Calluna vulgaris Crude Extract Reverses Liver Steatosis and Insulin Resistance-Associated-Brain Lesion Induced by CCl4 Administration
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Plant Collection, Extraction, and Polyphenolic Content Identification
2.2.1. Extraction Preparation
2.2.2. Phytochemical Identification
2.2.3. HPLC Analysis of Polyphenolic Compounds of CV Extract
2.3. Animal
Animal Design
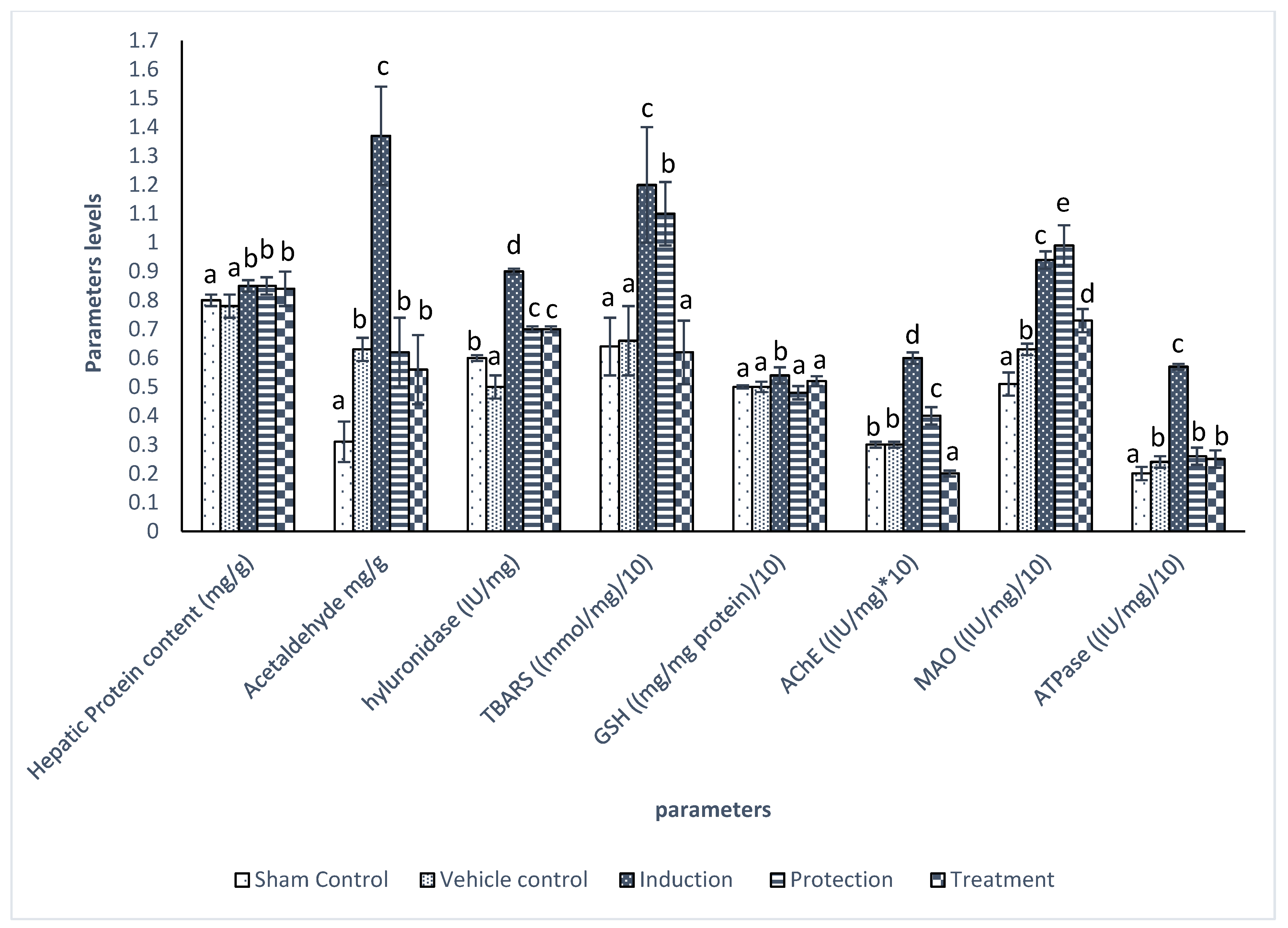
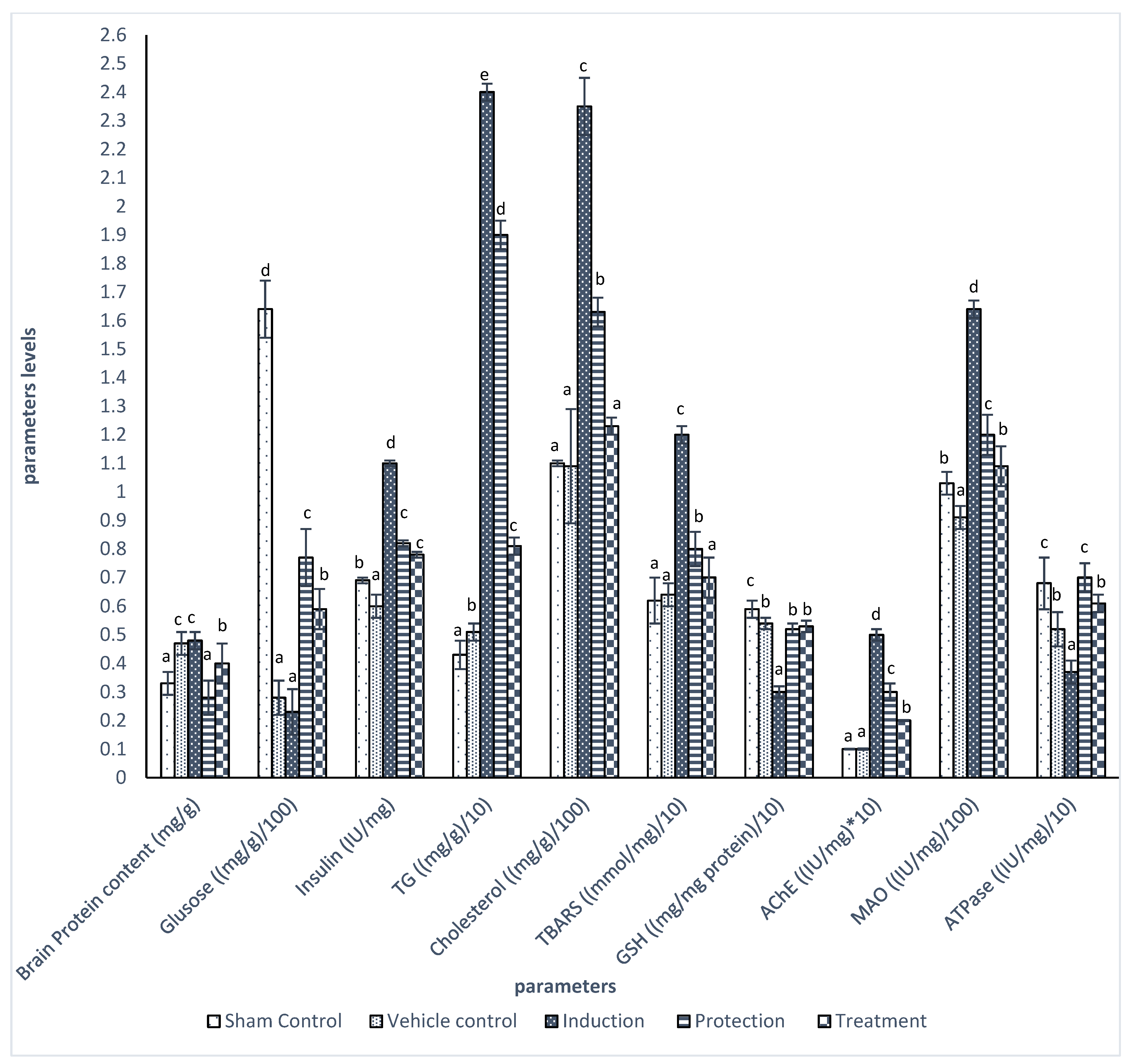
2.4. Biochemical Parameters
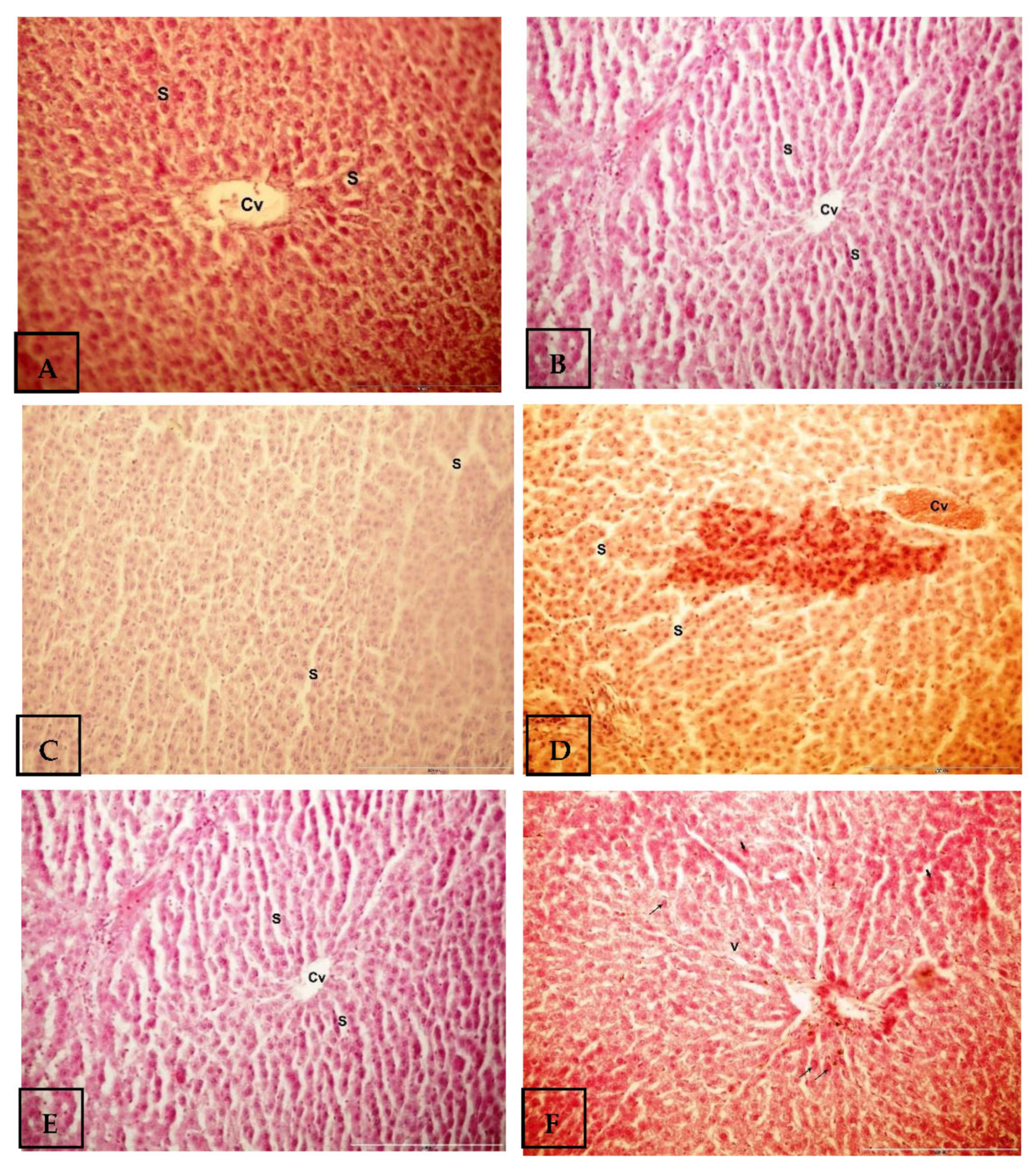
2.5. Histological Investigations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. GI epidemiology: Nonalcoholic fatty liver disease: Incidence and prevalence. Aliment. Pharmacol. Ther. 2007, 25, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Deary, I.J.; Ryan, C. Cognition and diabetes: A lifespan perspective. Lancet Neurol. 2008, 7, 184–190. [Google Scholar] [CrossRef]
- Kouvari, M.; Sergi, D.; D’Cunha, N.M.; Bulman, A.; Panagiotakos, D.B.; Naumovski, N. Is Non-Alcoholic Fatty Liver Disease Connected with Cognition? The Complex Interplay between Liver and Brain. Diabetology 2022, 3, 355–363. [Google Scholar] [CrossRef]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef]
- Dutta, B.J.; Singh, S.; Seksaria, S.; Gupta, G.D. Inside the diabetic brain: Insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacol. Res. 2022, 182, 106358. [Google Scholar] [CrossRef]
- Liddell, J.R.; Hoepken, H.H.; Crack, P.J.; Robinson, S.R.; Dringen, R. Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J. Neurosci. Res. 2006, 84, 578–586. [Google Scholar] [CrossRef]
- Carvalho, C.; Cardoso, S. Diabetes–Alzheimer’s Disease Link: Targeting Mitochondrial Dysfunction and Redox Imbalance. Antioxid. Redox Signal. 2020, 34, 631–649. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Bayram, H.; Majoo, F.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Filip, G.A.; Postescu, D.; Tatomir, C.; Muresan, A.; Clichici, S. Calluna vulgaris extract modulates NF-κB/ERK signaling pathway and matrix metalloproteinase expression in SKH-1 hairless mice skin exposed to ultraviolet B irradiation. J. Physiol. Pharmacol. 2012, 63, 423–432. [Google Scholar] [PubMed]
