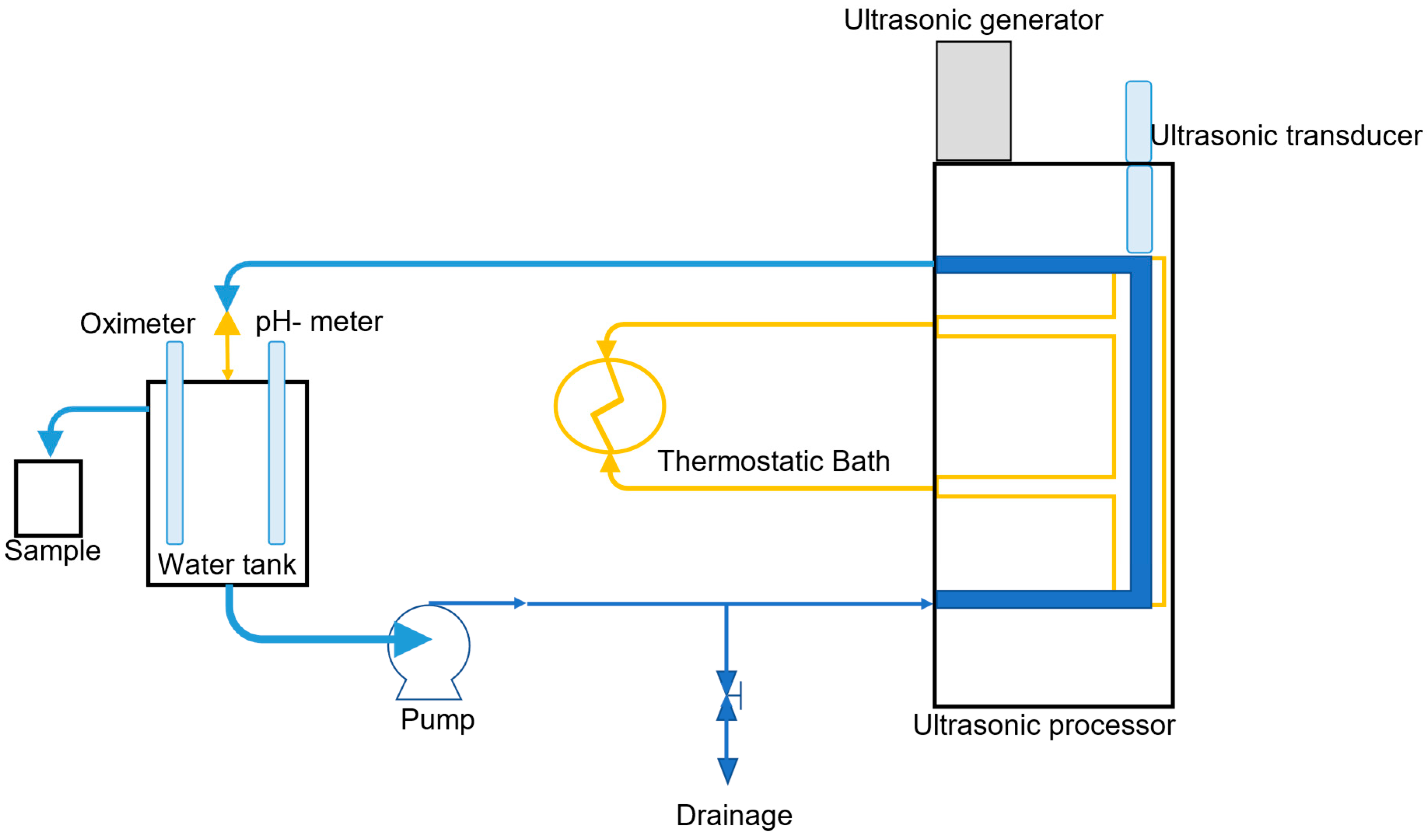
3.3. Sono Activated Persulfate
4. Important Factors Affecting the Electro, Sono and Thermal Activated Persulfate
4.1. Electrochemically Activated PDS
4.2. Thermally Activated PDS
4.3. Sono Activated PDS
5. Limitation and Future Perspective of Persulfate Activation by Electro, Sono and Thermal
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaffari, Z.; Abuabdou, S.; Ng, D.; Bashir, M. Insight into two-dimensional MXenes for environmental applications: Recent progress, challenges, and prospects. FlatChem 2021, 28, 100256. [Google Scholar] [CrossRef]
- Khan, N.; Khan, A.; Tiwari, P.; Zubair, M.; Naushad, M. New insights into the integrated application of Fenton-based oxidation processes for the treatment of pharmaceutical wastewater. Water Process Eng. 2021, 44, 102440. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, W.; Chen, X.; Lv, S. A dandelion-like NiCo2O4 microsphere with superior catalytic activity as the mediator of persulfate activation for high-efficiency degradation of emerging contaminants. J. Environ. Chem. Eng. 2022, 10, 106920. [Google Scholar] [CrossRef]
- Bashir, M.; Mojiri, A. Recent Developments in Emerging Contaminants Determination and Treatment Technologies. Separations 2022, 9, 434. [Google Scholar] [CrossRef]
- He, S.; Chen, Y.L.X.; Zeng, L.; Zhu, M. Heterogeneous photocatalytic activation of persulfate for the removal of organic contaminants in water: A critical review. ACS EST Eng. 2022, 2, 527–546. [Google Scholar] [CrossRef]
- Yang, R.; Chang, Q.; Li, N.; Yang, H. Synergistically enhanced activation of persulfate for efficient oxidation of organic contaminants using a microscale zero-valent aluminum/Fe-bearing clay composite. Chem. Eng. J. 2022, 433, 133682. [Google Scholar]
- Weng, M.; Cai, M.; Xie, Z.; Dong, C.; Zhang, Y.; Song, Z.; Shi, Y.; Jin, M.; Wang, Q.; Wei, Z. Hydrodynamic cavitation-enhanced heterogeneous activation of persulfate for tetracycline degradation: Synergistic effects, degradation mechanism and pathways. Chem. Eng. J. 2022, 431, 134238. [Google Scholar] [CrossRef]
- Checa-Fernández, A.; Santos, A.; Romero, A.; Domínguez, C. Remediation of real soil polluted with hexachlorocyclohexanes (α-HCH and β-HCH) using combined thermal and alkaline activation of persulfate: Optimization of the operating conditions. Sep. Purif. Technol. 2021, 270, 118795. [Google Scholar] [CrossRef]
- He, P.; Zhu, J.; Chen, Y.; Chen, F.; Zhu, J.; Liu, M.; Zhang, K.; Gan, M. Pyrite-activated persulfate for simultaneous 2, 4-DCP oxidation and Cr (VI) reduction. Chem. Eng. J. 2021, 406, 126758. [Google Scholar] [CrossRef]
- Ge, M.; Hu, Z.; Wei, J.; He, Q.; He, Z. Recent advances in persulfate-assisted TiO2-based photocatalysis for wastewater treatment: Performances, mechanism and perspectives. J. Alloys Compd. 2021, 888, 161625. [Google Scholar] [CrossRef]
- Giannakis, S.; Samoili, S.; Rodriguez-Chueca, J. A meta-analysis of the scientific literature on (photo) Fenton and persulfate advanced oxidation processes: Where do we stand and where are we heading to? Curr. Opin. Green Sustain. Chem. 2021, 29, 100456. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Sun, Z. Electro-enhanced degradation of atrazine via Co-Fe oxide modified graphite felt composite cathode for persulfate activation. Chem. Eng. J. 2022, 433, 133789. [Google Scholar] [CrossRef]
