Synthesis of Silica Gel Chelated with Alizarin and 1-Nitroso-2-Naphthol for Solid Phase Extraction of Lead in Ground Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
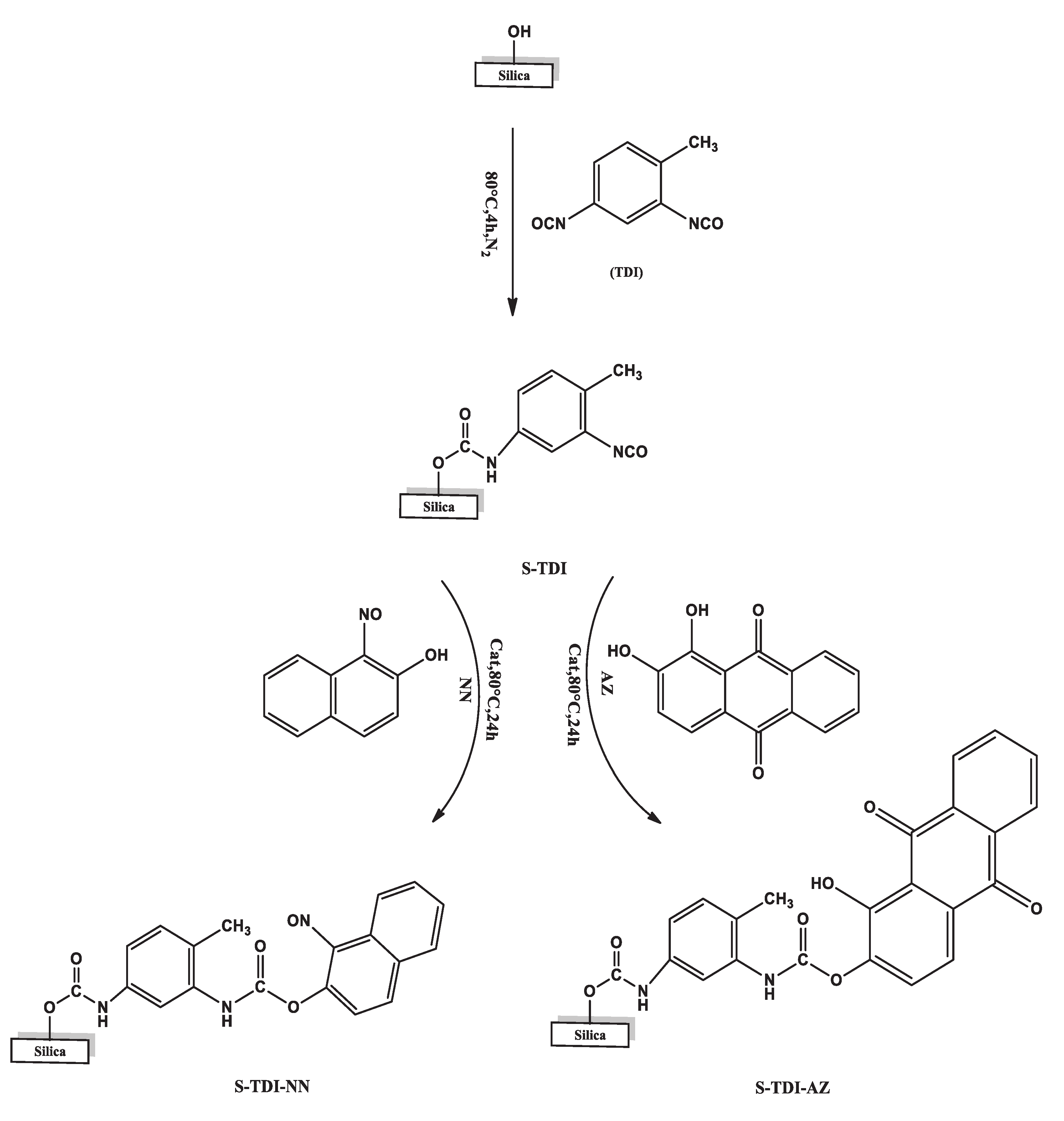
2.3. Preparation of Chelating Resin
2.4. Determination of Isocyanate Groups in the Reaction System
2.5. Screening Experiments
2.6. Effect of pH
2.7. Study of Sorption Capacity
2.8. Solid-Phase Extraction Process
3. Results
3.1. Characterization of Chelating Resins
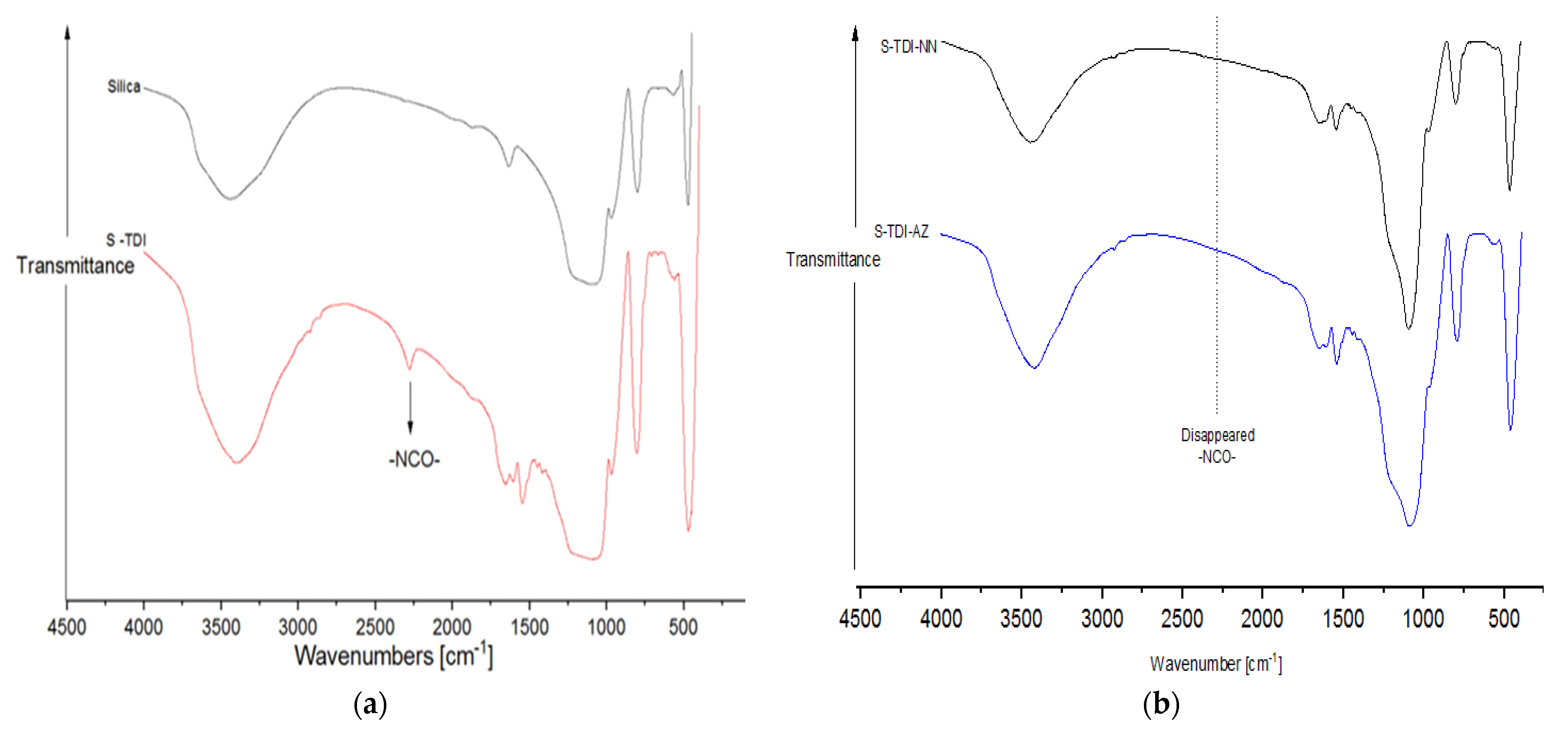
3.1.1. FT-IR Analysis
3.1.2. SEM Analysis
3.1.3. Elemental Analysis
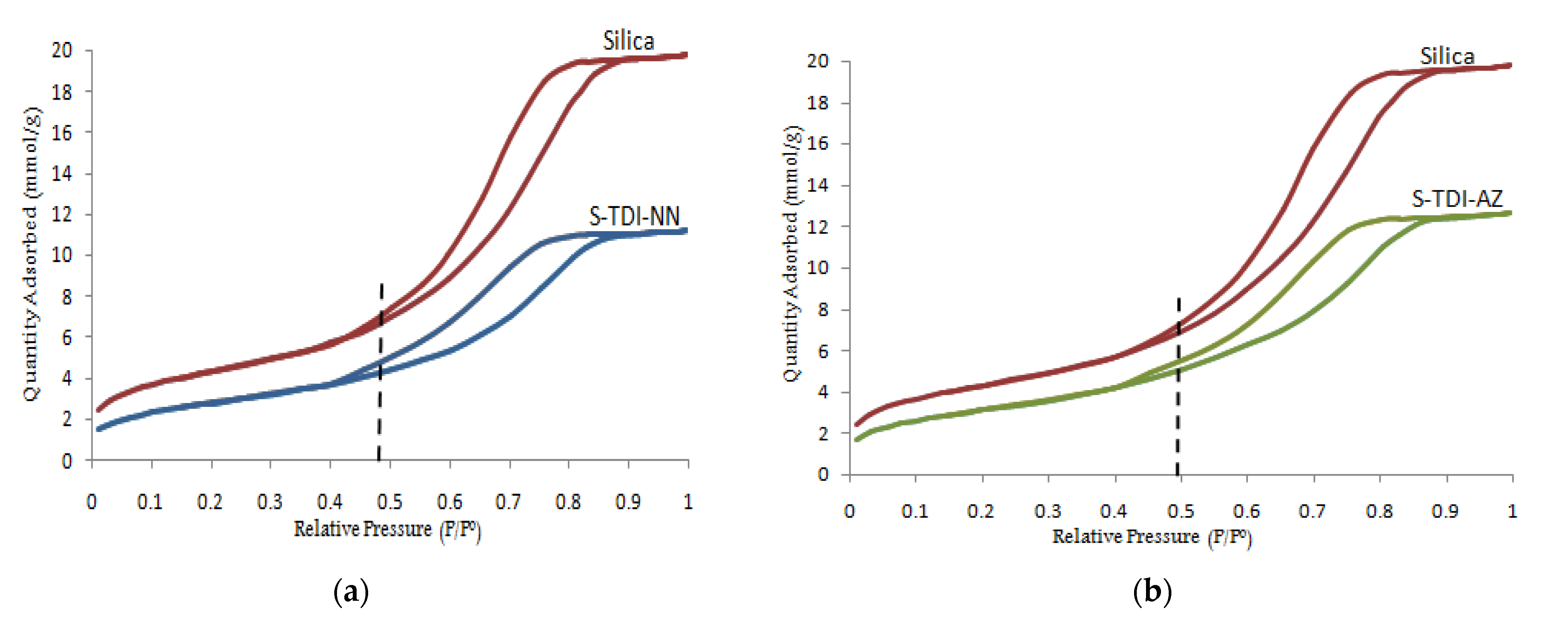
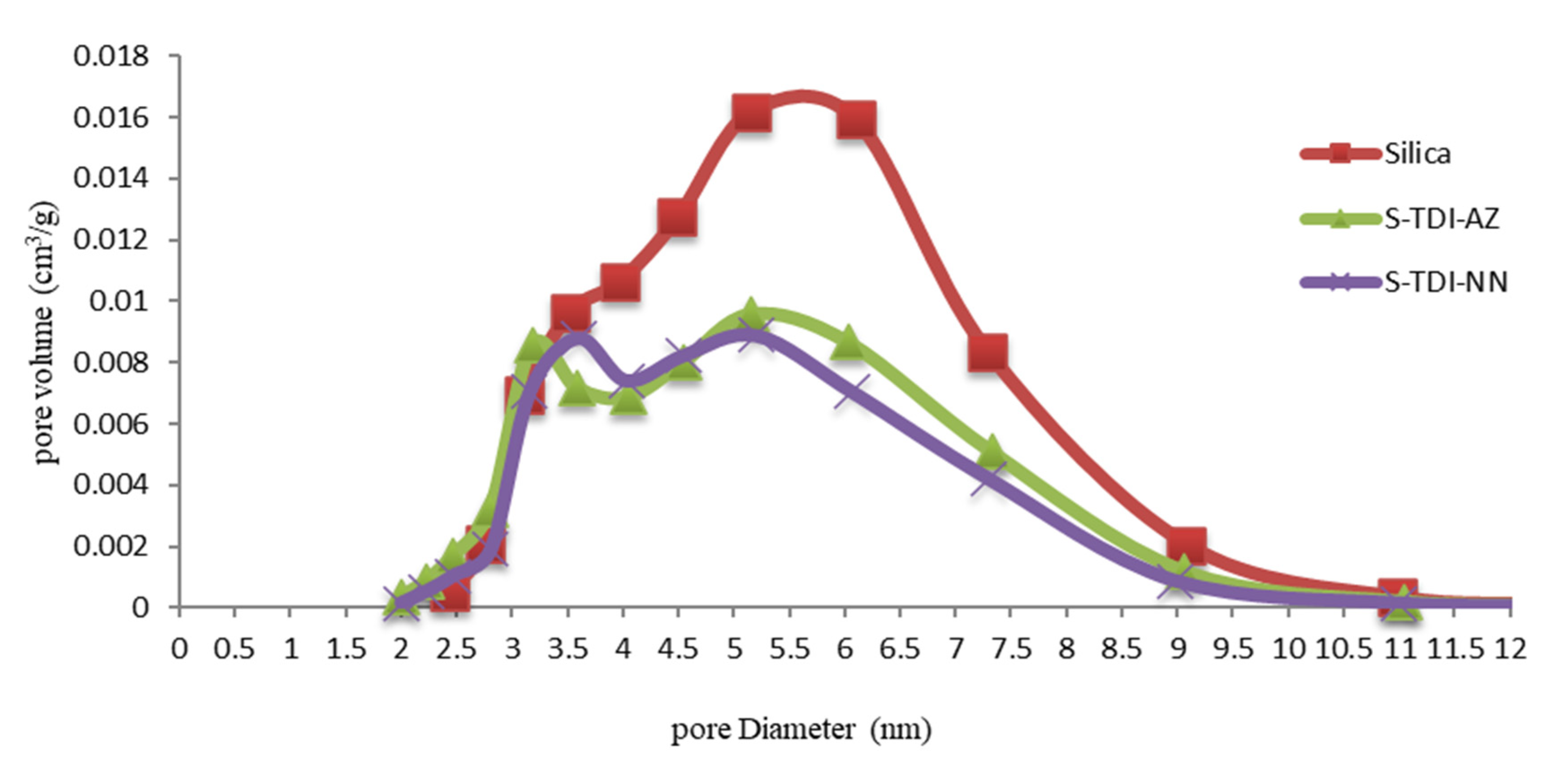
3.1.4. N2Adsorption-Desorption
3.2. Screening Result
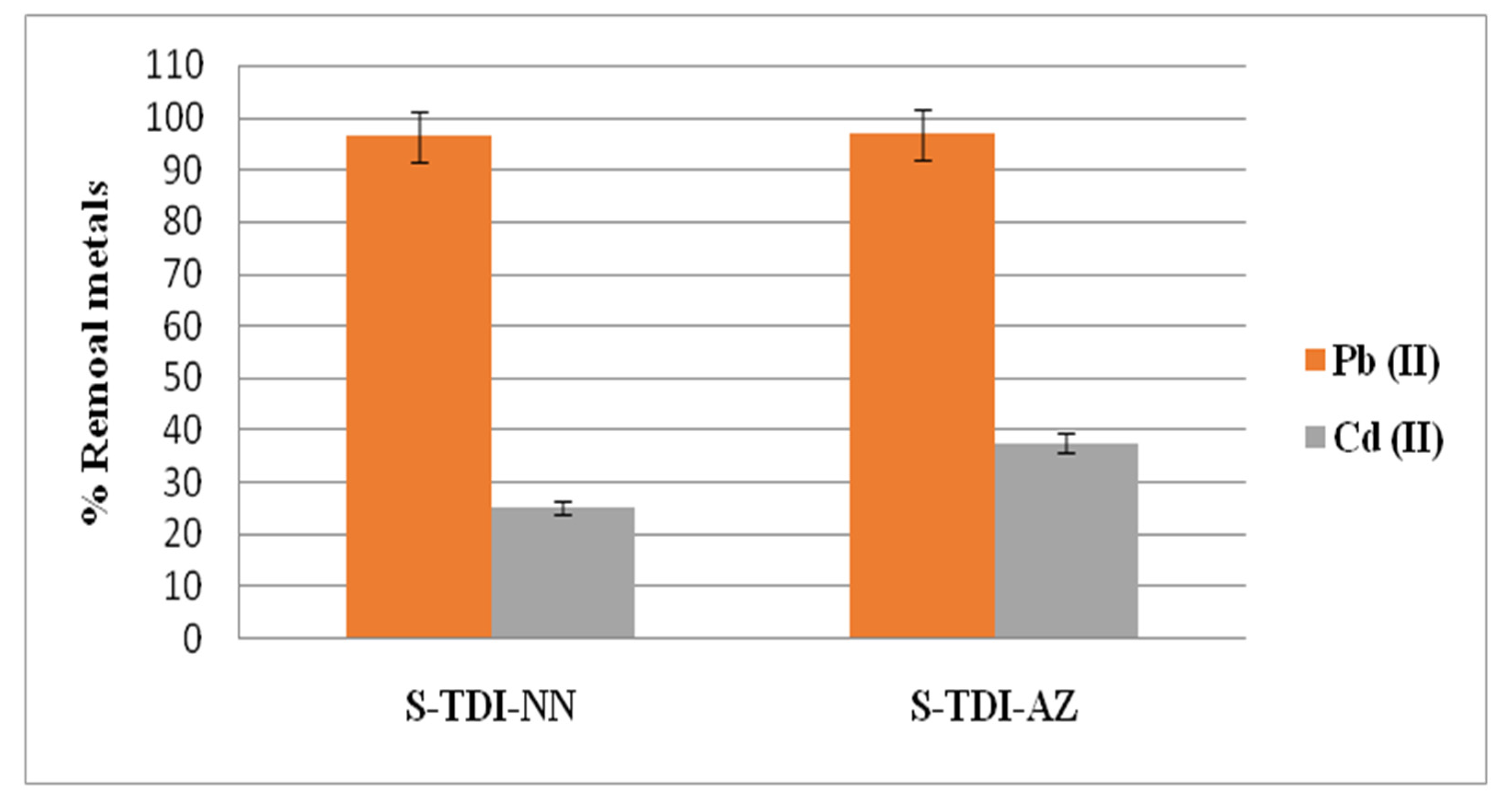
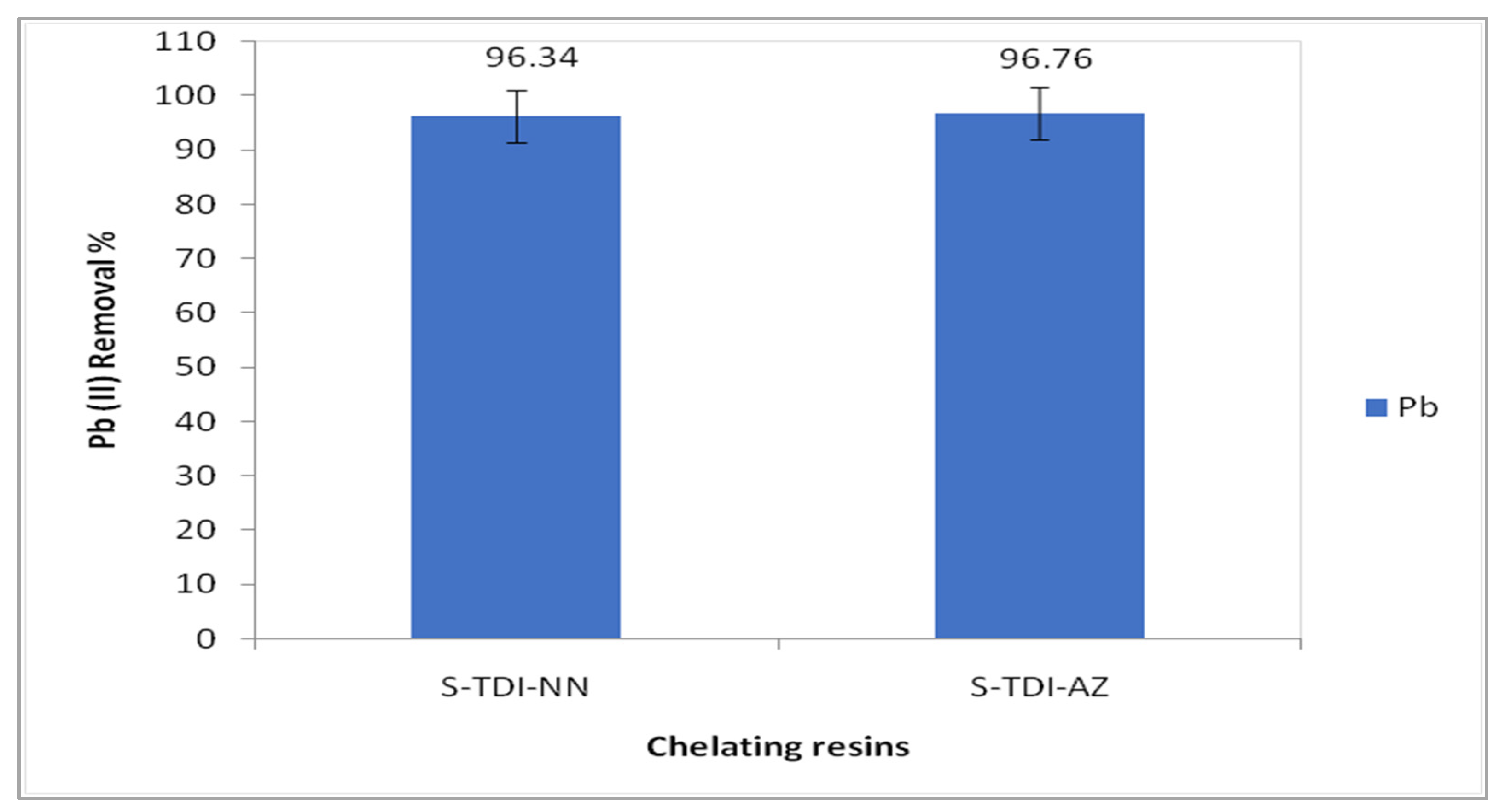
3.3. Efficiency of the Chelating Resins
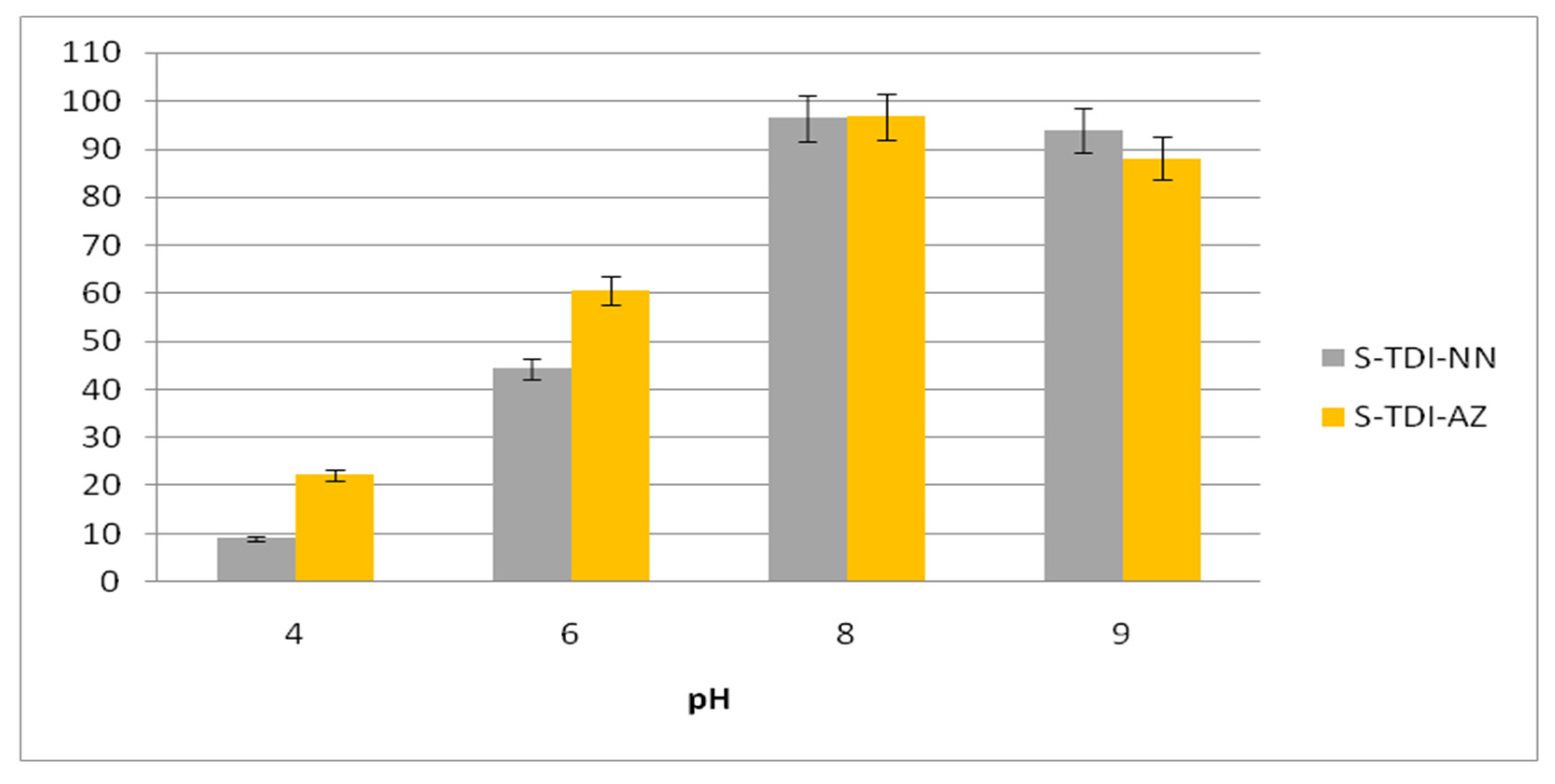
3.4. Influence of pH on Pb (II) Uptake
3.5. Adsorption Capacity
3.6. Analytical Features
3.7. Analytical Application
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabermahani, F.; Hassani, Z.; Faramarzpoor, M. The use of silica gel modified with allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate for selective separation and preconcentration of lead in environmental samples. Environ. Monit. Assess. 2013, 185, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Jal, P.K.; Patel, S.; Mishra, B.K. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 62, 1005–1028. [Google Scholar] [CrossRef] [PubMed]
- Camel, V. Solid phase extraction of trace elements. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Xie, F.; Lin, X.; Wu, X.; Xie, Z. Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta 2008, 74, 836–843. [Google Scholar] [CrossRef]
