Anaerobic Digestion of Phosphorus-Rich Sludge and Digested Sludge: Influence of Mixing Ratio and Acetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Sludge
2.2. Analyses
2.3. Experimental Set-Up
2.3.1. Anaerobic Digestion of PRS and DS with Different Mixing Ratios under the Same Acetic Acid Concentration
2.3.2. Anaerobic Digestion of PRS and DS with the Same Mixing Ratio under Different Acetic Acid Concentrations
2.3.3. Anaerobic Digestion of WAS and DS with Different Mixing Ratios under the Same Acetic Acid Concentration
3. Results and Discussion
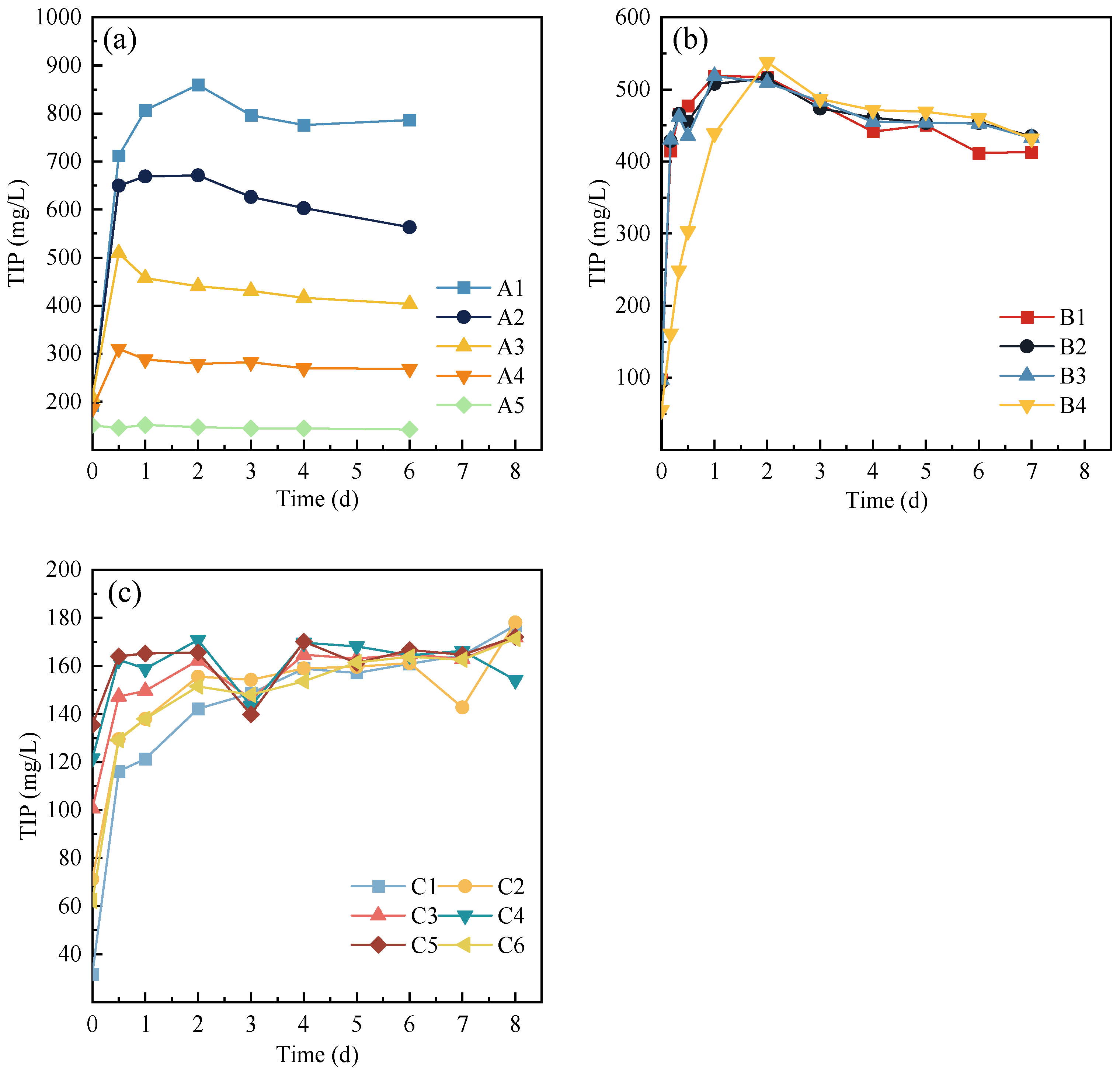
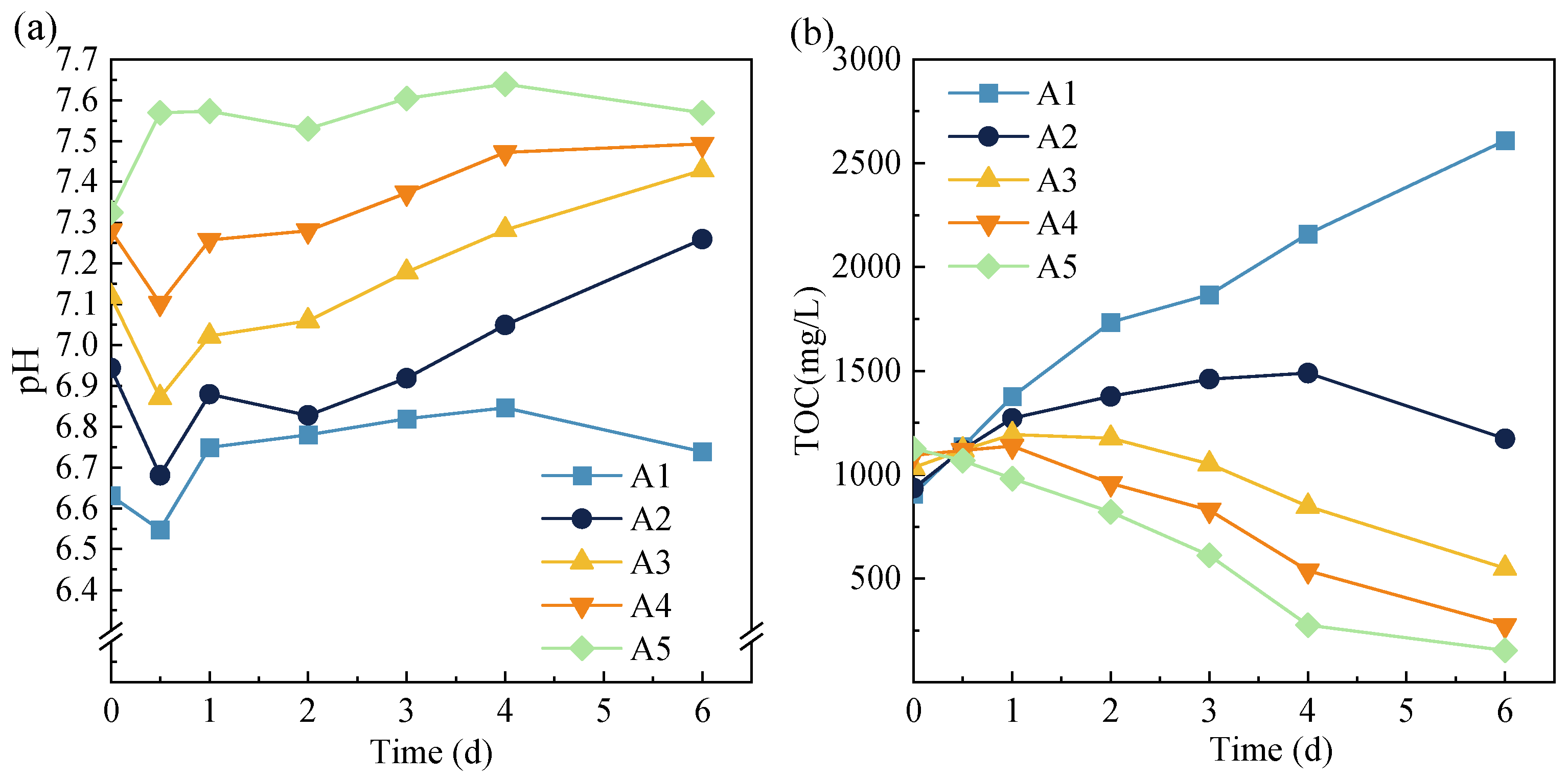
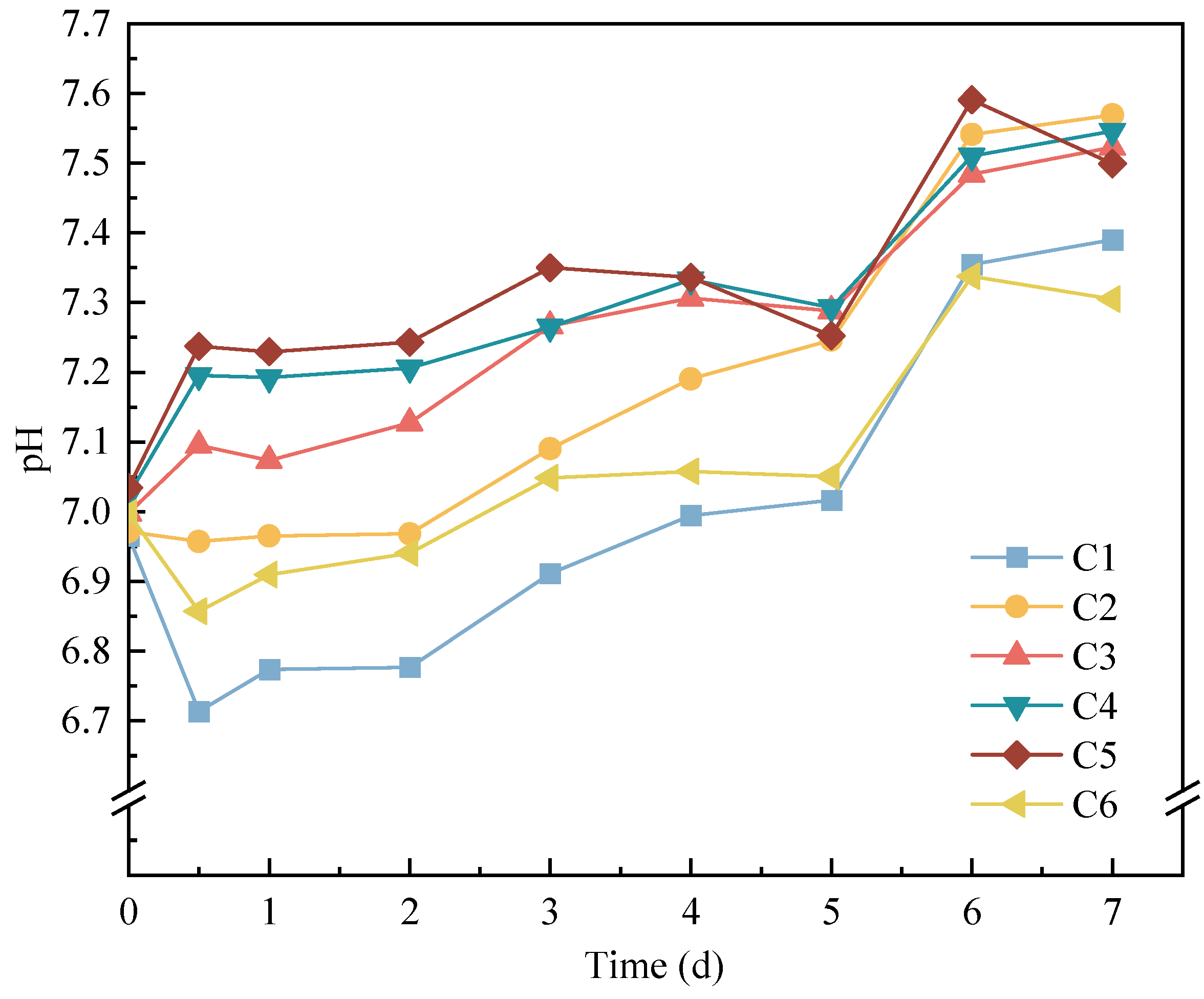
3.1. Phosphorus Release during Anaerobic Digestion
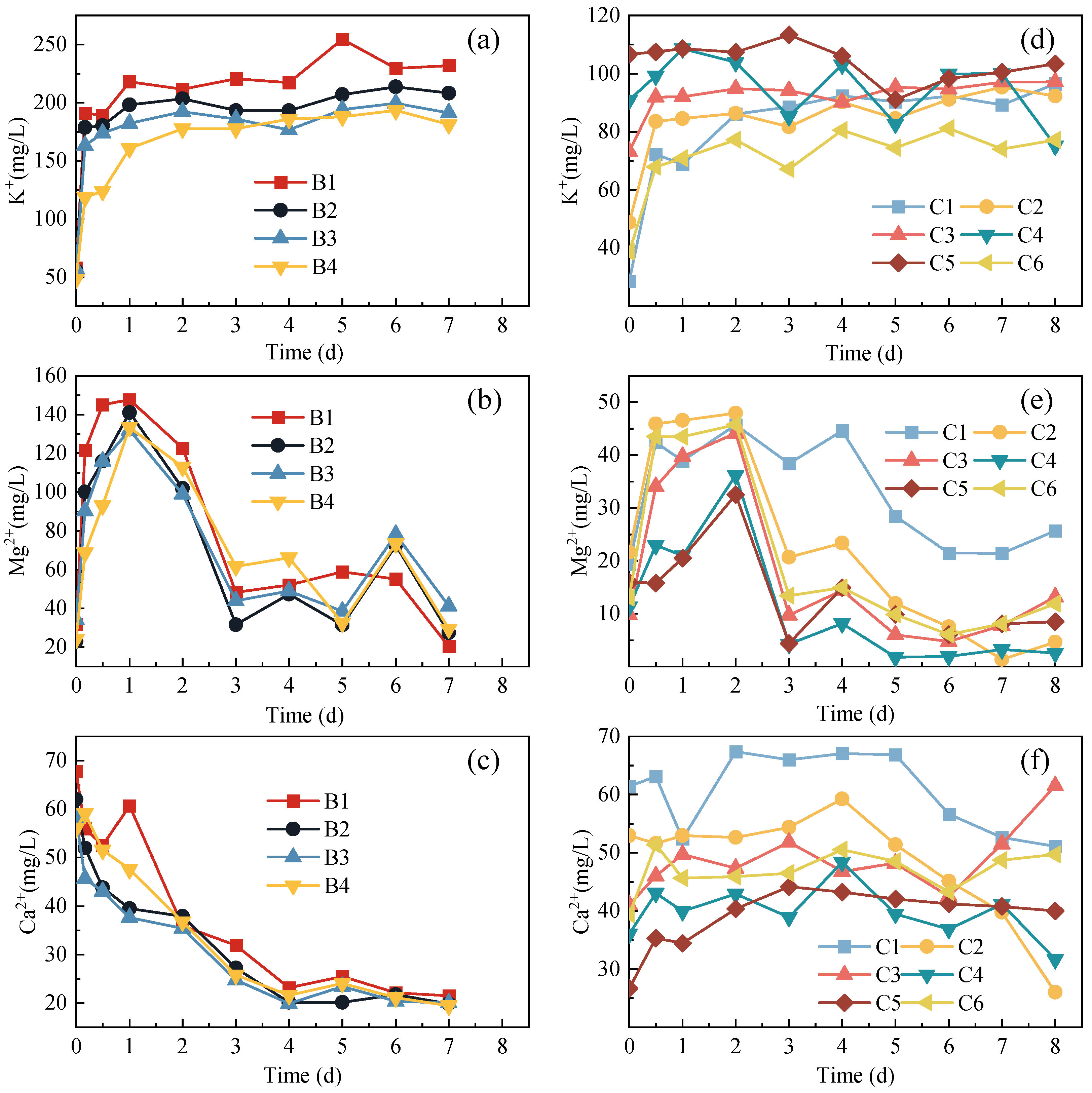
3.2. Variations of Metal Ions
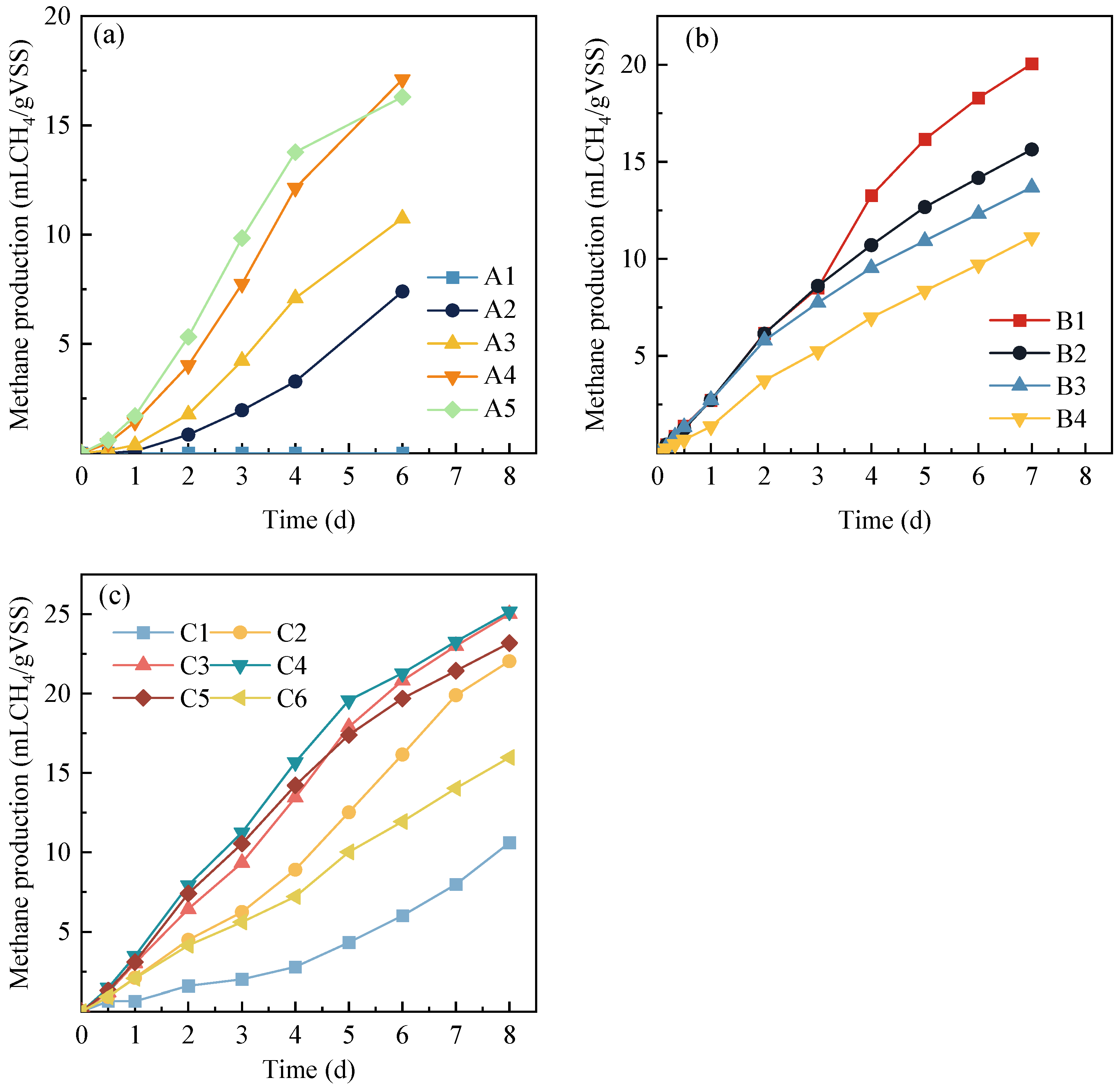
3.3. Methane Production Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, R.H.; Cui, J.L.; Hu, J.H.; Wang, W.J.; Li, B.; Li, X.D.; Li, X.Y. Transformation of Fe–P Complexes in Bioreactors and P Recovery from Sludge: Investigation by XANES Spectroscopy. Environ. Sci. Technol. 2020, 54, 4641–4650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Barjenbruch, M.; Kabbe, C.; Inial, G.; Remy, C. Phosphorus recovery from municipal and fertilizer wastewater: China’s potential and perspective. J. Environ. Sci. 2017, 52, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chen, H.; Zeng, X.; Li, Z.; Yu, W.