- Starchenko, G.; Hrytsyk, A.; Raal, A.; Koshovyi, O. Phytochemical profile and pharmacological activities of water and hydroethanolic dry extracts of Calluna vulgaris (L.) Hull. Herb. Plants 2020, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, D.A.; ElAhwany, A.M.; El-Mallawany, S.M.; Saif, A.A. In vitro screening for anti-acetylcholiesterase, anti-oxidant, anti-glucosidase, anti-inflammatory and anti-bacterial effect of three traditional medicinal plants. Biotechnol. Biotechnol. Equip. 2014, 28, 1155–1164. [Google Scholar] [CrossRef]
- Ismail, N.; Ghareeb, D.; El- Sohaimy, S.; EL-Demellawy, M.; El-Saied, M. Evaluation of the anti-Fusarium effect of Cinnamoum zeilanicum, Berberise vulgaris and Caluna vulgaris ethanolic extracts. Int. J. Cancer Biomed. Res. 2020, 4, 143–150. [Google Scholar] [CrossRef]
- Abdel-Latif, M.; Hegazy, A.; Khalil, S.; Ghareeb, D. Prophylactic effect of herbal extracts on LPS-induced inflammatory response in rat hepatocytes. Int. J. Phytomed. 2017, 9, 20–28. [Google Scholar]
- Orhan, I.; Küpeli, E.; Terzioğlu, S.; Yesilada, E. Bioassay-guided isolation of kaempferol-3-O-β-d-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L. J. Ethnopharmacol. 2007, 114, 32–37. [Google Scholar] [CrossRef]
- Burncoose Nurseries. HEATHERS Calluna. Available online: https://www.burncoose.co.uk/site/plants.cfm?pl_id=650 (accessed on 6 January 2020).
- US National Plant Germplasm System. Calluna vulgaris (L.) Hull. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=8605 (accessed on 6 January 2020).
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Tomah, S.; Hamdy, O.; Abuelmagd, M.; Hassan, A.; Alkhouri, N.; Al-Badri, M.; Gardner, H.; Eldib, A.; Eid, A. Prevalence of and risk factors for non-alcoholic fatty liver disease (NAFLD) and fibrosis among young adults in Egypt. BMJ Open Gastroenterol. 2021, 8, e000780. [Google Scholar] [CrossRef]