- Song, H.; Yan, L.; Ma, J.; Jiang, J.; Cai, G.; Zhang, W.; Zhang, Z.; Zhang, J.; Yang, T. Nonradical oxidation from electrochemical activation of peroxydisulfate at Ti/Pt anode: Efficiency, mechanism and influencing factors. Water Res. 2017, 116, 182–193. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Laha, J.; Hunjan, M. K 2 S 2 O 8 activation by glucose at room temperature for the synthesis and functionalization of heterocycles in water. Chem. Commun. 2021, 57, 8437–8440. [Google Scholar] [CrossRef]
- Laha, J.; Tinwala, U.; Hunjan, M. Minisci aroylation of N-heterocycles using choline persulfate in water under mild conditions. New J. Chem. 2021, 45, 22853–22859. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochemistry 2016, 29, 156–162. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Kim, J.; Ma, J.; Han, Z.; Khim, J. Improving sono-activated persulfate oxidation using mechanical mixing in a 35-kHz ultrasonic reactor: Persulfate activation mechanism and its application. Ultrason. Sonochem. 2021, 72, 105412. [Google Scholar] [CrossRef]
- Acero, J.; Benítez, F.; Real, F.; Rodríguez, E. Degradation of selected emerging contaminants by UV-activated persulfate: Kinetics and influence of matrix constituents. Sep. Purif. Technol. 2018, 201, 41–50. [Google Scholar] [CrossRef]
- Chekem, C.; Chiron, S.; Mancaux, J.; Plantard, G.; Goetz, V. Thermal activation of persulfates for wastewater depollution on pilot scale solar equipment. Sol. Energy 2020, 205, 372–379. [Google Scholar] [CrossRef]
- Miao, D.; Liu, G.; Wei, Q.; Hu, N.; Zheng, K.; Zhu, C.; Liu, T.; Zhou, K.; Yu, Z.; Ma, L. Electro-activated persulfate oxidation of malachite green by boron-doped diamond (BDD) anode: Effect of degradation process parameters. Water Sci. Technol. 2020, 81, 925–935. [Google Scholar] [CrossRef]
- Huang, W.; Bianco, A.; Brigante, M.; Mailhot, G. UVA-UVB activation of hydrogen peroxide and persulfate for advanced oxidation processes: Efficiency, mechanism and effect of various water constituents. J. Hazard. Mater. 2018, 347, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Duan, X.; Wang, C.; Sun, H.; Tan, X.; Tade, M.; Wang, S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Wang, L.; Luo, D.; Yang, J.; Wang, C. Metal-organic frameworks-derived catalysts for contaminant degradation in persulfate-based advanced oxidation processes. J. Clean. Prod. 2022, 375, 134118. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Rout, P.; Zhang, T.C.; Bhunia, P.; Surampallo, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Barbosa, M.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.; Silva, A.M. Occurance and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Pesqueira, J.; Pereira, M.; Silva, A. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: A review. J. Clean. Prod. 2020, 261, 121078. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.; Đolić, M.; Gernjak, W.; Heath, E. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Moreira, N.; Puma, G.; Silva, A. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Yap, H.; Pang, Y.; Lim, S.; Abdullah, A.; Ong, H.; Wu, C. A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int. J. Environ. Sci. Technol. 2019, 16, 601–628. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef]