- Hassanien, M.; Kenawy, I.; Mostafa, M.; El-Dellay, H. Extraction of gallium, indium and thallium from aquatic media using amino silica gel modified by gallic acid. Microchim. Acta 2011, 172, 137–145. [Google Scholar] [CrossRef]
- Sadeghi, S.; Sheikhzadeh, E. Solid phase extraction using silica gel functionalized with Sulfasalazine for preconcentration of uranium(VI) ions from water samples. Microchim. Acta 2008, 163, 313–320. [Google Scholar] [CrossRef]
- Durduran, E.; Altundag, H.; Imamoglu, M.; Yıldız, S.Z.; Tuzen, M. Simultaneous ICP-OES determination of trace metals in water and food samples after their preconcentration on silica gel functionalized with N-(2-aminoethyl)-2,3-dihydroxybenzaldimine. J. Ind. Eng. Chem. 2015, 27, 245–250. [Google Scholar] [CrossRef]
- Pereira, A.S.; Ferreira, G.; Caetano, L.; Martines, M.A.U.; Padilha, P.M.; Santos, A.; Castro, G.R. Preconcentration and determination of Cu(II) in a fresh water sample using modified silica gel as a solid-phase extraction adsorbent. J. Hazard. Mater. 2010, 175, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ferreira, G.; Caetano, L.; Castro, R.; Santos, A.; Padilha, P.; Castro, G. 4-amine-2-mercaptopyrimidine modified silica gel applied in Cd(II) and Pb(II) extraction from an aqueous medium. Pol. J. Chem. Technol. 2010, 12, 7. [Google Scholar] [CrossRef]
- Ozcelik, G.; Imamoglu, M.; Yildiz, S.; Kara, D. Chemically Modified Silica Gel with N-(2-aminoethyl)-Salicylaldimine for Simultaneous Solid Phase Extraction and Preconcentration of Cu(II), Ni(II), Cd(II) and Zn(II) in waters. Water Air Soil Pollut. 2012, 223, 5391–5399. [Google Scholar] [CrossRef]
