Progress in the Separation and Purification of Carbon Hydrocarbon Compounds Using MOFs and Molecular Sieves
Abstract
:1. Introduction
2. Separation Mechanism of Low-Carbon Hydrocarbons
3. Purification of Methane
3.1. Adsorption Separation of Methane and Nitrogen
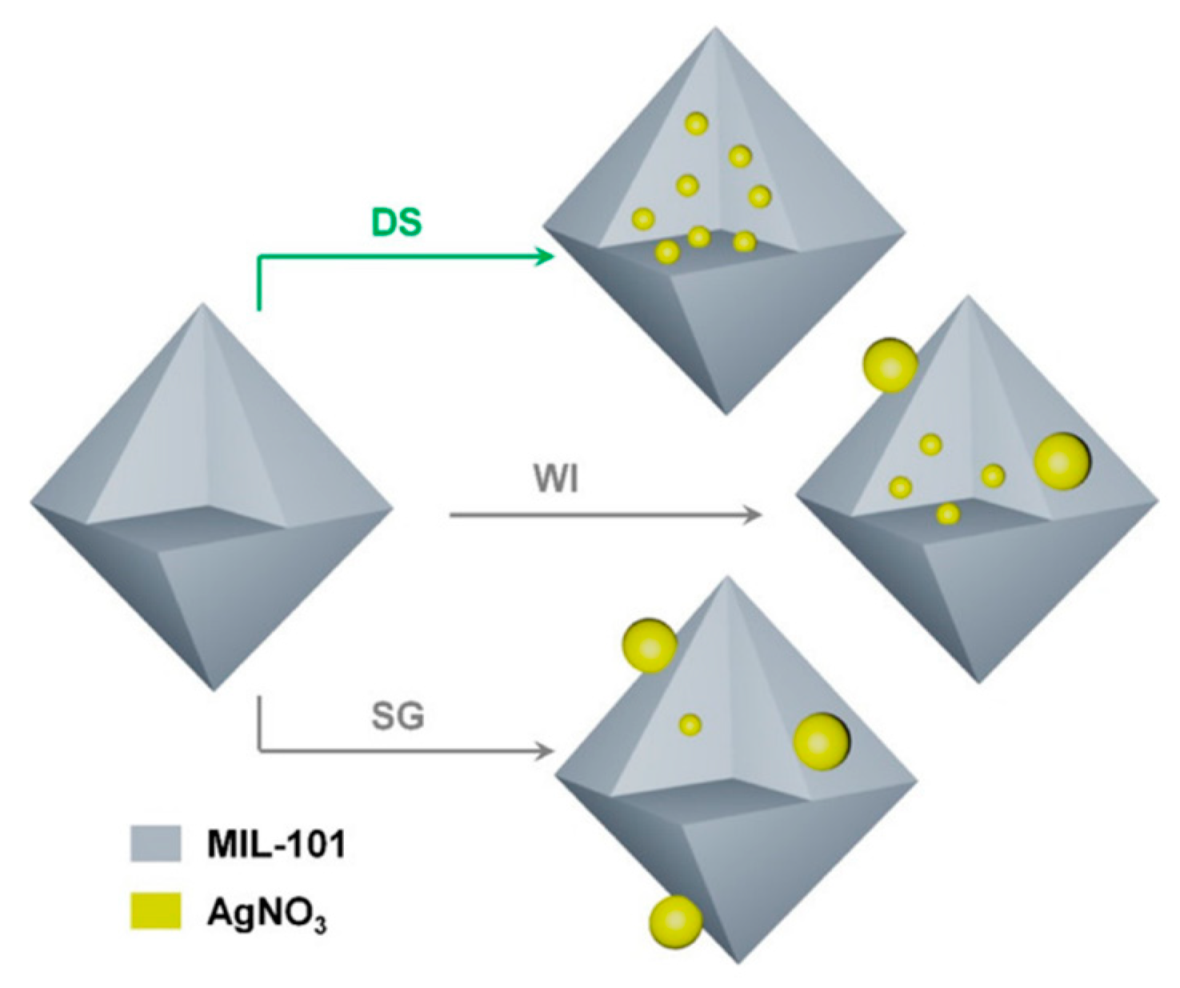
3.1.1. Metal–Organic Frameworks for Methane/Nitrogen Separation
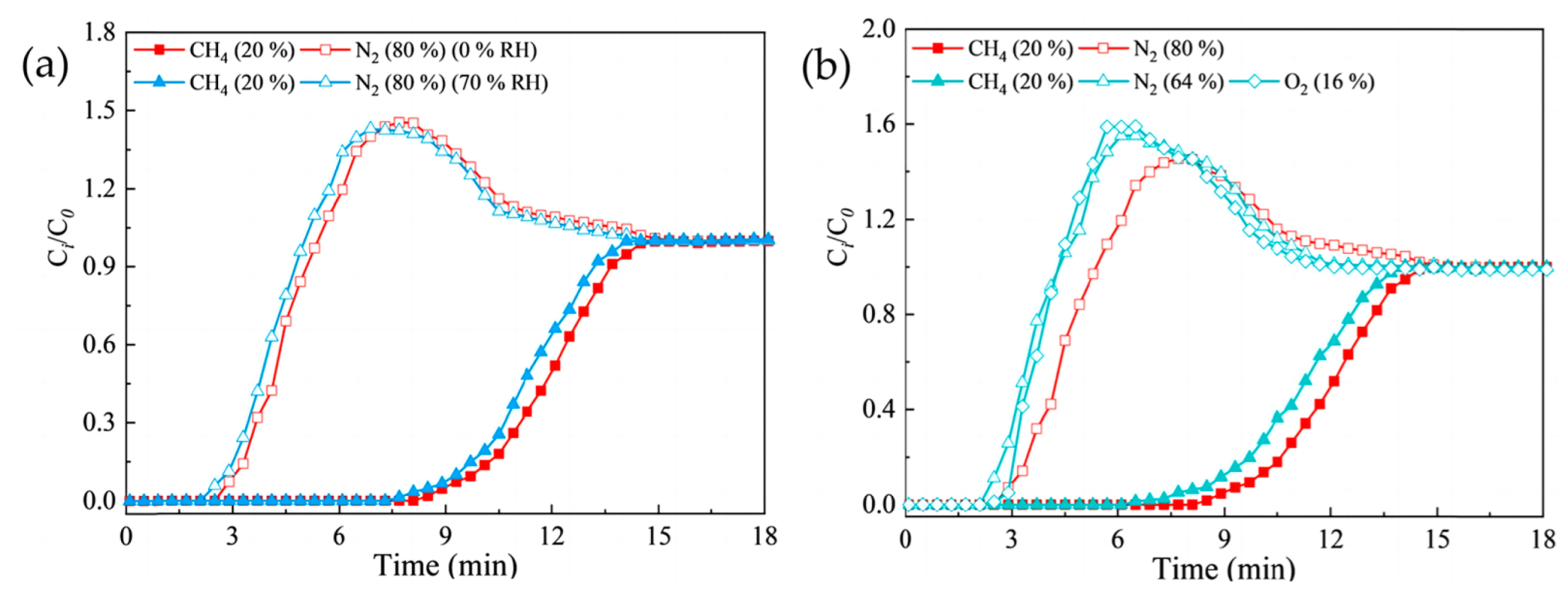
3.1.2. Molecular Sieves for Methane/Nitrogen Separation
3.2. Adsorption Separation of Methane and Carbon Dioxide
3.2.1. Metal–Organic Frameworks for Methane/Carbon Dioxide Separation
3.2.2. Molecular Sieves for Methane/Carbon Dioxide Separation
4. Adsorption Separation of Olefins and Alkanes
4.1. Adsorption Separation of Ethylene and Ethane
4.1.1. Metal–Organic Frameworks for Ethylene/Ethane Separation
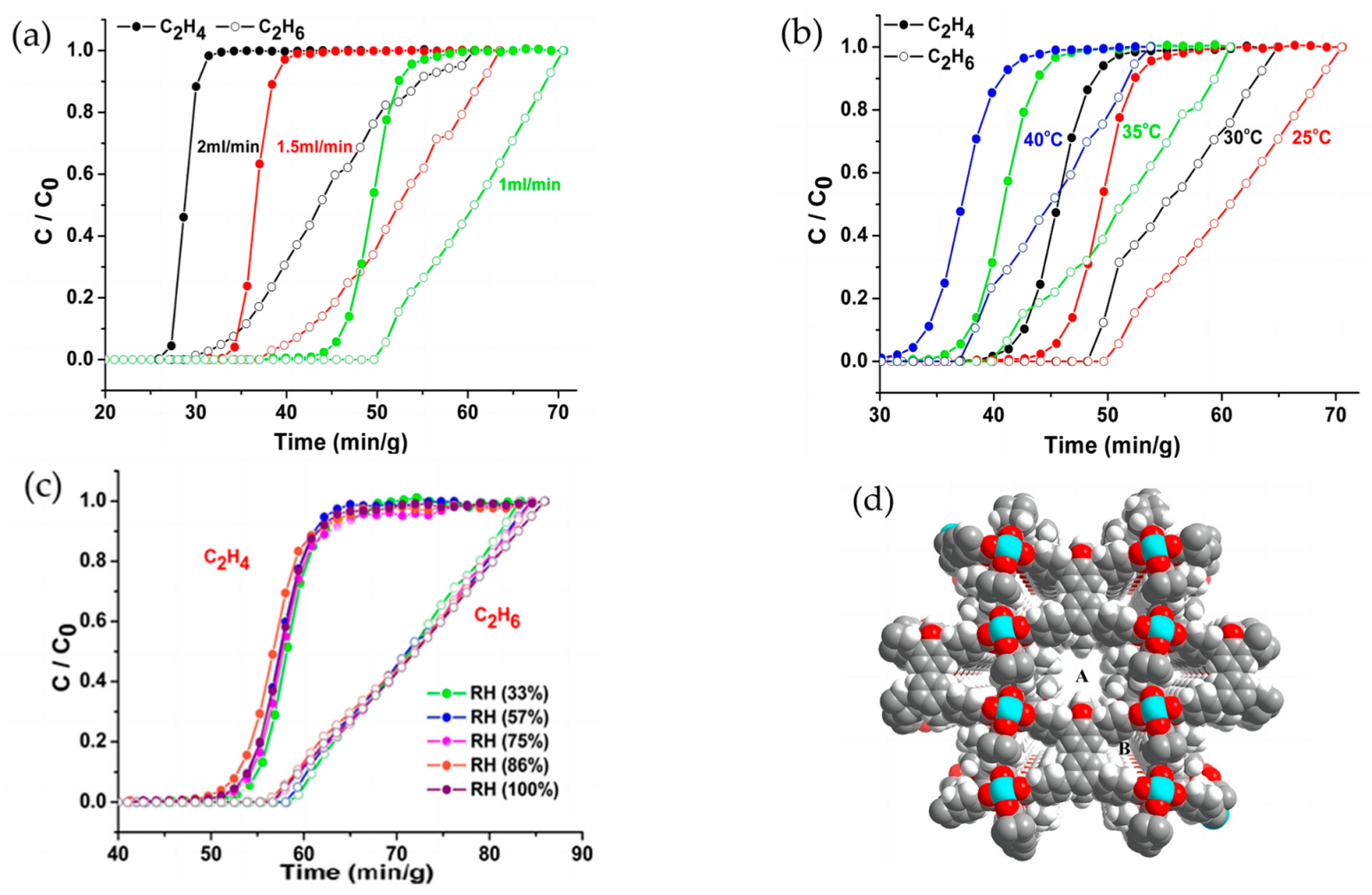
4.1.2. Molecular Sieves for Ethylene/Ethane Separation
4.2. Adsorption Separation of Propylene and Propane
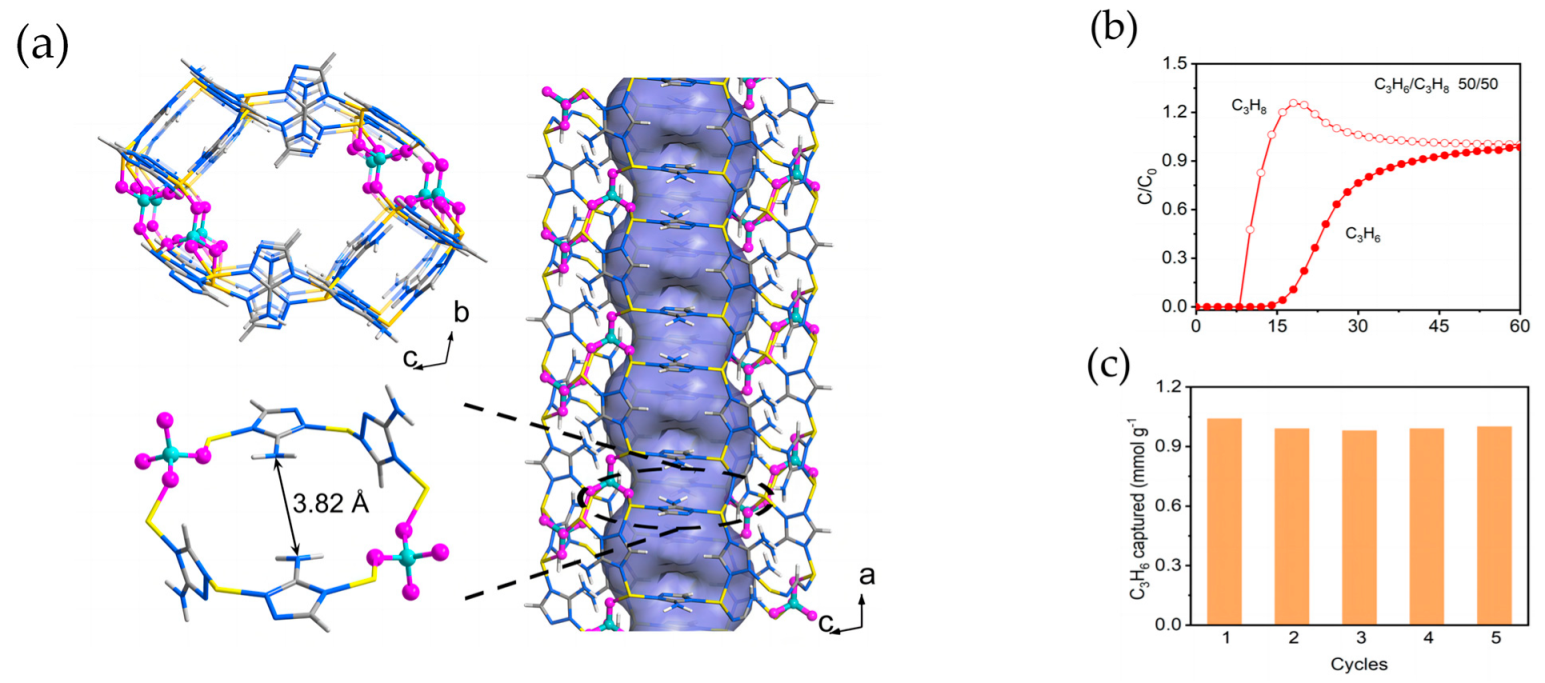
4.2.1. Metal–Organic Frameworks for Propylene/Propane Separation
4.2.2. Molecular Sieves for Propylene/Propane Separation
5. Adsorptive Separation of Isomers in High-Carbon Hydrocarbons (C6–C8) Using Metal–Organic Frameworks and Molecular Sieves
5.1. Metal–Organic Frameworks for High-Carbon Hydrocarbons (C6–C8) Isomer Separation
5.2. Molecular Sieves for High-Carbon Hydrocarbons (C6–C8) Isomer Separation
6. Perspectives
6.1. Challenges
- (1)
- Several effective adsorption separation materials have been created recently, although they have only been used in lab settings. Even if their hydrothermal stability is still subpar, their separation performance will be significantly compromised in humid and hot application scenarios. Furthermore, the use of organic solvents and precious metals for modification raises the cost of adsorbents, posing small environmental hazards. This makes large-scale manufacturing difficult. The research on the concept of carbon neutrality and the study on recyclability during the gas adsorption process still have certain gaps, nevertheless.