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Tao, S.; et al. A comparison between sulfuric acid and oxalic acid leaching with subsequent purification and precipitation for phosphorus recovery from sewage sludge incineration ash. Water Res. 2019, 159, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Q.; Li, J.S.; Poon, C.S.; Cheeseman, C.R.; Donatello, S.; Tsang DC, W. Feasibility of wet-extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 939–971. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Yu, H.Y.; Dai, X.H. Research Progress on Phosphorus in Sludge and Its Recovery Technology. China Water Wastewater 2022, 38, 29–34. [Google Scholar]
- Jardin, N.; Popel, H.J. Phosphate Release of Sludges from Enhanced Biological P-Removal during Digestion. Water Sci. Technol. 1994, 30, 281–292. [Google Scholar] [CrossRef]
- Mavinic, D.S.; Koch, F.A.; Hall, E.R.; Abraham, K.; Niedbala, D. Anaerobic co-digestion of combined sludges from a BNR wastewater treatment plant. Environ. Technol. 1998, 19, 35–44. [Google Scholar] [CrossRef]
- Carliell-Marquet, C.M.; Wheatley, A.D. Measuring metal and phosphorus speciation in P-rich anaerobic digesters. Water Sci. Technol. 2002, 45, 305–312. [Google Scholar] [CrossRef]
- Kuroda, A.; Takiguchi, N.; Gotanda, T.; Nomura, K.; Kato, J.; Ikeda, T.; Ohtake, H. A simple method to release polyphosphate from activated sludge for phosphorus reuse and recycling. Biotechnol. Bioeng. 2002, 78, 333–338. [Google Scholar] [CrossRef]