- Mendil, D.; Demirci, Z.; Uluozlu, O.D.; Tuzen, M.; Soylak, M. A new separation and preconcentration method for selenium in some foods using modified silica gel with 2,6-diamino-4-phenil-1,3,5-triazine. Food Chem. 2017, 221, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jing, M.; Lee, F.S.C.; Wang, X. Synthesis of 8-hydroxyquinoline Bonded Silica (SHQ) and Its Application in Flow Injection-inductively Coupled Plasma Mass Spectrometry Analysis of Trace Metals in Seawater. Chin. J. Anal. Chem. 2006, 34, 459–462. [Google Scholar] [CrossRef]
- Bilgiç, A.; Çimen, A. Removal of chromium (VI) from polluted wastewater by chemical modification of silica gel with 4-acetyl-3-hydroxyaniline. RSC Adv. 2019, 9, 37403–37414. [Google Scholar] [CrossRef] [PubMed]
- Al-Anber, M.A.; Hijazi, A.K.; Al-Momani, I.F.; Al-Matarneh, A.; Al-Bayed, M.t.; Alhalasah, W. Impregnation of Benzyl-L-cysteine into silica gel for the removal of cadmium (II) ion from water. J. Sol-Gel Sci. Technol. 2023, 106, 246–264. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Mentasti, E.; Sarzanini, C. Immobilized ligands on silica: Uptake of cobalt and other metals by 1-nitroso-2-naphthol. Polyhedron 1986, 5, 1013–1015. [Google Scholar] [CrossRef]
- Yun, J.; Choi, H. Micellar colorimetric determination of iron, cobalt, nickel and copper using 1-nitroso-2-naphthol. Talanta 2000, 52, 893–902. [Google Scholar] [CrossRef]
- Doskocz, M.; Kubas, K.; Frąckowiak, A.; Gancarz, R. NMR and ab initio studies of Mg2+, Ca2+, Zn2+, Cu2+ alizarin complexes. Polyhedron 2009, 28, 2201–2205. [Google Scholar] [CrossRef]