- (2)
- Even when achieving large adsorption capacity or high separation selectivity, it is currently not possible to accomplish both huge adsorption capacity and high separation selectivity in carbon hydrocarbon compounds separation and purification. More in-depth research should be performed on the transport mechanism and separation performance of molecules in MOFs and molecular sieves in order to improve material design, increase separation efficiency, design adsorbents with both a large adsorption capacity and high selectivity, and achieve more effective, energy-saving, and environmentally friendly low-carbon hydrocarbon separation.
6.2. Opportunities
- (1)
- Hierarchical combination design of different adsorbent materials
- (2)
- Computer-aided data processing and simulation for screening adsorbents
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, D.M. The light hydrocarbons in petroleum: A critical review. Org. Geochem. 1997, 26, 417–440. [Google Scholar] [CrossRef]
- Chen, X.Y.; Xiao, A.; Rodrigue, D. Polymer-based Membranes for Propylene/Propane Separation. Sep. Purif. Rev. 2021, 51, 130–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Lian, X.; Rajamani, K.; Yang, S.; Hu, T. An ultramicroporous metal-organic framework based on octahedral-like cages showing high-selective methane purification from a six-component C1/C2/C3 hydrocarbons mixture. Sep. Purif. Rev. 2023, 304, 122312. [Google Scholar] [CrossRef]
- Shi, R.F.; Lv, D.F.; Chen, Y.W.; Wu, H.X.; Liu, B.Y.; Xia, Q.B.; Li, Z. Highly selective adsorption separation of light hydrocarbons with a porphyrinic zirconium metal-organic framework PCN-224. Sep. Purif. Rev. 2023, 207, 262–268. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Hu, Y.Q.; Luan, B.Q.; Wang, L.Y.; Krishna, R.; Ni, H.F.; Hu, X.; Zhang, Y.B. Benchmark single-step ethylene purification from ternary mixtures by a customized fluorinated anion-embedded MOF. Nat. Commun. 2023, 14, 401. [Google Scholar] [CrossRef]
- Jiang, H.F.; Chen, Y.; Song, S.Q.; Guo, Z.Y.; Zhang, Z.Q.; Zheng, C.Y.; He, G.W.; Wang, H.J.; Wu, H.; Huang, T.; et al. Confined facilitated transport within covalent organic frameworks for propylene/propane membrane separation. Chem. Eng. J. 2022, 439, 135657. [Google Scholar] [CrossRef]
- Park, H.M.; Lee, J.Y.; Jee, K.Y.; Nakao, S.; Lee, Y.T. Hydrocarbon separation properties of a CVD-deposited ceramic membrane under single gases and binary mixed gas. Sep. Purif. Rev. 2021, 254, 117642–117651. [Google Scholar] [CrossRef]
- Lee, J.; Chuah, C.Y.; Kim, J.; Kim, Y.; Nakeun, K.; Seo, Y.; Kim, K.; Bae, T.H. Separation of acetylene from carbon dioxide and ethylene by a water-stable microporous metal–organic framework with aligned imidazolium groups inside the channels. Angew. Chem. Int. Ed. 2018, 130, 7995–7999. [Google Scholar] [CrossRef]
- Cui, X.L.; Xing, H.B. Research progress on separation of low-carbon hydrocarbons from metal-organic framework materials. CIESC J. 2018, 69, 2339–2352. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Yang, L.F.; Qian, S.H.; Wang, X.B.; Cui, X.L.; Chen, B.L.; Xing, H.B. Energy-efficient separation alternatives: Metal–organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef]
- Wu, N.; Xue, H. Comparative study on shallow cooling process of light hydrocarbon recovery from associated gas in oilfield. Chem. Eng. Des. Commun. 2020, 30, 6–11. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Bae, T.H. Recent advances in mixed-matrix membranes for light hydrocarbon (C1–C3) separation. Membranes 2022, 12, 201. [Google Scholar] [CrossRef]
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Ko, D. Optimization of vacuum pressure swing adsorption processes to sequester carbon dioxide from coalbed methane. Ind. Eng. Chem. Res. 2016, 55, 8967–8978. [Google Scholar] [CrossRef]
- Zhai, R.; Jiao, F.L.; Lin, H.J.; Hao, F.R.; Li, J.B.; Yan, H.; Li, N.N.; Wang, H.H.; Jin, Z.Y.; Zhang, Y.J.; et al. Research progress of metal-organic framework materials. Chin. J. Chromatogr. 2014, 32, 107–116. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhang, X.J.; Ba, Y.Q.; Li, T.Y.; Hao, G.P.; Lu, A.H. Recent advances in carbon-based adsorbents for adsorptive separation of light hydrocarbons. Research 2022, 2022, 9780864. [Google Scholar] [CrossRef]