- EL Tallawy, H.; Farghaly, W.; El Hamed, M.; Badry, R.; Usama, K.; Shehata, G.; Tohmy, A.; Abdulghani, K.; Ghanem, M.; Sayed, M.; et al. Prevalence of Alzheimer dementia in Upper Egypt (desert areas). Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 29. [Google Scholar] [CrossRef]
- Ghareeb, D.; Hussen, H. Vanadium improves brain acetylcholinesterase activity on early stage alloxan-diabetic rats. Neurosci. Lett. 2008, 436, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewicz-Hussain, A.; Moniuszko-Jakoniuk, J.; Rogalska, J. Assessment of lipid peroxidation in rat tissues in subacute chlorfenvinphos administration. Pol. J. Environ. Stud. 2007, 16, 233–236. [Google Scholar]
- Jollow, D.; Mitchell, J.; Zampaglione, N.; Gillette, J. Bromobenze induced liver necrosis: Protective role of glutathione and evidence for 3, 4-bromobenzenoxide as the hepatotoxic intermediate. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Stroev, E.; Makarova, V. Carbohydrate metabolism. In Laboratory Manual in Biochemistry; Mir Publishers: Moscow, Russia, 1989; pp. 144–146. [Google Scholar]
- Leaback, D.; Walker, P. On the Morgan-elson reaction. Biochim. Biophys. Acta 1963, 74, 297–300. [Google Scholar]
- Ellman, G.; Courtney, K.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sandler, M.; Reveley, M.; Glover, V. Human platelet monoamine oxidase activity in health and disease: A review. J. Clin. Pathol. 1981, 34, 292–302. [Google Scholar] [CrossRef]
- Litwack, G.; Bothwell, J.W.; Williams, J.N.; Elvehjem, J. A colorimetric assay for xanthine oxidase in rat liver homogenates. J. Biol. Chem. 1953, 200, 303–310. [Google Scholar] [CrossRef]
- Bancroft, D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: London UK, 2008. [Google Scholar]
- Malik, A.; Arooj, M.; Butt, T.; Zahid, S.; Zahid, F.; Jafar, T.; Waquar, S.; Gan, S.; Ahmad, S.; Mirza, M. n silico and in vivo characterization of cabralealactone, solasodin and salvadorin in a rat model: Potential anti-inflammatory agents. Drug Des. Dev. Ther. 2018, 12, 1431. [Google Scholar] [CrossRef]