- Yang, M.; Gao, Y.; He, J.P.; Li, H.M. Preparation of polyamide 6/silica nanocomposites from silica surface initiated ring-opening anionic polymerization. Express Polym. Lett. 2007, 1, 433–442. [Google Scholar] [CrossRef]
- Alahmadi, S.M.; Mohamad, S.; Maah, M.J. Synthesis and Characterization of Mesoporous Silica Functionalized with Calix[4]arene Derivatives. Int. J. Mol. Sci. 2012, 13, 13726. [Google Scholar] [CrossRef]
- AlSuhaimi, A.O.; AlRadaddi, S.M.; Al-Sheikh Ali, A.K.; Shraim, A.M.; AlRadaddi, T.S. Silica-based chelating resin bearing dual 8-Hydroxyquinoline moieties and its applications for solid phase extraction of trace metals analysis from seawater prior to their analysis by ICP-MS. Arab. J. Chem. 2017, 12, 360–369. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Puhakka, V.; Repo, E.; Sillanpää, M. Selective separation of scandium from iron, aluminium and gold rich wastewater using various amino and non-amino functionalized silica gels—A comparative study. J. Clean. Prod. 2018, 170, 890–901. [Google Scholar] [CrossRef]
- Arnold, R.G.; Nelson, J.A.; Verbanc, J.J. Recent Advances In Isocyanate Chemistry. Chem. Rev. 1957, 57, 47–76. [Google Scholar] [CrossRef]
- Simons, D.M.; Arnold, R.G. Relative Reactivity of the Isocyanate Groups in Toluene-2,4-diisocyanate. J. Am. Chem. Soc. 1956, 78, 1658–1659. [Google Scholar] [CrossRef]
- Dev, K.; Rao, G.N. Preparation and analytical properties of a chelating resin containing bicine groups. Talanta 1995, 42, 591–596. [Google Scholar] [CrossRef]
- Bulut, V.N.; Gundogdu, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Elci, L.; Tufekci, M. A multi-element solid-phase extraction method for trace metals determination in environmental samples on Amberlite XAD-2000. J. Hazard. Mater. 2007, 146, 155–163. [Google Scholar] [CrossRef]