- Qin, Y.; Gao, X.; Zhang, H.T.; Zhang, S.H.; Zheng, L.G.; Li, Q.; Mo, Z.S.; Duan, L.H.; Zhang, X.T.; Song, L.J. Measurements and distinguishment of mass transfer processes in fluid catalytic cracking catalyst particles by uptake and frequency response methods. Catal. Today 2015, 245, 147–154. [Google Scholar] [CrossRef]
- He, Y.; Xiang, S.C.; Chen, B.L. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 2011, 133, 14570–14573. [Google Scholar] [CrossRef]
- Lin, R.; Wu, H.; Li, L.B.; Tang, X.; Li, Z.Q.; Gao, J.K.; Cui, H.; Zhou, W.; Chen, B.L. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks. J. Am. Chem. Soc. 2018, 140, 2940–12946. [Google Scholar] [CrossRef]
- Li, L.B.; Lin, R.; Wang, X.Q.; Zhou, W.; Jia, L.T.; Li, J.P.; Chen, B.L. Kinetic Separation of Propylene over Propane in a Microporous Metal-Organic Framework. Chem. Eng. J. 2018, 354, 977–982. [Google Scholar] [CrossRef]
- Xu, S.; Liu, R.S.; Zhang, M.Y.; Lu, A. Designed synthesis of porous carbons for the separation of light hydrocarbons. Chin. J. Chem. Eng. 2022, 42, 130–150. [Google Scholar] [CrossRef]
- Hao, X.F. Preparation of Modified Clinoptilolite Zeolite and Its CH4/N2 Adsorption and Separation Performance. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2020. [Google Scholar]
- Liu, J.Q. Synthesis of Nanozeolite and Study on the Adsorption and Separation Performance of CH4/N2. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2021. [Google Scholar]
- Freitas, M.; Figueiredo, J. Preparation of carbon molecular sieves for gas separations by modification of the pore sizes of activated carbons. Fuel 2001, 80, 1–6. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Towards a molecular understanding of shape selectivity. Nature 2008, 451, 671–678. [Google Scholar] [CrossRef]
- Zhang, W.C.; Cheng, Y.H.; Guo, C.S.; Xie, C.P. Cobalt Incorporated Porous Aromatic Framework for CO2/CH4 Separation. Ind. Eng. Chem. Res. 2018, 57, 10985–10991. [Google Scholar] [CrossRef]
- Guo, P.; Li, M. Research Progress on CH4/N2 Separation Process in Coal-bed Methane. Chem. Ind. Eng. Prog. 2008, 7, 963. [Google Scholar] [CrossRef]
- He, Y.B.; Zhou, W.; Qian, G.D.; Chen, B.L. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.L.; Pham, T.; Lan, P.C.; Xing, H.B.; Forrest, K.A.; Wojtas, L.; Space, B.; Ma, S.Q. A Metal-Organic Framework Based Methane Nano-trap for the Capture of Coal-Mine Methane. Angew. Chem. Int. Ed. 2019, 58, 10138–10141. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.H.; Yang, Q.Y.; Liu, D.H. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation. Chem. Eng. J. 2021, 408, 127294–127301. [Google Scholar] [CrossRef]
- Chang, M.; Yan, T.G.; Wei, Y.; Wang, J.X.; Liu, D.H.; Chen, J.F. Enhancing CH4 Capture from Coalbed Methane through Tuning van der Waals Affinity within Isoreticular Al-Based Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2022, 14, 25374–25384. [Google Scholar] [CrossRef]
- Wang, S.M.; Shivanna, M.; Yang, Q.Y. Nickel-Based Metal-Organic Frameworks for Coal-Bed Methane Purification with Record CH4/N2 Selectivity. Angew. Chem. Int. Ed. 2022, 61, 202201017–202201024. [Google Scholar] [CrossRef]
- Li, T.; Jia, X.X.; Cheng, H.; Chang, Z.Y.; Li, L.B.; Wang, Y.; Li, J.P. Tuning the Pore Environment of MOFs toward Efficient CH4/N2 Separation under Humid Conditions. ACS Appl. Mater. Interfaces 2022, 14, 15830–15839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H. Synthesis and Modification of SAPO Molecular Sieve and Its Separation Performance for Small Molecules. Ph.D. Thesis, Jilin University, Changchun, China, 2021. [Google Scholar]
- Yang, J.F.; Zhao, Q.; Xu, H. Adsorption of CO2, CH4 and N2 on Gas Diameter Grade Ion-Exchange Small Pore Zeolites. J. Chem. Eng. Data 2012, 57, 3701–3709. [Google Scholar] [CrossRef]
- Yang, J.F.; Liu, J.Q.; Liu, P.X.; Li, L.B.; Tang, X.; Shang, H.; Li, J.P.; Chen, B.L. K-Chabazite Zeolite Nanocrystal Aggregates for Highly Efficient Methane Separation. Angew. Chem. Int. Ed. 2021, 61, 202116850–202116856. [Google Scholar] [CrossRef]
- Chen, Y.H.; Peng, Q.L.; Jiang, H.; Sun, H.; Shen, B.X.; Zhang, J.; Li, W.L.; Liu, Y.J.; Guo, L. Sr2+ exchange regulates the selective adsorption of N2 in CH4 by ETS-4 zeolite. Acta Pet. Sin. Pet. Process. Sect. 2022, 38, 1–10. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Yang, W.; Wang, N.; Chu, W.; Liu, D.J. Effect of nitrogen-containing groups on methane adsorption behaviors of carbon spheres. J. Anal. Appl. Pyrolysis 2014, 107, 204–210. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, D.C.; Meng, Z.Y.; Li, Y.Y. Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve. Sep. Purif. Rev. 2019, 218, 1505–1511. [Google Scholar] [CrossRef]