- Qu, R.; Feng, M.; Wang, X.; Huang, Q.; Lu, J.; Wang, L.; Wang, Z. Rapid removal of tetrabromobisphenol A by ozonation in water: Oxidation products, reaction pathways and toxicity assessment. PLoS ONE 2015, 10, 139580. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Lee, J.; Von Gunten, U.; Kim, J. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Oh, W.; Dong, Z.; Lim, T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Guo, D.; You, S.; Li, F.; Liu, Y. Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation: A review. Chin. Chem. Lett. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidaton: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Chen, N.; Shi, Y.; Zhang, L. Pyrite enables persulfate activation for efficient atrazine degradation. Chemosphere 2019, 244, 125568. [Google Scholar] [CrossRef]
- Karim, A.; Jiao, Y.; Zhou, M.; Nidheesh, P. Iron-based persulfate activation process for environmental decontamination in water and soil. Chemosphere 2021, 265, 129057. [Google Scholar] [CrossRef]
- Long, Y.; Feng, Y.; Li, X.; Suo, N.; Chen, H.; Wang, Z.; Yu, Y. Removal of diclofenac by three-dimensional electro-Fenton-persulfate (3D electro-Fenton-PS). Chemosphere 2019, 219, 1024–1031. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.; Dincer, K.; Demir, A. Concentrated leachate treatment by electro-Fenton and electro-persulfate processes. Int. J. Environ. Res. 2020, 14, 439–461. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, S.; Bu, L.; Shi, Z.; Zhu, S. Degradation of diuron by electrochemically activated persulfate. Water Air Soil Pollut. 2016, 227, 279. [Google Scholar] [CrossRef]
- Ding, J.; Bu, L.; Zhao, Q.; Kabutey, F.; Wei, L.; Dionysiou, D. Electrochemical activation of persulfate on BDD and DSA anodes: Electrolyte influence, kinetics and mechanisms in the degradation of bisphenol A. J. Hazard. Mater. 2020, 388, 121789. [Google Scholar] [CrossRef]
- Fernandes, A.; Nunes, M.; Rodrigues, A.; Pacheco, M.; Ciríaco, L.; Lopes, A. Electro-Persulfate Processes for the Treatment of Complex Wastewater Matrices: Present and Future. Molecules 2021, 26, 4821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, Z.; Cui, J. Research on the mechanism and reaction conditions of electrochemical preparation of persulfate in a split-cell reactor using BDD anode. RSC Adv. 2020, 10, 33928–33936. [Google Scholar] [CrossRef] [PubMed]
- Matzek, L.; Carter, K. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Onn, S.; Bashir, M.; Sethupathi, S.; Amr, S.; Nguyen, T. Colour and COD removal from mature landfill leachate using electro-persulphate oxidation process. Mater. Today Proc. 2020, 31, 69–74. [Google Scholar] [CrossRef]
- Varank, G.; Guvenc, S.Y.; Demir, A.; Kavan, N.; Donmez, N.; Onen, Z. Modeling and optimizing electro-persulfate processes using Fe and Al electrodes for paper industry wastewater treatment. Water Sci. Technol. 2020, 81, 345–357. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, S.; Song, Y.; Wang, B.; Zhang, F. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. J. Electroanal. Chem. 2018, 809, 74–79. [Google Scholar] [CrossRef]
- Banerjee, M.; Panjikar, P.; Das, D.; Iyer, S.; Bhosle, A.; Chatterjee, A. Grindstone chemistry: A “green” approach for the synthesis and derivatization of heterocycles. Tetrahedron 2022, 112, 132753. [Google Scholar] [CrossRef]