- Godoy-Matos, A.; Silva Júnior, W.; Valerio, C. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60–79. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin resistance and oxidative stress in the brain: What’s new? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Ta, Q.; Nguyen, T.; Nguyen, T.; Giau, V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, W.; Yin, Y.; Zhang, P.; Tao, K. 4-OI attenuates carbon tetrachloride-induced hepatic injury via regulating oxidative stress and the inflammatory response. Front. Pharmacol. 2021, 12, 1238–1250. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguuayo, C.; Salomon, C.; Zuniga, F. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Ouerghi, N.; Feki, M.; Bragazzi, N.L.; Knechtle, B.; Hill, L.; Nikolaidis, P.T.; Bouassida, A. Ghrelin Response to Acute and Chronic Exercise: Insights and Implications from a Systematic Review of the Literature. Sport. Med. 2021, 51, 2389–2410. [Google Scholar] [CrossRef]
- Farzaei, M.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, E.; Ochiai, H.; Shirasawa, T.; Yoshimoto, T.; Nagahama, S.; Muramatsu, J.; Chono, T.; Ito, T.; Inoue, H.; Kokaze, A. Eating quickly is associated with a low aspartate aminotransferase to alanine aminotransferase ratio in middle-aged adults: A large-scale cross-sectional survey in Japan. Arch. Public Health 2020, 78, 101. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative stress is a key modulator in the development of nonalcoholic fatty liver disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.; Mayes, W.; Bracy, D.; Bookbinder, L.; Hasty, A.; Thompson, C.; Wasserman, D. Hyaluronan Accumulates with High-Fat Feeding and. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef]
- Chajara, A.; Raoudi, M.; Delpech, B.; Delpech, M.; Basuyau, J.; Levesque, H. Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler. Thromb. Vasc. 2000, 20, 1480–1487. [Google Scholar] [CrossRef]