- Zhang, J.H.; Qu, S.D.; Li, L.T.; Wang, P.; Li, X.F.; Che, Y.F.; Li, X.L. Preparation of Carbon Molecular Sieves Used for CH4/N2 Separation. J. Chem. Eng. Data 2018, 63, 1737–1744. [Google Scholar] [CrossRef]
- Toledo-cervantes, A.; Estrada, J.; Lebrero, R.; Raul, M. A comparative analysis of biogas upgrading technologies: Photosynthetic vs. physical/chemical processes. Algal Res. 2017, 25, 237–243. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Liu, H.; Liu, P.F.; Wu, P.P.; Yang, Z.H.; Yang, Q.Y.; Liu, X.H. Research Progress of Metal-Organic Framework Materials in CO2/CH4 Adsorption and Separation. CIESC J. 2014, 65, 1563–1570. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective Gas Adsorption Separation in Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Gil, M.V.; Rubiera, F.; Pevida, C. Adsorption performance indicators for the CO2/CH4 separation: Application to biomass-based activated carbons. Fuel Process. Technol. 2016, 142, 361–369. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.C.; Pang, J.D.; Bosch, M.; Lollar, C.; Sun, Y.J.; Qin, J.S.; Yang, X.Y.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Li, X.J.; Duan, J.; Fu, Y.; Wang, Z.Y.; Zhu, M.; Li, N. Investigation of highly efficient adsorbent based on Ni-MOF-74 in the separation of CO2 from natural gas. Chem. Eng. J. 2021, 419, 129653–129662. [Google Scholar] [CrossRef]
- Zhang, J.W.; Qu, P.; Hu, M.C.; Li, S.N.; Jiang, Y.C.; Zhai, Q.G. Self-Assembly of a Rare Nanocage-based Fe-MOF toward High Methane Purification Performance. Cryst. Growth Des. 2020, 20, 5657–5663. [Google Scholar] [CrossRef]
- Zheng, B.S.; Luo, X.; Wang, Z.X.; Zhang, S.W.; Yun, R.R.; Huang, L.; Zeng, W.J.; Liu, W.L. An unprecedented water stable acylamide-functionalized metal-organic framework for highly efficient CH4/CO2 gas storage/separation and acid–base cooperative catalytic activity. Inorg. Chem. Front. 2018, 5, 2355–2363. [Google Scholar] [CrossRef]
- Ko, D. Comparison of carbon molecular sieve and zeolite 5A for CO2 sequestration from CH4/CO2 mixture gas using vacuum pressure swing adsorption. Korean J. Chem. Eng. 2021, 38, 1043–1051. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Gao, H. Purification of CH4 from CH4/CO2 mixture using carbon-based adsorbents. Trans. Chin. Soc. Agric. Mach. 2013, 44, 154–157. [Google Scholar] [CrossRef]
- Lei, L.F.; Bai, L.; Lindbråthen, A.; Pan, F.J.; Zhang, X.P.; He, X.Z. Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chem. Eng. J. 2020, 401, 126084. [Google Scholar] [CrossRef]
- Liang, J.P.; Ma, B.W. Preparation of Coconut Shell Activated Carbon for CH4/CO2 Separation in Coalbed Methane. Chieh Ching Mei Chi Shu 2021, 27, 254–261. [Google Scholar] [CrossRef]
- Kaya, M.; Atelge, M.R.; Bekirogullari, M.; Eskicioglu, C.; Atabani, A.E.; Kumar, G.L.; Yildiz, Y.S.; Unalan, S. Carbon molecular sieve production from defatted spent coffee ground using ZnCl2 and benzene for gas purification. Fuel 2020, 277, 118183–118194. [Google Scholar] [CrossRef]
- Orlando, F.C.; Ignacio, C.G.; Manuel, M.E.; Carlos, R.R.; Joaquín, S.A. Activated carbon from polyurethane residues as molecular sieves for kinetic adsorption/separation of CO2/CH4. Colloids Surf. 2022, 652, 129882–129887. [Google Scholar]
- Wang, Y.X.; Shing, B.P.; Zhao, D. Alternatives to Cryogenic Distillation: Advanced Porous Materials in Adsorptive Light Olefin/Paraffin Separations. Small 2019, 15, 1900058–1900096. [Google Scholar] [CrossRef]
- Wu, H.X. Synthesis of Several Microporous MOFs and Their Adsorption Separation for C2H4/C2H6 and C3H6/C3H8. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2021. [Google Scholar]
- Wu, C.H.; Zhang, K.X.; Wang, H.L.; Fan, Y.Q.; Zhang, S.W.; He, S.F.; Wang, F.; Tao, Y.; Zhao, X.W.; Zhang, Y.B.; et al. Enhancing the Gas Separation Selectivity of Mixed-Matrix Membranes Using a Dual-Interfacial Engineering Approach. J. Am. Chem. Soc. 2022, 14, 18503–18512. [Google Scholar] [CrossRef]
- Suo, C.; Ma, J.; Zhao, N.; Wang, R.C.; Zhu, R.Z.; Wang, L.; He, J.X. Research on carbon peaking and carbon neutrality path of chemical enterprises. China Rubber 2022, 48, 14–20. [Google Scholar] [CrossRef]
- Yu, J.J.; Zhang, X.; Wu, X.X.; Liu, T.; Zhang, Z.Q.; Wu, J.; Zhu, C. Metal-free radical difunctionalization of ethylene. Chem 2023, 9, 472–482. [Google Scholar] [CrossRef]