- Battistoni, P.; Fava, G.; Pavan, P.; Musacco, A.; Cecchi, F. Phosphate removal in anaerobic liquors by struvite crystallization without addition of chemicals: Preliminary results. Water Res. 1997, 31, 2925–2929. [Google Scholar] [CrossRef]
- Petzet, S.; Cornel, P. Prevention of Struvite Scaling in Digesters Combined with Phosphorus Removal and Recovery-The FIX-Phos Process. Water Environ. Res. 2012, 84, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.G.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Rudolfs, W.; Stahl, G.W. Phosphates in sewage and sludge treatment. III. Effects on sludge digestion. Sewage Work. J. 1947, 19, 415–422. [Google Scholar]
- Takiguchi, N.; Kishino, M.; Kuroda, A.; Kato, J.; Ohtake, H. A laboratory-scale test of anaerobic digestion and methane production after phosphorus recovery from waste activated sludge. J. Biosci. Bioeng. 2004, 97, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Kleybocker, A.; Liebrich, M.; Kasina, M.; Kraume, M.; Wittmaier, M.; Wurdemann, H. Comparison of different procedures to stabilize biogas formation after process failure in a thermophilic waste digestion system: Influence of aggregate formation on process stability. Waste Manag. 2012, 32, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, A.J.; Ma, L.S.; Jing, Z.T.; Yang, J.; Tang, Y.; Hu, B. A comparison on phosphorus release and struvite recovery from waste activated sludge by different treatment methods. Int. Biodeterior. Biodegrad. 2020, 148, 104878. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, Z.P.; Dai, X.H.; Li, Y.M. Novel CaO2 beads used in the anaerobic fermentation of iron-rich sludge for simultaneous short-chain fatty acids and phosphorus recovery under ambient conditions. Bioresour. Technol. 2021, 322, 124553. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Zhang, J.Y.; Tang, R.J.; Liao, S.T.; Zhang, Z.P.; Li, Y.M. Effect of surfactants on phosphorus release and acidogenic fermentation of waste activated sludge containing different aluminium phosphate forms. Chemosphere 2022, 287, 132213. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Wu, B.R.; Liu, Z.G.; Dai, X.H. Simultaneous enhancing phosphorus recovery and volatile fatty acids production during anaerobic fermentation of sewage sludge with peroxydisulfate pre-oxidation. Bioresour. Technol. 2022, 357, 127164. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Ping, Q.; Guo, W.J.; Cai, C.; Li, Y.M. A novel approach using protein-rich biomass as co-fermentation substrates to enhance phosphorus recovery from FePs-bearing sludge. Water Res. 2022, 218, 118479. [Google Scholar] [CrossRef]