- Weng, C.; Tsai, K. Ultrasound and heat enhanced persulfate oxidation activated with Fe0 aggregate for the decolorization of CI Direct Red 23. Ultrason. Sonochem. 2016, 29, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids-New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern—A review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef]
- Johnson, R.; Tratnyek, P.; Johnson, R. Persulfate persistence under thermal activation conditions. Environ. Sci. Technol. 2008, 42, 9350–9356. [Google Scholar] [CrossRef]
- Ji, Y.; Shi, Y.; Dong, W.; Wen, X.; Jiang, M.; Lu, J. Thermo-activated persulfate oxidation system for tetracycline antibiotics degradation in aqueous solution. Chem. Eng. J. 2016, 298, 225–233. [Google Scholar] [CrossRef]
- Jorfi, S.; Pourfadakari, S.; Ahmadi, M.; Akbari, H. Thermally activated persulfate treatment and mineralization of a recalcitrant high TDS petrochemical wastewater. Pol. J. Chem. Technol. 2017, 19, 72–77. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Chokejaroenrat, C.; Satapanajaru, T.; Chirasatienpon, T.; Angkaew, A. Removal of 17β-estradiol using persulfate synergistically activated using heat and ultraviolet light. Water Air Soil Pollut. 2020, 231, 247. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Li, L.; Deng, J.; Zhou, S. Kinetic oxidation of antipyrine in heat-activated persulfate. Desalination Water Treat. 2015, 53, 263–271. [Google Scholar] [CrossRef]
- Bashir, M.; Sheen, O.; Ng, C.; Abujazar, M.; Alazaiza, M.; Amr, S.A. Advanced Treatment of Palm Oil Mill Effluent Using Thermally Activated Persulfate Oxidation. Separations 2022, 9, 171. [Google Scholar] [CrossRef]
- Nie, M.; Yang, Y.; Zhang, Z.; Yan, C.; Wang, X.; Li, H.; Dong, W. Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem. Eng. J. 2014, 246, 373–382. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef]
- Liu, L.; Lin, S.; Zhang, W.; Farooq, U.; Shen, G.; Hu, S. Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem. Eng. J. 2018, 346, 515–524. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water. Chemosphere 2018, 190, 296–306. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, N.; Deng, Y.; Yin, D.; Zhang, Y.; Rong, W.; Zhou, S. Heat-activated persulfate oxidation of sulfamethoxazole in water. Desalination Water Treat. 2015, 56, 2225–2233. [Google Scholar] [CrossRef]
- Zhou, R.; Li, T.; Su, Y.; Li, C.; Jin, X.; Ren, H. Removal of sulfanilic acid from wastewater by thermally activated persulfate process: Oxidation performance and kinetic modeling. J. Chem. Technol. Biotechnol. 2019, 94, 3208–3216. [Google Scholar] [CrossRef]
- Kodavatiganti, S.; Bhat, A.; Gogate, P. Intensified degradation of Acid Violet 7 dye using ultrasound combined with hydrogen peroxide, Fenton, and persulfate. Sep. Purif. Technol. 2021, 279, 119673. [Google Scholar] [CrossRef]
- Miklos, D.; Remy, C.; Jekel, M.; Linden, K.; Drewes, J.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Aseman-Bashiz, E.; Sayyaf, H. Metformin degradation in aqueous solutions by electro-activation of persulfate and hydrogen peroxide using natural and synthetic ferrous ion sources. J. Mol. Liq. 2020, 300, 112285. [Google Scholar] [CrossRef]