- Lv, D.F.; Zhou, P.J.; Xu, J.H.; Tu, S.; Xu, F.; Yan, J.; Xi, H.X.; Yuan, W.B.; Fu, Q.; Chen, X.; et al. Recent advances in adsorptive separation of ethane and ethylene by C2H6-selective MOFs and other adsorbents. Chem. Eng. J. 2022, 431, 133208. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.Q.; Wang, X. Ethylene/ethane separation by CuCl/AC adsorbent prepared using CuCl2 as a precursor. Adsorption 2016, 22, 1013–1022. [Google Scholar] [CrossRef]
- Chang, G.G.; Huang, M.H.; Su, Y.; Xing, H.B.; Su, B.Z.; Zhang, Z.G.; Yang, Q.W.; Yang, Y.W.; Ren, Q.L.; Bao, Z.B.; et al. Immobilization of Ag(i) into a metal-organic framework with -SO3H sites for highly selective olefin-paraffin separation at room temperature. Chem. Commun. 2015, 51, 2859–2862. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Z.Z.; Xu, C.L.; Wu, H.; Shi, L.; Wang, S.J.; Xu, X.Y.; Yuan, A.H.; Wang, S.B.; Sun, H.Q. Confinement of Ag(I) Sites within MIL-101 for Robust Ethylene/Ethane Separation. ACS Sustain. Chem. Eng. 2019, 8, 823–830. [Google Scholar] [CrossRef]
- Bao, Z.B.; Wang, J.W.; Zhang, Z.G.; Xing, H.B.; Yang, Q.W.; Yang, Y.W.; Wu, H.; Krishna, R.; Zhou, W.; Chen, B.L.; et al. Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-Based Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2018, 57, 16020–16025. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, X.J.; Xie, M.; Huang, Y.L.; Luo, D.; Wang, T.; Zhao, Y.F.; Lu, W.G.; Li, D. Cage-Interconnected Metal-Organic Framework with Tailored Apertures for Efficient C2H6/C2H4 Separation under Humid Conditions. AICHE J. 2019, 141, 20390–20396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wu, Y.; Liang, W.W.; Peng, J.J.; Li, H.; Wang, H.H.; Janik, M.; Xiao, J. Bimetallic ions regulate pore size and chemistry of zeolites for selective adsorption of ethylene from ethane. Chem. Eng. Sci. 2020, 220, 115636–115648. [Google Scholar] [CrossRef]
- Bereciartua, P.; Cantin, A.; Corma, A.; Jorda, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W., Jr.; Kortunov, P.; Casty, G.L. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.Y.; Liu, C.P.; Pang, J.D.; Zou, S.X.; Ji, Z.Y.; Hu, F.L.; Chen, C.; Yuan, D.Q.; Hong, M.C.; Wu, M.Y. A Metal-Organic Framework with Nonpolar Pore Surfaces forthe One-Step Acquisition of C2H4 from a C2H4 and C2H6 Mixture. Angew. Chem. Int. Ed. 2022, 61, e202210343. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.M.; Xin, M.D.; Zou, K.; Xu, G.T. Research Progress of Molecular Sieve and Metal-Organic Framework Materials for Ethylene/Ethane Separation. Acta Pet. Sin. Pet. Process. Sect. 2020, 36, 866–877. [Google Scholar] [CrossRef]
- Wang, Q.X. Design, Preparation and Gas Separation Performance Evaluation of Carbon Molecular Sieve Membranes. Master’s Thesis, China University of Petroleum, Beijing, China, 2020. [Google Scholar]
- Zandvoort, I.V.; Klink, G.V.; Jong, E.; Waal, J. Selectivity and stability of zeolites [Ca]A and [Ag]A towards ethylene adsorption and desorption from complex gas mixtures. Microporous Mesoporous Mater. 2018, 263, 142–149. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Peng, Q.L.; Fang, D.Y.; Liu, C.L.; Wu, K.G.; Chen, Y.X.; Gao, W.K.; Wang, H.; Yang, F.J.; et al. Successively separating C3H6 and C2H4 from C2H4/C2H6/C3H6/C3H8 mixture in tandem fixed beds involving two zeolites LTA well-regulated via low transition-metal doping. Chem. Eng. J. 2023, 47, 145151. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, N.Y.; Zhang, X.W.; He, H.; Huang, R.K.; Ye, Z.M.; Li, Y.; Zhou, D.D.; Liao, P.Q.; Chen, X.M.; et al. Selective Aerobic Oxidation of a Metal-Organic Framework Boosts Thermodynamic and Kinetic Propylene/Propane Selectivity. Angew. Chem. Int. Ed. 2019, 58, 7692–7696. [Google Scholar] [CrossRef]
- Yang, P.; Meng, X.H.; Guo, P.H.; Zhou, R.J.; Zhang, Y.H.; Cao, S.; Zhang, D.; Ji, H.B.; Duan, L.H. Highly selective separation of C3H6/C3H8 within hierarchical metal–organic CuxOy@HP–Cu–BTCs. Mater. Chem. Phys. 2023, 294, 127024–127037. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.Q.; Yu, C.; Zhang, P.X.; Wang, J.; Kong, L.Y.; Cui, X.L.; He, C.H.; Deng, S.G.; Xing, H.B. Separation of propylene and propane with a microporous metal-organic framework via equilibrium-kinetic synergetic effect. AICHE J. 2020, 67, 17094–17102. [Google Scholar] [CrossRef]