- Hu, X.B.; Zhao, X.; Ye, X.; Zhong, M.Y. Enhancement of mixing with waste activated sludge to anaerobic digestion of flocculated sludge. Chin. J. Environ. Eng. 2016, 10, 1456–1462. [Google Scholar]
- Sun, C.; Guo, L.; Zheng, Y.K.; Yu, D.; Jin, C.J.; Zhao, Y.G.; Yao, Z.W.; Gao, M.C.; She, Z.L. Effect of mixed primary and secondary sludge for two-stage anaerobic digestion (AD). Bioresour. Technol. 2022, 343, 126160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. The Study on Phosphorus Discharge Capability of High Phosphatic Sludge and Phosphorus Recycle Potency. Master’s Thesis, Chongqing University, Chongqing, China, November 2002. [Google Scholar]
- Xiao, F. Phosphate release by polyphosphate accumulating organisms under different acetate concentration. Environ. Sci. Manag. 2009, 34, 90–94. [Google Scholar]
- APHA. Standard Method for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Luo, G.Y.; Zhang, Y.; Xu, X.Y. Characteristics of Activated Sludge and Stability of Denitrifying Phosphorus Removal in SUFR System. Res. Environ. Sci. 2011, 24, 85–89. [Google Scholar]
- Jardin, N.; Popel, H.J. Behavior of waste activated sludge from enhanced biological phosphorus removal during sludge treatment. Water Environ. Res. 1996, 68, 965–973. [Google Scholar] [CrossRef]
- Chen, W.L.; Wang, R.Y.; Li, Y.M. Effect of acetate concentration on phosphate release during anaerobic fermentation of phosphorus-rich sludge. China Environ. Sci. 2015, 35, 1763–1770. [Google Scholar]