- Ferkous, H.; Merouani, S.; Hamdaoui, O.; Pétrier, C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: Evidence of sulfate radical formation. Ultrason. Sonochem. 2017, 34, 580–587. [Google Scholar] [CrossRef]
- Monteagudo, J.; El-Taliawy, H.; Durán, A.; Caro, G.; Bester, K. Sono-activated persulfate oxidation of diclofenac: Degradation, kinetics, pathway and contribution of the different radicals involved. J. Hazard. Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef]
- Qian, Y.; Guo, X.; Zhang, Y.; Peng, Y.; Sun, P.; Huang, C.; Niu, J.; Zhou, X.; Crittenden, J. Perfluorooctanoic acid degradation using UV–persulfate process: Modeling of the degradation and chlorate formation. Environ. Sci. Technol. 2016, 50, 772–781. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Wang, Y.; Zhang, H. The UV/peroxymonosulfate process for the mineralization of artificial sweetener sucralose. Chem. Eng. J. 2017, 317, 561–569. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Lin, K.; Zhang, W.; Lu, S.; Luo, Q. Removal of 1, 1, 1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochem. 2013, 20, 855–863. [Google Scholar] [CrossRef]
- Chakma, S.; Praneeth, S.; Moholkar, V. Mechanistic investigations in sono-hybrid (ultrasound/Fe2+/UVC) techniques of persulfate activation for degradation of Azorubine. Ultrason. Sonochem. 2017, 38, 652–663. [Google Scholar] [CrossRef]
- Hossein, P.A.; Meshkinian, A.; Ashrafi, S.; Khan, M.; Naghizadeh, A.; Abi, G.; Kamani, H. Survey of sono-activated persulfate process for treatment of real dairy wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 93–98. [Google Scholar] [CrossRef]
- Chen, L.; Lei, C.; Li, Z.; Yang, B.; Zhang, X.; Lei, L. Electrochemical activation of sulfate by BDD anode in basic medium for efficient removal of organic pollutants. Chemosphere 2018, 210, 516–523. [Google Scholar] [CrossRef]
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef]
- Matzek, L.; Tipton, M.; Farmer, A.; Steen, A.; Carter, K. Understanding electrochemically activated persulfate and its application to ciprofloxacin abatement. Environ. Sci. Technol. 2018, 52, 5875–5883. [Google Scholar] [CrossRef]
- Frontistis, Z.; Mantzavinos, D.; Meriç, S. Degradation of antibiotic ampicillin on boron-doped diamond anode using the combined electrochemical oxidation-Sodium persulfate process. J. Environ. Manag. 2018, 223, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, L.; Xie, L.; Deng, P.; Deng, D. Compatibility of surfactants and thermally activated persulfate for enhanced subsurface remediation. Environ. Sci. Technol. 2017, 51, 7055–7064. [Google Scholar] [CrossRef]
- Park, S.; Lee, L.; Medina, V.; Zull, A.; Waisner, S. Heat-activated persulfate oxidation of PFOA, 6: 2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere 2016, 145, 376–383. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.; Rahdar, S. The application of thermally activated persulfate for degradation of Acid Blue 92 in aqueous solution. Int. J. Ind. Chem. 2019, 10, 249–260. [Google Scholar] [CrossRef]
- Pokhalekar, P.; Chakraborty, M. Degradation of bisphenol A and 4-tert-octylphenol: A comparison between ultrasonic and photocatalytic technique. Desalination Water Treat. 2016, 57, 10370–10377. [Google Scholar] [CrossRef]