- de Medeiros, I.; de Lima, G. s nonalcoholic fatty liver disease an endogenous alcoholic fatty liver disease?–A mechanistic hypothesis. Med. Hypotheses 2015, 85, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Katunga, L.; Willis, M. itochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin. Exp. Pharmacol. Physiol. 2012, 39, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Haddad, A.; Osme, A.; Kim, C.; Borzou, A.; Ilchenko, S.; Allende, D.; Dasarathy, S.; McCullough, A.; Sadygov, R.G.; et al. Hepatic mitochondrial defects in a nonalcoholic fatty liver disease mouse model are associated with increased degradation of oxidative phosphorylation subunits. Mol. Cell. Proteom. 2018, 17, 2371–2386. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Vives-Bauza, C.; Gonzalo, R.; Manfredi, G.; Garcia-Arumi, E.; Andreu, A. Enhanced ROS production and antioxidant defenses in cybrids harbouring mutations in mtDNA. Neurosci. Lett. 2006, 391, 136–141. [Google Scholar] [CrossRef]
- Caro, A.; Evans, L.; Cederbaum, I. CYP2E1 overexpression inhibits microsomal Ca2+−ATPase activity in HepG2 cells. Toxicology 2009, 255, 171–176. [Google Scholar] [CrossRef]
- Ghareeb; Hafez, H.; Hussien, H.; Kabapy, N. Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab. Brain Dis. 2011, 26, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Colognesi, M.; Gabbia, D.; De Martin, S. Depression and cognitive impairment—Extrahepatic manifestations of NAFLD and NASH. Biomedicines 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Manzella, N.; Santin, Y.; Maggiorani, D.; Martini, H.; Douin-Echinard, V.; Passos, J.; Lezoualc’h, F.; Binda, C.; Parini, A.; Mialet-Perez, J. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell 2018, 17, e12811. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.; Backman, L.; Alfredson, H.; Scott, A.; Danielson, P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-β1. PLoS ONE 2017, 12, e0174101. [Google Scholar] [CrossRef]
- Bekkai, D.; Oulad El Majdoub, Y.; Bekkai, H.; Cacciola, F.; Miceli, N.; Taviano, M.; Cavò, E.; Errabii, T.; Vinci, R.; Mondello, L.; et al. Determination of the Phenolic Profile by Liquid Chromatography, Evaluation of Antioxidant Activity and Toxicity of Moroccan Erica multiflora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules 2022, 27, 3979. [Google Scholar] [CrossRef]