- Kumar, M.; Rathore, D.P.S.; Singh, A.K. Amberlite XAD-2 functionalized with o-aminophenol: Synthesis and applications as extractant for copper(II), cobalt(II), cadmium(II), nickel(II), zinc(II) and lead(II). Talanta 2000, 51, 1187–1196. [Google Scholar] [CrossRef]
- Duran, C.; Senturk, H.B.; Gundogdu, A.; Bulut, V.N.; Elci, L.; Soylak, M.; Tufekci, M.; Uygur, Y. Determination of Some Trace Metals in Environmental Samples by Flame AAS Following Solid Phase Extraction with Amberlite XAD-2000 Resin after Complexing with 8-Hydroxyquinoline. Chin. J. Chem. 2007, 25, 196–202. [Google Scholar] [CrossRef]
- Tian, Y.; Yin, P.; Qu, R.; Wang, C.; Zheng, H.; Yu, Z. Removal of transition metal ions from aqueous solutions by adsorption using a novel hybrid material silica gel chemically modified by triethylenetetraminomethylenephosphonic acid. Chem. Eng. J. 2010, 162, 573–579. [Google Scholar] [CrossRef]
- Ou, B.; Huang, R.; Zhou, H.; Li, Z.; Chen, L.; Zhou, Z.; Li, D. Preparation of doubly responsive polymer functionalized silica hybrid nanoparticles via a one-pot thiol-isocyanate click reaction at room temperature. Polym. Compos. 2017, 38, 1454–1461. [Google Scholar] [CrossRef]
- Rosu, M.-C.; Suciu, R.-C.; Lazar, M.D.; Bratu, I. The influence of alizarin and fluorescein on the photoactivity of Ni, Pt and Ru-doped TiO2 layers. Mater. Sci. Eng. B 2013, 178, 383–390. [Google Scholar] [CrossRef]