- Wild, D.; Kisliakova, A.; Siegrist, H. Prediction of recycle phosphorus loads from anaerobic digestion. Water Res. 1997, 31, 2300–2308. [Google Scholar] [CrossRef]
- Bi, D.S.; Guo, X.P. on the effects of nitrogen and phosphorus release on the anaerobic digestion of PRWAS with pH-adjusted by Mg(OH)2. J. Saf. Environ. 2012, 67, 94–97. [Google Scholar]
- Wild, D.; Kisliakova, A.; Siegrist, H. P-fixation by Mg, Ca and zeolite A during stabilization of excess sludge from enhanced biological P-removal. Water Sci. Technol. 1996, 34, 391–398. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, Y.M. Effect of pH on phosphorus release from chemical-biological mixed sludge during anaerobic fermentation. China Environ. Sci. 2014, 34, 650–657. [Google Scholar]
- Marti, N.; Ferrer, J.; Seco, A.; Bouzas, A. Optimisation of sludge line management to enhance phosphorus recovery in WWTP. Water Res. 2008, 42, 4609–4618. [Google Scholar] [CrossRef]
- Fujimoto, N.; Mizuochi, T.; Togami, Y. Phosphorus Fixation in the Sludge Treatment System of a Biological Phosphorus Removal Process. Water Sci. Technol. 1990, 23, 635–640. [Google Scholar] [CrossRef]
- Barat, R.; Bouzas, A.; Marti, N.; Ferrer, J.; Seco, A. Precipitation assessment in wastewater treatment plants operated for biological nutrient removal: A case study in Murcia, Spain. J. Environ. Manag. 2009, 90, 850–857. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.G.; Jiang, S.; Yuan, H.Y.; Zhou, Q.; Gu, G.W. Hydrolysis and acidification of waste activated sludge at different pHs. Water Res. 2007, 41, 683–689. [Google Scholar] [CrossRef]