- Fukuzawa, Y.; Kapoor, M.; Yamasaki, K.; Okubo, T.; Hotta, Y.; Juneja, L. Effects of green tea catechins on nonalcoholic steatohepatitis (NASH) patients. J. Funct. Foods 2014, 9, 48–59. [Google Scholar] [CrossRef]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of tea catechins on Alzheimer’s disease: Recent updates and perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Chang, W.C.; Wu JS, B.; Shih, R.W.; Shen, S.C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Kaczmarczyk-Sedlak, I.; Wojnar, W.; Folwarczna, J. The effects of sinapic acid on the development of metabolic disorders induced by estrogen deficiency in rats. Oxidative Med. Cell. Longev. 2018, 2018, 9274246. [Google Scholar] [CrossRef]
- Verma, V.; Singh, D. Sinapic acid alleviates oxidative stress and neuro-inflammatory changes in sporadic model of Alzheimer’s disease in rats. Brain Sci. 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
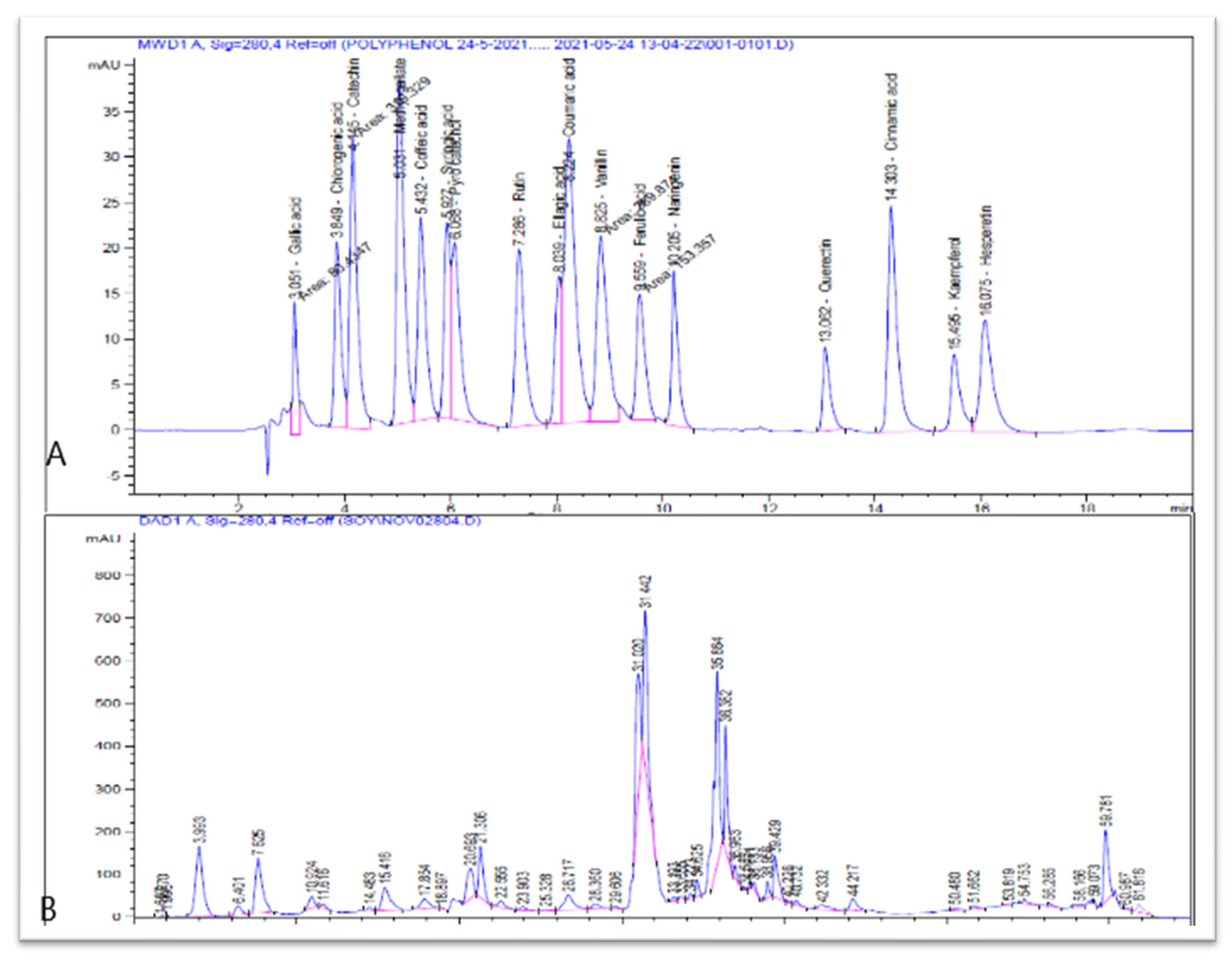
| Phenolic Compound Name | µg/g Extract | Monoisotopic (Da) | Theoretical m/z (Da) | Error of the Calculation (ppm) |
|---|---|---|---|---|
| Total Phenolic Content | 135.6 ± 2.563 | ChemSpider Webpage | WARWICK/Chemistry Webpage | |
| Apigenin-7-glucoside | 0.145 ± 0.002 | 432.105652 | 432.106195 | −1.256636 |
| Cateachin | 5.501 ± 0.056 | 290.079041 | 290.079587 | −1.88 |
| Chlorogenic | 1.593 ± 0.003 | 354.095093 | 354.095631 | −1.52 |
| Chrysin | 1.835 ± 0.123 | 254.057907 | 254.058457 | −2.164856 |
| Cinnamic acid | 0.625 ± 0.056 | 148.052429 | 148.052978 | −3.708132 |
| Ferulic acid | 0.101 ± 0.003 | 194.057907 | 194.058457 | −2.83 |
| Gallic acid | 3.525 ± 0.143 | 170.021530 | 170.022072 | −3.19 |
| Kaempferol | 0.044 ± 0.001 | 286.047729 | 286.048287 | −1.950720 |
| p- hydroxybenzoic acid | 1.136 ± 0.093 | 138.031693 | 138.032243 | −3.984576 |
| p-coumaric acid | 1.751 ± 0.081 | 164.047348 | 164.047893 | −3.322201 |
| Protocatechuic acid | 2.719 ± 0.132 | 154.026611 | 154.027157 | −3.544829 |