- Foretić, B.; Burger, N.; Hankonyi, V. Reactions of the aquapentacyanoferrate(II) ion with 2-nitroso-1-naphthol and 2-nitroso-1-naphthol-4-sulphonic acid. Polyhedron 1995, 14, 605–609. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mishra, M.; Sharma, S.; Dutta, S. Zinc(II) complex immobilized on amine functionalized silica gel: A novel, highly efficient and recyclable catalyst for multicomponent click synthesis of 1,4-disubstituted 1,2,3-triazoles. J. Coord. Chem. 2016, 69, 1152–1165. [Google Scholar] [CrossRef]
- Khan, A.A.; Baig, U. Preparation, characterization, and properties of polyacrylonitrile–silica gel anion exchange composite fibers. Polym. Eng. Sci. 2013, 53, 2027–2033. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, Y.; Qu, W.; Sun, C.; Chen, J.; Ping, Y.; Chen, H.; Niu, Y. Mercury adsorption by sulfur- and amidoxime-containing bifunctional silica gel based hybrid materials. Chem. Eng. J. 2013, 219, 51–61. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, R.; Sun, C.; Chen, H.; Wang, C.; Ji, C.; Yin, P.; Sun, Y.; Zhang, H.; Niu, Y. Comparison of synthesis of chelating resin silica-gel-supported diethylenetriamine and its removal properties for transition metal ions. J. Hazard. Mater. 2009, 163, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, L.; Ling, P.; Jing, X.; Li, C.; Li, A.; You, X. Interaction mechanism of aqueous heavy metals onto a newly synthesized IDA-chelating resin: Isotherms, thermodynamics and kinetics. Chem. Eng. J. 2011, 173, 106–114. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Niu, Y.; Qu, R.; Chen, H.; Mu, L.; Liu, X.; Wang, T.; Zhang, Y.; Sun, C. Synthesis of silica gel supported salicylaldehyde modified PAMAM dendrimers for the effective removal of Hg(II) from aqueous solution. J. Hazard. Mater. 2014, 278, 267–278. [Google Scholar] [CrossRef]