| Sludge | pH | MLSS (g/L) | MLVSS (g/L) | TP (mg/L) | TN (mg/L) | TOC (mg/L) |
|---|---|---|---|---|---|---|
| PRS | 7.40 | 12.10 | 8.71 | 944.58 | 8.39 | 22.23 |
| DS | 7.33 | 17.38 | 10.75 | - | 391.40 | 192.06 |
| Experimental Group | Added Acetic Acid (mg COD/L) | The Mixing Ratios of Sludge | MLSS (g/L) | MLVSS (g/L) | |
|---|---|---|---|---|---|
| PRS (%) | DS (%) | ||||
| A1 | 3000 | 100 | 0 | 16.91 | 12.79 |
| A2 | 3000 | 75 | 25 | 17.48 | 12.86 |
| A3 | 3000 | 50 | 50 | 16.61 | 11.37 |
| A4 | 3000 | 25 | 75 | 15.18 | 9.37 |
| A5 | 3000 | 0 | 100 | 14.48 | 7.95 |
| Experimental Group | Added Acetic Acid (mg COD/L) | The Mixing Ratios of Sludge | MLSS (g/L) | MLVSS (g/L) | |
|---|---|---|---|---|---|
| PRS (%) | DS (%) | ||||
| B1 | 2000 | 75 | 25 | 13.42 | 9.26 |
| B2 | 1000 | 75 | 25 | 13.42 | 9.26 |
| B3 | 500 | 75 | 25 | 13.42 | 9.26 |
| B4 | 0 | 75 | 25 | 13.42 | 9.26 |
| Experimental Group | Added Acetic Acid (mg COD/L) | The Mixing Ratios of Sludge | MLSS (g/L) | MLVSS (g/L) | |
|---|---|---|---|---|---|
| WAS (%) | DS (%) | ||||
| C1 | 3000 | 100 | 0 | 23.27 | 17.30 |
| C2 | 3000 | 75 | 25 | 22.97 | 16.12 |
| C3 | 3000 | 50 | 50 | 22.66 | 14.88 |
| C4 | 3000 | 25 | 75 | 22.34 | 13.65 |