| Quercetin | 0.105 ± 0.003 | 302.042664 | 302.043201 | −1.777891 |
| Rosmarinic acid | 0.392 ± 0.004 | 360.084503 | 360.085066 | −1.563519 |
| Rutin | 0.858 ± 0.002 | 610.153381 | 610.153933 | −0.9 |
| Sinapic acid | 9.365 ± 0.873 | 224.068466 | 224.069022 | −2.481378 |
| Syringic acid | 0.682 ± 0.005 | 198.052826 | 198.053372 | −2.76 |
| Vanillic acis | 0.246 ± 0.002 | 152.047348 | 152.047893 | −3.58 |
| Groups | Body Weight Percentage Gain (%) | % of LIVER to Body Mass | % of Brain to Body Mass |
|---|---|---|---|
| Sham Control | 32.6 ± 3.2 b | 5.4 ± 0.4 b | 1.25 ± 0.12 b |
| Vehicle control | 37.5 ± 1.6 b | 3.9 ± 0.3 a | 0.87 ± 0.08 a |
| Induction | 24.5 ± 3.9 a | 3.6 ± 0.25 a | 0.81 ± 0.07 a |
| Protection | 32.7 ± 3.6 b | 4.9 ± 0.34 b | 1.05 ± 0.09 b |
| Treatment | 23.5 ± 3.5 a | 3.5 ± 0.35 a | 0.99 ± 0.09 ab |
| p Value | 0.00 |
| Groups | ALT (U/l) | AST (U/l) | AST/ALT | ALP (U/l) | LDH (U/l) | Protein (g/dL) |
|---|---|---|---|---|---|---|
| Sham Control | 142.3 ± 4.42 ab | 245.4 ± 2.47 a | 1.72 ± 0.08 b | 112.9 ± 1.27 b | 144.1 ± 2.40 b | 8.2 ± 0.53 b |
| Vehicle control | 135.4 ± 8.01 a | 250.4 ± 4.26 a | 1.84 ± 0.06 b | 92.81 ± 2.84 a | 123.3 ± 7.23 a | 7.9 ± 0.41 a |
| Induction | 260.3 ± 7.76 c | 350.3 ± 2.98 b | 1.34 ± 0.02 a | 220.9 ± 2.20 c | 345.2 ± 6.23 c | 9.9 ± 0.21 c |
| Protection | 145.9 ± 5.54 b | 262.6 ± 10.51 a | 1.80 ± 0.01 b | 108.2 ± 1.13 b | 118.4 ± 3.17 a | 7.7 ± 0.31 a |
| Treatment | 149.6 ± 1.56 b | 254.1 ± 11.64 a | 1.7 ± 0.07 b | 109.9 ± 3.68 b | 144.9 ± 10.42 b | 8.1 ± 0.41 b |
| p Value | 0.000 * | 0.014 | 0.005 | 0.000 | 0.000 | 0.000 |
| Groups | Glucose (mg/dL) | Insulin (µIU/mL) | HOMA-IR | TG (mg/dL) | Cholesterol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| Sham Control | 62.04 ± 6.46 a | 6.93 ± 0.45 a | 1.1 ± 0.01 a | 114.90 ± 4.04 a | 114.38 ± 0.47 a | 41.12 ± 0.59 b | 50.28 ± 0.93 a | 22.98 ± 0.8 a |
| Vehicle control | 65.64 ± 3.81 a | 6.79 ± 0.53 a | 1.1 ± 0.02 a | 109.31 ± 9.00 a | 118.50 ± 6.16 a | 39.87 ± 3.10 b | 56.77 ± 3.26 a | 21.86 ± 1.80 a |