- Zeng, H.; Xie, M.; Wang, T.; Wei, R.J.; Xie, X.J.; Zhao, Y.F.; Lu, W.G.; Li, D. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 2021, 595, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Huang, H.L.; Peng, Y.G.; Chang, Y.J.; Zhang, Z.; Liu, D.H.; Zhong, C.L. A temperature-responsive smart molecular gate in a metal–organic framework for task-specific gas separation. J. Mater. Chem. A 2019, 7, 26574–26579. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhang, S.Z.; Wang, H.T.; Zhang, Y. Research Progress on Acrylic/Propane Adsorption and Separation Materials. Fine Chem. 2020, 37, 1327–1333. [Google Scholar] [CrossRef]
- Moradi, H.; Azizpour, H.; Bahmanyar, H.; Emamian, H. Emamian Molecular dynamic simulation of carbon dioxide, methane, and nitrogen adsorption on Faujasite zeolite. Chin. J. Chem. Eng. 2022, 43, 70–76. [Google Scholar] [CrossRef]
- Olson, D.; Camblor, M.; Villaescusa, L.; Kuehl, G.H. Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous Mesoporous Mater. 2003, 67, 27–33. [Google Scholar] [CrossRef]
- Fu, D.L.; Park, Y.; Davis, M.E. Zinc containing small-pore zeolites for capture of low concentration carbon dioxide. Angew. Chem. Int. Ed. 2021, 134, 2–9. [Google Scholar] [CrossRef]
- Xiong, Y.; Woodward, R.T.; Danaci, D.; Evans, A.; Tian, T.; Azzan, H.; Ardakani, M.; Petit, C. Understanding trade-offs in adsorption capacity, selectivity and kinetics for propylene/propane separation using composites of activated carbon and hypercrosslinked polymer. Chem. Eng. J. 2021, 426, 131628–131636. [Google Scholar] [CrossRef]
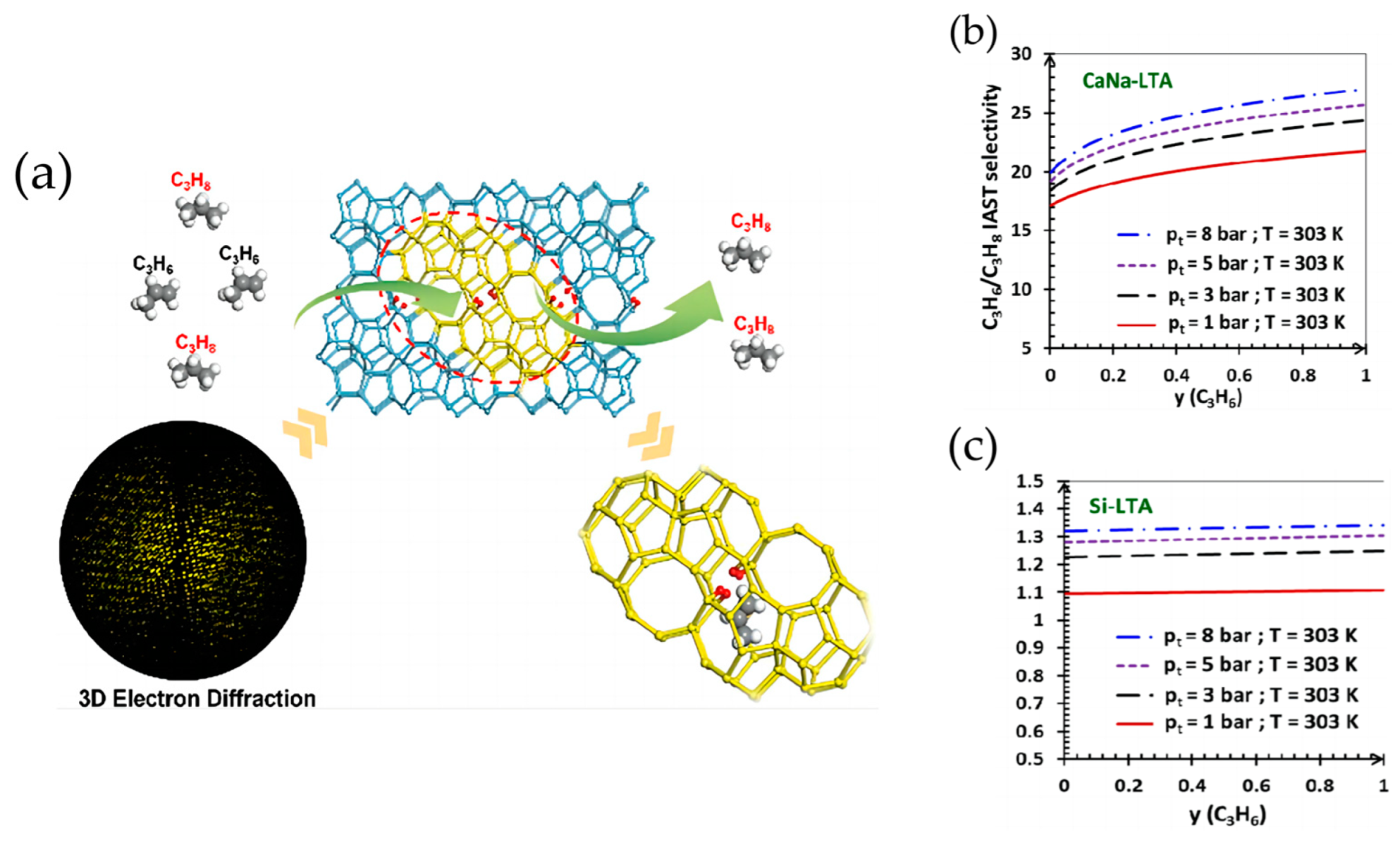
- Wang, J.; Ma, C.; Liu, J.Q.; Liu, Y.; Xu, X.Q.; Xie, M.; Wang, H.; Wang, L.; Guo, P.; Liu, Z.M. Pure Silica with Ordered Silanols for Propylene/Propane Adsorptive Separation Unraveled by Three-Dimensional Electron Diffraction. AICHE J. 2023, 145, 6853–6860. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Miao, G.; Xu, G.D.; Luo, J.Z.; Yang, C.T.; Xiao, J. Mixed (Ag+, Ca2+)-LTA zeolite with suitable pore feature for effective separation of C3H6/C3H8. Chem. Eng. J. 2022, 45, 137913–137923. [Google Scholar] [CrossRef]
- Benchaabane, M.A.; Goncalves, G.T.; Bloch, E.; Palllaudean, J.L.; Daou, T.J.; Bourrelly, S.; Chaplais, G.; Llewellyn, P.L. An initial evaluation of the thermodynamic or kinetic separation performance of cation-exchanged LTA zeolites for mixtures of propane and propylene. Microporous Mesoporous Mater. 2022, 344, 112211–112227. [Google Scholar] [CrossRef]
- Song, Z.N.; Huang, Y.; Wang, L.; Li, S.G.; Yu, M. Composite 5A zeolite with ultrathin porous TiO2 coating for selective gas adsorption. Chem. Commun. 2015, 51, 373–375. [Google Scholar] [CrossRef]
- Zhou, X.P.; Falconer, J.L.; Medlin, J.W. Mechanism of selectivity control for zeolites modified with organic monolayers. Microporous Mesoporous Mater. 2022, 337, 111913–111920. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Peh, S.B.; Kang, C.J.; Chai, K.G.; Zhao, D. Metal-organic frameworks for C6–C8 hydrocarbon separations. J. Energy Chem. 2021, 3, 100057–100099. [Google Scholar] [CrossRef]
- Han, X.; Chen, Y.T.; Su, B.G.; Bao, Z.B.; Zhang, Z.G.; Yang, Y.W.; Ren, Q.L.; Yang, Q.W. Research Progress on Adsorption and Separation Materials for Hexane Isomers. CIESC J. 2021, 72, 3445–3465. [Google Scholar] [CrossRef]
- Xia, H.L.; Zhou, K.; Yu, L.; Wang, H.; Liu, X.Y.; Proserpio, D.M.; Li, J. Customized Synthesis: Solvent- and Acid-Assisted Topology Evolution in Zirconium-Tetracarboxylate Frameworks. Inorg. Chem. 2022, 61, 7980–7988. [Google Scholar] [CrossRef]
- Yu, Q.C.; Guo, L.D.; Lai, D.; Zhang, Z.G.; Yang, Q.W.; Yang, Y.W.; Ren, Q.L.; Bao, Z.B. A pore-engineered metal-organic framework with mixed ligands enabling highly efficient separation of hexane isomers for gasoline upgrading. Sep. Purif. Technol. 2021, 268, 118646–118657. [Google Scholar] [CrossRef]
- Guo, F.A.; Wang, J.; Chen, C.L.; Dong, X.L.; Li, X.Y.; Wang, H.; Guo, P.; Han, Y.; Li, J. Linker Vacancy Engineering of a Robust FTW-type Zr-MOF for Hexane Isomers Separation. Angew. Chem. Int. Ed. 2023, 62, 202219053. [Google Scholar] [CrossRef]
- Lal, B.; Idress, K.B.; Xie, H.M.; Smoljan, C.S.; Shafaie, S.; Islamoglu, T.; Farha, O.K. Pore Aperture Control toward Size-Exclusion-Based Hydrocarbon Separations. Angew. Chem. Int. Ed. 2023, 62, 2022190533–2022190539. [Google Scholar] [CrossRef]
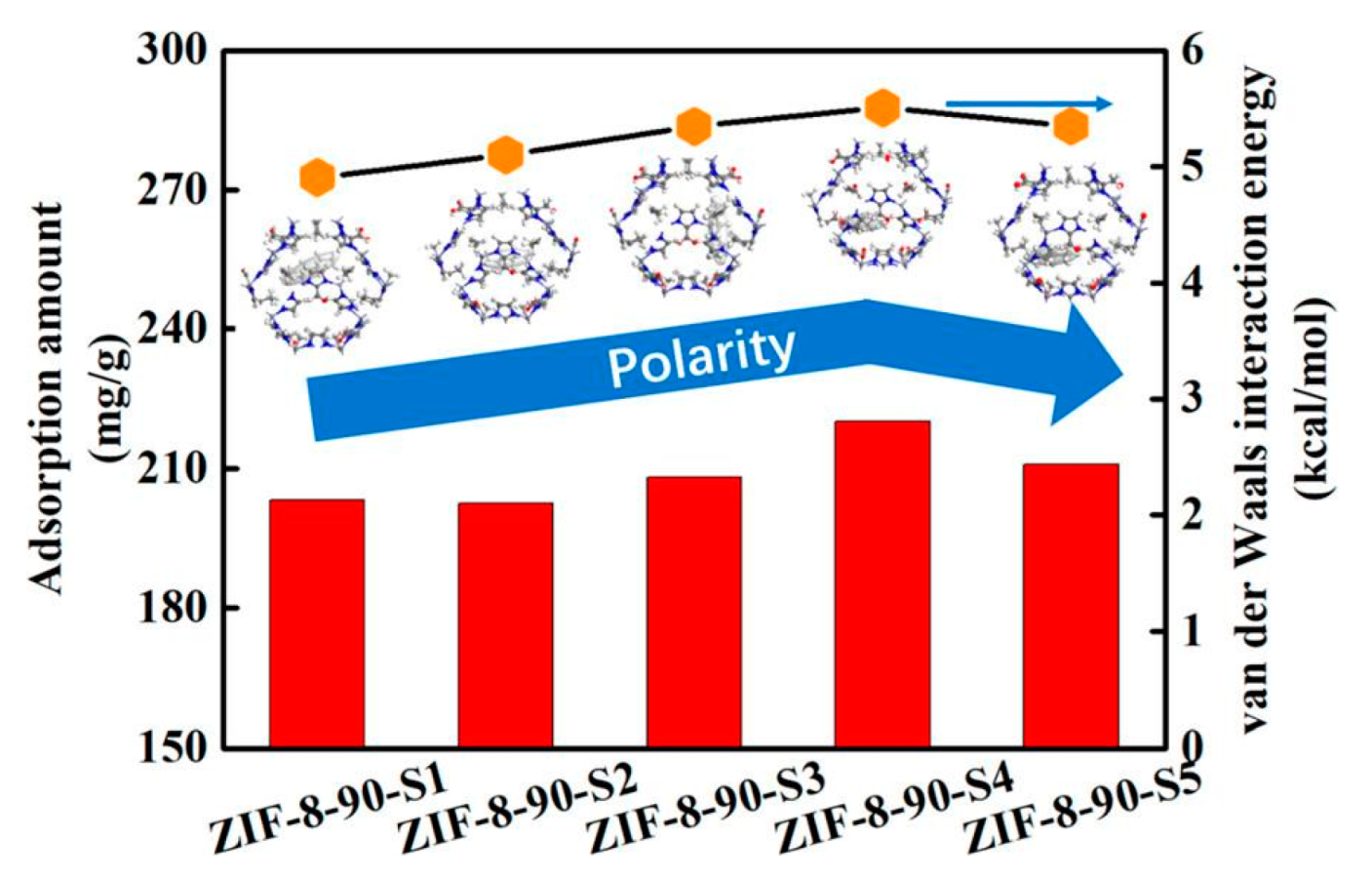