| C5 | 3000 | 0 | 100 | 22.05 | 12.47 |
| C6 | 0 | 75 | 25 | 23.55 | 16.50 |
| Experimental Group | SPRR (mg P/(g SS•h)) |
|---|---|
| A1 | 2.56 |
| A2 | 3.06 |
| A3 | 3.12 |
| A4 | 2.73 |
| B1 | 2.66 |
| B2 | 2.70 |
| B3 | 2.75 |
| B4 | 2.28 |
| C1 | 0.30 |
| C2 | 0.69 |
| C3 | 0.83 |
| C4 | 1.50 |
| C6 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, Z.; Wang, W.; Ping, Q.; Wang, L.; Pu, X.; Wang, B.; Li, Y. Anaerobic Digestion of Phosphorus-Rich Sludge and Digested Sludge: Influence of Mixing Ratio and Acetic Acid. Separations 2023, 10, 539. https://doi.org/10.3390/separations10100539
Xi Z, Wang W, Ping Q, Wang L, Pu X, Wang B, Li Y. Anaerobic Digestion of Phosphorus-Rich Sludge and Digested Sludge: Influence of Mixing Ratio and Acetic Acid. Separations. 2023; 10(10):539. https://doi.org/10.3390/separations10100539
Chicago/Turabian StyleXi, Zhicheng, Wenhan Wang, Qian Ping, Lin Wang, Xiangkai Pu, Bin Wang, and Yongmei Li. 2023. "Anaerobic Digestion of Phosphorus-Rich Sludge and Digested Sludge: Influence of Mixing Ratio and Acetic Acid" Separations 10, no. 10: 539. https://doi.org/10.3390/separations10100539
APA StyleXi, Z., Wang, W., Ping, Q., Wang, L., Pu, X., Wang, B., & Li, Y. (2023). Anaerobic Digestion of Phosphorus-Rich Sludge and Digested Sludge: Influence of Mixing Ratio and Acetic Acid. Separations, 10(10), 539. https://doi.org/10.3390/separations10100539