| Induction | 220.5 ± 1.53 c | 10.76 ± 0.33 c | 5.9 ± 0.03 d | 255.84 ± 9.50 d | 238.86 ± 6.98 c | 25.47 ± 3.92 a | 167.21 ± 1.16 c | 45.32 ± 1.90 c |
| Protection | 90.68 ± 2.56 b | 8.03 ± 0.26 b | 1.8 ± 0.02 b | 136.29 ± 8.25 c | 136.37 ± 0.62 b | 44.12 ± 2.75 c | 64.81 ± 3.78 b | 27.53 ± 1.65 b |
| Treatment | 89.33 ± 1.59 b | 8.40 ± 0.10 b | 1.9 ± 0.04 c | 126.73 ± 1.98 b | 132.76 ± 2.51 b | 44.17 ± 1.67 c | 64.24 ± 1.6 b | 25.35 ± 0.40 b |
| p Value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Groups | TBARS nmol/mL | GSH mg/dL | AChE IU/mg | MAO IU/mg | ATPase IU/mg |
|---|---|---|---|---|---|
| Sham Control | 8.30 ± 1.11 a | 5.92 ± 0.38 b | 2.58 ± 0. 3 a | 1.85 ± 0.18 b | 0.15 ± 0.02 a |
| Vehicle control | 8.36 ± 1.87 a | 5.61 ± 0.35 b | 2.62 ± 0.94 a | 1.79 ± 0.14 b | 0.14 ± 0.01 a |
| Induction | 16.29 ± 2.96 c | 3.78 ± 1.02 a | 4.79 ± 0.14 b | 1.14 ± 0.12 a | 0.14 ± 0.02 a |
| Protection | 10.55 ± 0.92 b | 5.52 ± 0.29 b | 2.60 ± 0.34 a | 1.81 ± 0.18 b | 0.16 ± 0.01 a |
| Treatment | 9.78 ± 0.30 b | 5.33 ± 0.39 b | 2.90 ± 0.64 a | 1.78 ± 0.12 b | 0.15 ± 0.01 a |
| p Value | 0.000 | 0.000 | 0.001 | 0.000 | 0.150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhujaily, M. Calluna vulgaris Crude Extract Reverses Liver Steatosis and Insulin Resistance-Associated-Brain Lesion Induced by CCl4 Administration. Separations 2023, 10, 94. https://doi.org/10.3390/separations10020094
Alhujaily M. Calluna vulgaris Crude Extract Reverses Liver Steatosis and Insulin Resistance-Associated-Brain Lesion Induced by CCl4 Administration. Separations. 2023; 10(2):94. https://doi.org/10.3390/separations10020094
Chicago/Turabian StyleAlhujaily, Muhanad. 2023. "Calluna vulgaris Crude Extract Reverses Liver Steatosis and Insulin Resistance-Associated-Brain Lesion Induced by CCl4 Administration" Separations 10, no. 2: 94. https://doi.org/10.3390/separations10020094
APA StyleAlhujaily, M. (2023). Calluna vulgaris Crude Extract Reverses Liver Steatosis and Insulin Resistance-Associated-Brain Lesion Induced by CCl4 Administration. Separations, 10(2), 94. https://doi.org/10.3390/separations10020094