- Herrmann, H. On the photolysis of simple anions and neutral molecules as sources of O−/OH, SO x− and Cl in aqueous solution. Phys. Chem. Chem. Phys. 2007, 9, 3935–3964. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.; Peralta-Hernandez, J.; Goonetilleke, A.; Bandala, E. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A review study on sulfate-radical-based advanced oxidation processes for domestic/industrial wastewater treatment: Degradation, efficiency, and mechanism. Front. Chem. 2020, 8, 1092. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.; Bashir, M.; Amr, S.A.; Alazaiza, M. Factorial design and optimization of thermal activation of persulfate for stabilized leachate treatment. Desalination Water Treat. 2022, 250, 211–220. [Google Scholar] [CrossRef]
- Ozturk, D.; Dagdas, E.; Fil, B.A.; Bashir, M.J. Central composite modeling for electrochemical degradation of paint manufacturing plant wastewater: One-step/two-response optimization. Environ. Technol. Innov. 2021, 21, 101264. [Google Scholar] [CrossRef]
- Mojiri, A.; Bashir, M.J.K. Wastewater treatment: Current and future techniques. Water 2022, 14, 448. [Google Scholar] [CrossRef]
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J. An overview of per-and polyfluoroalkyl substances (PFAS) in the environment: Source, fate, risk and regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]



| Type of Effluent | Anode/Cathode | PS Added | Applied Current | Electrolysis Time/Min | pH | Pollutant | Removal (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Landfill Leachate | Al/Al | 0.88 g/L | 44.66 mA cm−2 | 68.30 | 4.00 | COD | 45.70 | [50] |
| Landfill Leachate | Fe/Fe | PS/COD Ratio 2.50 PS/COD Ratio 1.90 | 1.80 A 2.10 A | 35.90 | 6.40 | COD | 84.20 | [51] |
| Landfill Leachate | Fe/Fe | PDS/COD Ratio 1.72 | 1.26 A | 34.80 | 5.00 | COD | 72.60 | [44] |
| Stock solution | Fe/Fe | 0.50 mM | 30 mA | 15.00 | 7.00 | Diuron | >77 | [45] |
| Stock solution | BDD/Stainless steel | 0.02 M | 1.70 mA/cm2 | 30.00 | 4.40 | Malachite Green (MG) | >95 | [21] |
| Stock solution | Pt/Pt | 12.60 mM | 13.33 mA/cm2 | 240.00 | 4.42 | Tetracycline Hydrochloride (TCH) | 81.10 | [52] |
| Landfill Leachate | BDD/BDD | 1.00 mM | 5.00 mA/cm−2 | 10.00 | NA | Bisphenol A | 85.00 | [46] |
| Dimension Stable Anode (DSA) | 60.00 | |||||||
| Paper industry wastewater | Fe | PDS/COD Ratio 1.25 | 4.14 A | 5.00 | 6.00 | COD | 53.50 | [51] |
| Al | PDS/COD Ratio 0.50 | 4.25 | 25.00 | 7.25 | 72.80 |
| Pollutant | Pollutant Concentration | Oxidant Concentration | Temperature (°C) | pH | Reaction Time (h) | Degradation (%) | References |
|---|---|---|---|---|---|---|---|
| Acid orange 7 | 20.00 mg L−1 | 0.57 PS | 80.00 | 6.50 | 3.00 | 99.00 | [60] |
| Antipyrine | 0.0265 mM | 0.125 mM PS | 60.00 | 4.50 | 2.00 | 73.00 | [61] |
| Carbamazepine | 0.04 mM | 1.00 mM PS | 70.00 | 5.10 | 2.00 | ~100.00 | [62] |
| Chloramphenicol | 0.20 mM | 16.00 mM PS | 70.00 | 5.40 | 2.70 | 96.30 | [63] |
| Atrazine | 0.05 mM | 1.00 mM PS | 60.00 | 7.00 | 2.00 | 100.00 | [57] |
| Sulfamethazine | 0.03 mM | 2.00 mM PS | 60.00 | 7.00 | 6.00 | 100.00 | [64] |
| Sulfachloropyridazine | 1.00 µM | 140.00 µM | 40.00 | 3.00 | 1.10 | 85.00 | [65] |
| Azole Fungicide Fluconazole | 10.00 mg L−1 | 20.00 mM | 60.00 | 3.00 | 4.00 | 87.00 | [66] |
| Benzene, toluene, ethylbenzene, xylenes (BTEX) | 0.10 mM | 20.00 mM | 50.00 | 3.50 | 6.00 | >90.00 | [67] |