- Sun, H.; Jiang, H.; Kong, R.Q.; Ren, D.N.; Wan, D.; Tan, J.L.; Wu, D. Tuning n-Alkane Adsorption on Mixed-linker ZIF-8-90 via Controllable Ligand Hybridization: Insight into the Confinement from an Energetics Perspective. Ind. Eng. Chem. Res. 2019, 29, 13274–13283. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon, M.A.; Barthelett, K.; Pirngrubert, G.D. Separation of Cgparaffins using zeolitic imidazolate frameworks: Comparison with zeolite 5A. Ind. Eng. Chem. Res. 2012, 51, 4692–4702. [Google Scholar] [CrossRef]
- Liu, J.C.; Yang, X.M.; Wang, C.; Ye, L.; Sun, H. Synthesis of hierarchical 5A zeolites to improve the separation efficiency of n-paraffins. Adsorp. Sci. Technol. 2019, 37, 530–544. [Google Scholar] [CrossRef]
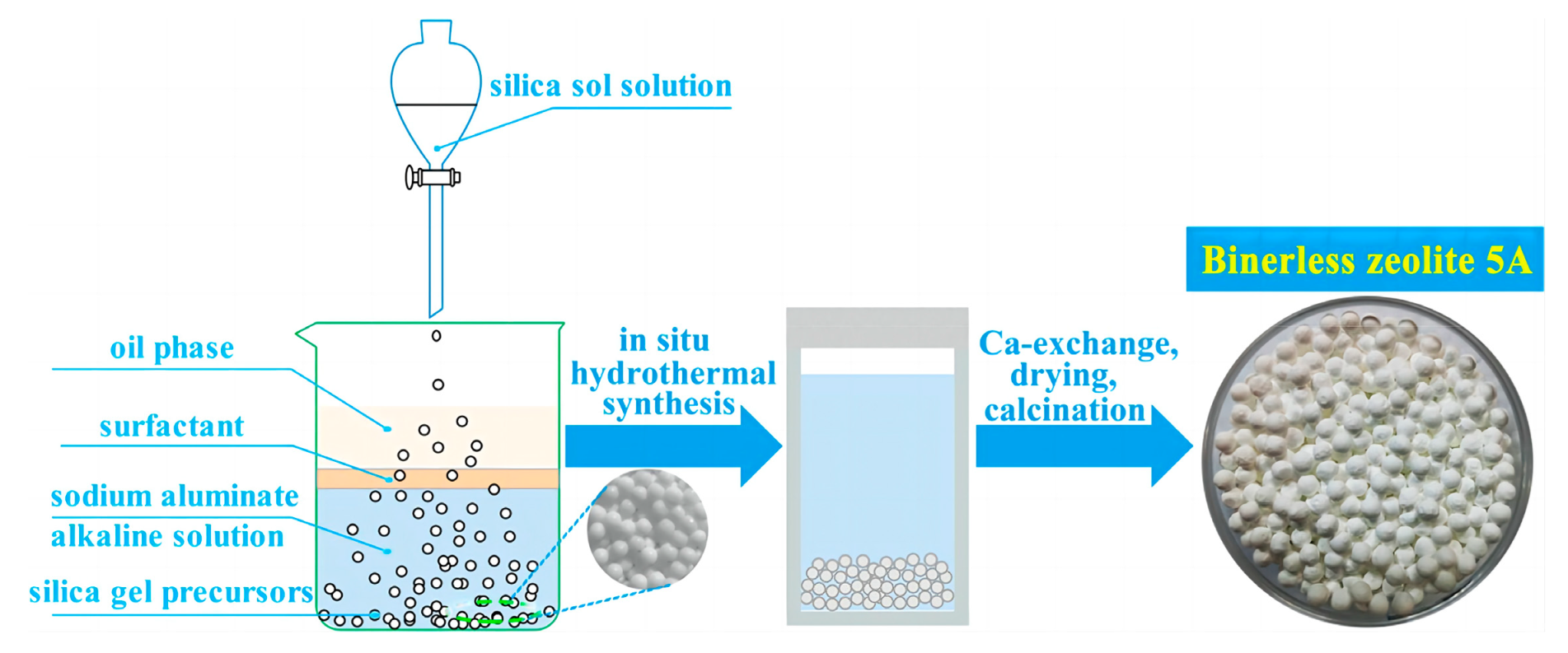
- Sun, H.; Sun, Z.W.; Shen, B.X.; Liu, J.C.; Li, G.N.; Wu, D.; Zhang, Y.X. One-pot synthesis of binderless zeolite A spheres via in situ hydrothermal conversion of silica gel precursor. AICHE J. 2018, 64, 4027–4038. [Google Scholar] [CrossRef]
- Sun, H.; Shen, B.X. Experimental study on coking, deactivation, and regeneration of binderless 5A zeolite during 1-hexene adsorption. Adsorption 2013, 19, 111–120. [Google Scholar] [CrossRef]




| Adsorbate | Boiling Point (K) | Dynamic Diameter (Å) | Dipole Moment (×1018/ ESU cm) | Polarizability (×10−25/cm3) |
|---|---|---|---|---|
| CH4 | 111.6 | 3.8 | 0 | 25.9 |
| CO2 | 194.6 | 3.3 | 0 | 2.76 |
| N2 | 77.15 | 3.6 | 0 | 1.53 |
| C2H6 | 184.6 | 4.4 | 0 | 44.3 |
| C2H4 | 169.4 | 4.2 | 0 | 42.5 |
| C3H8 | 231.0 | 4.3–5.1 | 0.084 | 62.9 |
| C3H6 | 225.5 | 4.7 | 0.366 | 62.9 |
| Name | Al-BPDC | Al-NDC | Al-BDC | Al-FUM-Me | Al-FUM |
|---|---|---|---|---|---|
| Ligand Structure | 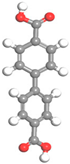 | 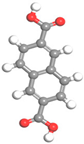 | 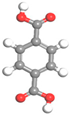 | 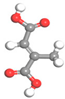 | 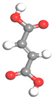 |
| Ligand Polarity (kcal/mol) | 10.683 | 10.729 | 11.467 | 12.585 | 14.291 |
| Aperture (Å) | 11.5 | 9.2 | 8.2 | 5.0 | 6.3 |
| CH4 Uptake (cm3/g) | 5.9 | 10.86 | 15.98 | 27.19 | 20.44 |
| CH4/N2 Selectivity | 2.2 | 3.1 | 3.4 | 8.6 | 5.1 |
| Adsorbents | T (K) | C2H4 Uptake (mmol/g) | C2H6 Uptake (mmol/g) | Selectivity | Qst (C2H4/C2H6, kJ mol−1) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| C2H4/C2H6 | C2H6/C2H4 | |||||||
| MOFs | UTSA-280 | 298 | 2.5 | 0.098 | >10,000 | - | 34.1/- | [47] |
| Fe-MOF-74 | 318 | 6.24 | 5.19 | 13.6 | - | 45/25 | [47] | |
| ZIF-7 | 298 | 1.90 | 2.00 | - | 2.5 | -/27 | [47] | |
| MUF-15 | 298 | 4.15 | 4.69 | - | 1.96 | -/29.2 | [47] | |
| Co-gallate | 298 | 3.37 | 0.31 | 52 | - | 44/- | [66] | |
| JNU-2 | 298 | 3.62 | 4.11 | - | 1.6 | -/30 | [67] | |
| Zeolites | DDR | 303 | 0.94 | 0.97 | - | 1.49 | -/25 | [62] |
| Silicalite-1 | 305 | 1.84 | 2.00 | - | 2.90 | -/28.9 | [62] | |
| Ag-Ca-4A | 298 | 3.7 | - | 17,568 | - | - | [68] | |
| ITQ-55 | 303 | 1.30 a | 0.80 b | 6.36 | - | - | [69] | |
| COFs | COF-102 | 298 | 1.73 | 1.90 | 1.48 | -/28.9 | [62] | |
| COF-320 | 298 | 1.79 | 2.35 | - | 1.52 | -/28.0 | [62] | |
| CTF-DCTC-400 | 298 | 1.68 | 1.82 | - | 1.04 | -/22.7 | [62] | |
| Carbon-Based Adsorbents | MC-S-Ag-3 | 298 | 3.4 | 2.6 | 2.4 | - | - | [17] |
| C-PDA-3 | 298 | 5.1 | 6.6 | 1.83 | - | 22/22 | [17] | |
| CuCl(8.0)/AC | 303 | 2.6 | 0.7 | 69.4 | - | - | [17] | |
| MGA-750–3 | 298 | 5.68 | 7.02 | - | 2.00 | 28.4/- | [62] | |
| FAU-ZTC | 303 | 3.86 | 4.71 | - | 1.48 | 25.0/- | [62] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Li, P.; Wang, Y.; Zhao, Q.; Sun, H. Progress in the Separation and Purification of Carbon Hydrocarbon Compounds Using MOFs and Molecular Sieves. Separations 2023, 10, 543. https://doi.org/10.3390/separations10100543
Zhou Y, Li P, Wang Y, Zhao Q, Sun H. Progress in the Separation and Purification of Carbon Hydrocarbon Compounds Using MOFs and Molecular Sieves. Separations. 2023; 10(10):543. https://doi.org/10.3390/separations10100543
Chicago/Turabian StyleZhou, Yousheng, Peicheng Li, Yifan Wang, Qiyue Zhao, and Hui Sun. 2023. "Progress in the Separation and Purification of Carbon Hydrocarbon Compounds Using MOFs and Molecular Sieves" Separations 10, no. 10: 543. https://doi.org/10.3390/separations10100543
APA StyleZhou, Y., Li, P., Wang, Y., Zhao, Q., & Sun, H. (2023). Progress in the Separation and Purification of Carbon Hydrocarbon Compounds Using MOFs and Molecular Sieves. Separations, 10(10), 543. https://doi.org/10.3390/separations10100543