- Ragab, M.A.; Korany, M.A.; Ibrahim, H.Z.; Abdel-Kawi, M.A.; Abd El Aal, A. Adsorption behavior of some metal ions on nanoparticles used in pharmaceutical matrices: Application to laboratory made drug formulation. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 155–162. [Google Scholar] [CrossRef]
- Liu, C.; He, S.; Shen, K.; Feng, X.; Fang, G.; Wang, S. l-Cysteine Functionalized Silica Gel as an Efficient Adsorbent for the Determination of Heavy Metals in Foods by ICP-MS. Food Anal. Methods 2014, 8, 1785–1793. [Google Scholar] [CrossRef]
- Ekinci, C.; Köklü, Ü. Determination of vanadium, manganese, silver and lead by graphite furnace atomic absorption spectrometry after preconcentration on silica-gel modified with 3-aminopropyltriethoxysilane. Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 1491–1495. [Google Scholar] [CrossRef]

| Chelating Resins | %C | %N | %H |
|---|---|---|---|
| S-TDI | 8.85 | 1.94 | 1.21 |
| S-TDI-NN | 10.62 | 2.44 | 1.29 |
| S-TDI-AZ | 10.94 | 1.40 | 2.51 |
| Sample | BET Surface Area (m2/g) | BJH Desorption Cumulative Volume of Pores (cm3/g) | BJH Desorption Average Pore Diameter (nm) |
|---|---|---|---|
| Silica | 520 | 0.69 | 5.34 |
| S-TDI-AZ | 353 | 0.44 | 5.06 |
| S-TDI-NN | 319 | 0.39 | 4.95 |
| Chelating Resins | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
|
Amberlite
XAD-2000-diethyldithio carbamate | 6.42 | [25] |
|
Silica gel modified by allyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetra hydropyrimidine-5-carboxylate | 5.0 | [1] |
| Amberlite XAD-2-o-aminophenol | 3.32 | [26] |
| Amberlite XAD-2000-HQ | 7.20 | [27] |
| S-TDI-AZ | 9.56 | Current study |