| Sulfamethoxazole | 40.00 µM | 2.40 mM | 60.00 | 7.00 | 2.00 | >80.00 | [68] |
| Sulfanilic Acid | 50.00 mg/L | 20.00 mmol/L | 60.00 | 7.00 | 4.00 | 57.40 | [69] |
| [Ultraviolet Activated Persulfate] | |||||||
| Pollutant | Pollutant Concentration | Oxidant Concentration | Wavelength (nm) | pH | Reaction Time (h) | Degradation (%) | References |
| Perfluorooctanoic Acid | 0.15 mM | 15.00 mM PS | 254.00 | 7.10 | 8.00 | >80.00 | [75] |
| 2-methylisoborneol | 0.238 μM | 10.00 μM PS | 254.00 | 7.00 | 0.14 | >90.00 | [76] |
| Sulfamethoxazole | 20.00 μM | 1.00 mM PS | 254.00 | 8.00 | 2.00 | 100.00 | [66] |
| Sucralose | 0.126 mM | 3.78 mM PMS | 254.00 | 7.00 | 1.00 | >95.00 (TOC) | [77] |
| [Ultrasonic activated persulfate] | |||||||
| Pollutant | Pollutant concentration | Oxidant concentration | Frequency (kHz) | pH | Reaction time (h) | Degradation (%) | References |
| 1,1,1-trichloroethane | 50.00 mg L−1 | 0.94 mM | 400.00 (bath) | 6.90 | 2.00 | 100.00 | [78] |
| Carbamazepine | 0.025 mmol/L | 1.00 mmol/L | 40.00 (bath) | 5.00 | 2.00 | 89.40 | [17] |
| Naphthol | 5.00 mg L−1 | 9.00 mg L−1 PDS | 585.00 (probe) | 6.00 | 0.33 | >90.00 | [73] |
| Azorubine | 20.00 mg L−1 | 4.00 mM PDS | 40.00 (bath) | 3.50 | 1.00 | 66.54 | [79] |
| COD | 720.00 mg/L | 500.00 mg/L | 130.00 | 3.00 | 1.00 | 74.50 | [80] |
| Diclofenac | 5.00 mg/L | 120.00 mg/L |
| 6.00 | 4.00 | 95.00 | [74] |
| Ibuprofen | 24.00 µm | 0.80 mM | 35 | 4.90 | 1.00 | Tap–90.40 River–85.30 | [18] |
| Carbamazepine | 2.50 mmol/L | 5.00 mmol/L |
| 5.00 | 2.00 | 89.40 | [17] |
| Influencing Factor | Electrochemically-Activated Persulfate | Thermally-Activated Persulfate | Sono-Activated Persulfate |
|---|---|---|---|
| Electrode Material |
| No electrode involved | No electrode involved |
| Current Density/amplitude |
| No current density/amplitude involved |
|
| pH | Formation of persulfate was higher in acid solution than in alkaline solution |
|
|
| Persulfate Concentration | Reaction rates are directly proportional to the concentration of persulfate |
|
|
| Temperature | PDS output and current efficiency decreased with the increase of temperature |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juni, F.; Bashir, M.J.K.; Haider Jaffari, Z.; Sethupathi, S.; Wong, J.W.C.; Zhao, J. Recent Advancements in the Treatment of Emerging Contaminants Using Activated Persulfate Oxidation Process. Separations 2023, 10, 154. https://doi.org/10.3390/separations10030154
Juni F, Bashir MJK, Haider Jaffari Z, Sethupathi S, Wong JWC, Zhao J. Recent Advancements in the Treatment of Emerging Contaminants Using Activated Persulfate Oxidation Process. Separations. 2023; 10(3):154. https://doi.org/10.3390/separations10030154
Chicago/Turabian StyleJuni, Farrandie, Mohammed J. K. Bashir, Zeeshan Haider Jaffari, Sumathi Sethupathi, Jonathan W. C. Wong, and Jun Zhao. 2023. "Recent Advancements in the Treatment of Emerging Contaminants Using Activated Persulfate Oxidation Process" Separations 10, no. 3: 154. https://doi.org/10.3390/separations10030154
APA StyleJuni, F., Bashir, M. J. K., Haider Jaffari, Z., Sethupathi, S., Wong, J. W. C., & Zhao, J. (2023). Recent Advancements in the Treatment of Emerging Contaminants Using Activated Persulfate Oxidation Process. Separations, 10(3), 154. https://doi.org/10.3390/separations10030154