| S-TDI-NN | 9.43 | Current study |
| Calibration Parameters | Pb (II) (μg/L) | ||
|---|---|---|---|
| Chelating Resins | S-TDI-AZ | S-TDI-NN | |
| Concentration range (μg/L) (n = 5) | 0.1–18 | 0.1–18 | |
| % RSD at 18 μg/L (n = 4) | 1.742 | 2.018 | |
| Correlation coefficient, R2 | 0.997 | 0.996 | |
| Sensitivity, CPS ratio/(μg/L) | 98,900 | 98,679 | |
| LOD (μg/L) | 0.0025 | 0.0026 | |
| Chelating Resins | Pb (II) Spiked (μg/L) | (Found ± RSD) a | Recovery (n = 4) (%) |
|---|---|---|---|
| S-TDI-AZ | 0 | 2.315 ± 0.061 | - |
| 5 | 7.550 ± 0.023 | 104.70 | |
| S-TDI-NN | 0 | 2.729 ± 0.053 | - |
| 5 | 7.860 ± 0.013 | 102.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahmadi, S.M.; Aljuhani, S.S. Synthesis of Silica Gel Chelated with Alizarin and 1-Nitroso-2-Naphthol for Solid Phase Extraction of Lead in Ground Water Samples. Separations 2023, 10, 544. https://doi.org/10.3390/separations10100544
Alahmadi SM, Aljuhani SS. Synthesis of Silica Gel Chelated with Alizarin and 1-Nitroso-2-Naphthol for Solid Phase Extraction of Lead in Ground Water Samples. Separations. 2023; 10(10):544. https://doi.org/10.3390/separations10100544
Chicago/Turabian StyleAlahmadi, Sana M., and Salwa S. Aljuhani. 2023. "Synthesis of Silica Gel Chelated with Alizarin and 1-Nitroso-2-Naphthol for Solid Phase Extraction of Lead in Ground Water Samples" Separations 10, no. 10: 544. https://doi.org/10.3390/separations10100544
APA StyleAlahmadi, S. M., & Aljuhani, S. S. (2023). Synthesis of Silica Gel Chelated with Alizarin and 1-Nitroso-2-Naphthol for Solid Phase Extraction of Lead in Ground Water Samples. Separations, 10(10), 544. https://doi.org/10.3390/separations10100544






