Abstract
Syzygium aromaticum L. is an aromatic plant with a significant amount of essential oil (EO), which is used in food, medicine, for flavoring, and in the fragrance industry. The purpose of this study was to comparatively evaluate the chemical composition, yield, and antioxidant and antifungal activities of Syzygium aromaticum essential oils extracted by the conventional hydro-distillation, steam distillation, and the emerging superheated steam distillation methods. It was noticed that the extraction methods significantly influenced the yield, chemical composition, and antioxidant and antimicrobial activities of essential oils. The maximum yield was obtained using superheated steam distillation, followed by hydro-distillation and steam distillation. The antioxidant potential of EO extracts was evaluated following the scavenging of 2,2-dipenyl-1-picrylhydrazyl radicals, hydrogen peroxide scavenging activity and ferric reducing power assays. Results revealed that EO extracted superheated steam distillation exhibited the highest antioxidant activity. GC-MS analysis depicted eugenol (47.94–26.50%) and caryophyllene (20.24–9.25%) as the major compounds of Syzygium aromaticum EOs. The antimicrobial activity of EO extracts was evaluated, via the resazurin microtiter plate assay, microdilution broth assay, and disc diffusion methods, against normal and food pathogenic bacterial and fungal strains. After comparative evaluation, it was observed that superheated steam extracted EO exhibited the highest antimicrobial potential. Overall, methodical evaluation disclosed that superheated steam distillation is an effective method to extract EOs from plant sources, with greater yield and promising biological activities.
1. Introduction
Syzygium aromaticum L. (S. aromaticum), commonly named clove, is a normal-sized tree about 8-10 m long that originates from the Myrtaceae family. Flower buds are an important part of the plant, and develop after the plant matures for 4 years. S. aromaticum is a spice that has been widely used as a flavoring agent, food preservative, and as a medical product in medicinal industries [1]. The main bioactive component of S. aromaticum essential oil (EO) is eugenol, which has applications in medicine, as well as the agriculture and food industries. It has antiseptic, anti-analgesic and anti-esthetic effects, and is largely used in dental pain-relieving medicines. Additionally, S. aromaticum EO also possesses antiviral, insecticidal, antioxidant, and antimicrobial activity because of its major compound, eugenol [2]. S. aromaticum EO contains eugenol, eugenyl acetate, and β-caryophyllene as major compounds, so it is preferred due to its safety and non-toxic effects at low concentrations [3].
Foodborne diseases have become a serious concern in spreading health problems, caused by the ingestion of pathogenic bacteria, viruses, or other protozoa [4]. There are several factors which tend to spread foodborne diseases in humans. Due to this issue, contamination of food occurs from pathogens, and is further reproduced to produce toxins. Improper handling and preservation of food and contaminated equipment may also enhance the chances of diseases [5]. To reduce the rate of microbial growth and food spoilage, food preservatives are added to the foodstuff at a safer concentration. There are two types of food preservatives, natural and synthetic. Natural preservatives are preferred over synthetic/chemical preservatives due to their low toxicity and high efficiency [6]. EOs can be used as a natural food preservative, due to their excellent antibacterial, antifungal, and antiviral activities [4,7,8].
Natural antioxidants are the compounds synthesized by plants, animals, fungi, and other microorganisms [9]. They can inhibit the propagation of free radical chain reactions, or the formation of free radicals [10]. Essential oils are a class of natural antioxidants made of a complex mixture of hundreds of compounds, with almost every class of functional group [11]. It has been reported that some EO compounds, such as rosmanol, rosmaridiphenol, and carnosol, have higher antioxidant activity than synthetic phenolic antioxidant BHA [12]. Recently, public interest in the use of natural antioxidants from plant sources has increased, due to their nontoxicity at low concentrations [11,13].
Hydro-distillation, steam distillation, supercritical fluid CO2 extraction, superheated steam distillation, and subcritical water extraction are the techniques mostly used for the extraction of EOs from plant biomass [14,15,16]. Subcritical water extraction serves as a green extraction technique, in which water is used as an extracting solvent at high pressure and temperature. It has been reported that the polarity and dielectric constant of subcritical water changes with changes in the temperature and pressure; it behaves like an organic solvent, and helps in the extraction of a variety of compounds [17]. Subcritical water extraction is preferred over hydro-distillation due to its high speed, efficiency, and environmentally friendly nature [18]. Supercritical fluid extraction (SCFE) is also an advanced technique for the extraction of bioactive compounds from plant biomass [19]. CO2 is most commonly used as an extracting fluid in SCFE, due to its benefits over other solvents such as its inertness, cheapness, easy availability, and high selectivity, suitable for heat-sensitive compounds, lower critical temperature, pressure, and its non-toxic and environmentally friendly nature [20]. SCFE is preferred, due to its low contamination, high selectivity, speed, and better recovery of compounds [21]. Cannabis sativa and Cannabis indica EOs were extracted with hydro-distillation, steam distillation, and supercritical fluid extraction methods [22]; they reported that supercritical fluid extraction technique showed the highest EO yields (0.039 to 0.031%), followed by hydro-distillation (0.035 to 0.021%), and steam distillation (0.032% to 0.015%). Similarly, supercritical fluid extraction showed a higher clove EO yield (19.6%) than hydro-distillation (11.5%), and steam distillation (10.1%) [23]. In another study, supercritical fluid extraction extracted a higher percentage of eugenol (73.1%) from clove, followed by steam distillation (54.0%), and microwave oven distillation (34.6%) [24].
Superheated steam distillation is an emerging extraction technique, in which superheated steam is used as an extracting medium. Superheated steam is steam that is heated to a temperature higher than its boiling point at a given pressure. The temperature of superheated steam (101 °C to above 1000 °C) depends on the pressure and material of the container in which the steam is produced. This type of steam comprises a number of beneficial characteristics, including higher thermal conductivity, low oxygen capacity, and higher extraction efficiency [15,25]. The polarity and dielectric constant of superheated steam reduce with an increase in temperature, allowing it to extract a variety of polar and non-polar substances. The higher temperature of the superheated steam helps to break the plant cell wall and release the EO [15]. It has been used for the extraction of EOs from Boswellia serrata oleo gum resin, thyme, and black pepper [15,26].
The literature study showed that superheated steam distillation is still not used for the extraction of EOs from S. aromaticum. Therefore, the present study planned and executed the extraction of S. aromaticum EOs, using superheated steam distillation to compare yields with conventional hydro-distillation and steam distillation methods. The variations in the chemical composition of EOs were examined using gas chromatography–mass spectrometry. The effect of extraction methods on antioxidant activity was evaluated via different antioxidant assays. Furthermore, the antimicrobial potential of EOs against normal and food-borne disease-causing bacteria and fungi was observed using the resazurin microtiter plate assay, microdilution broth assay, and disc diffusion methods.
2. Results and Discussion
2.1. Essential Oil Yield
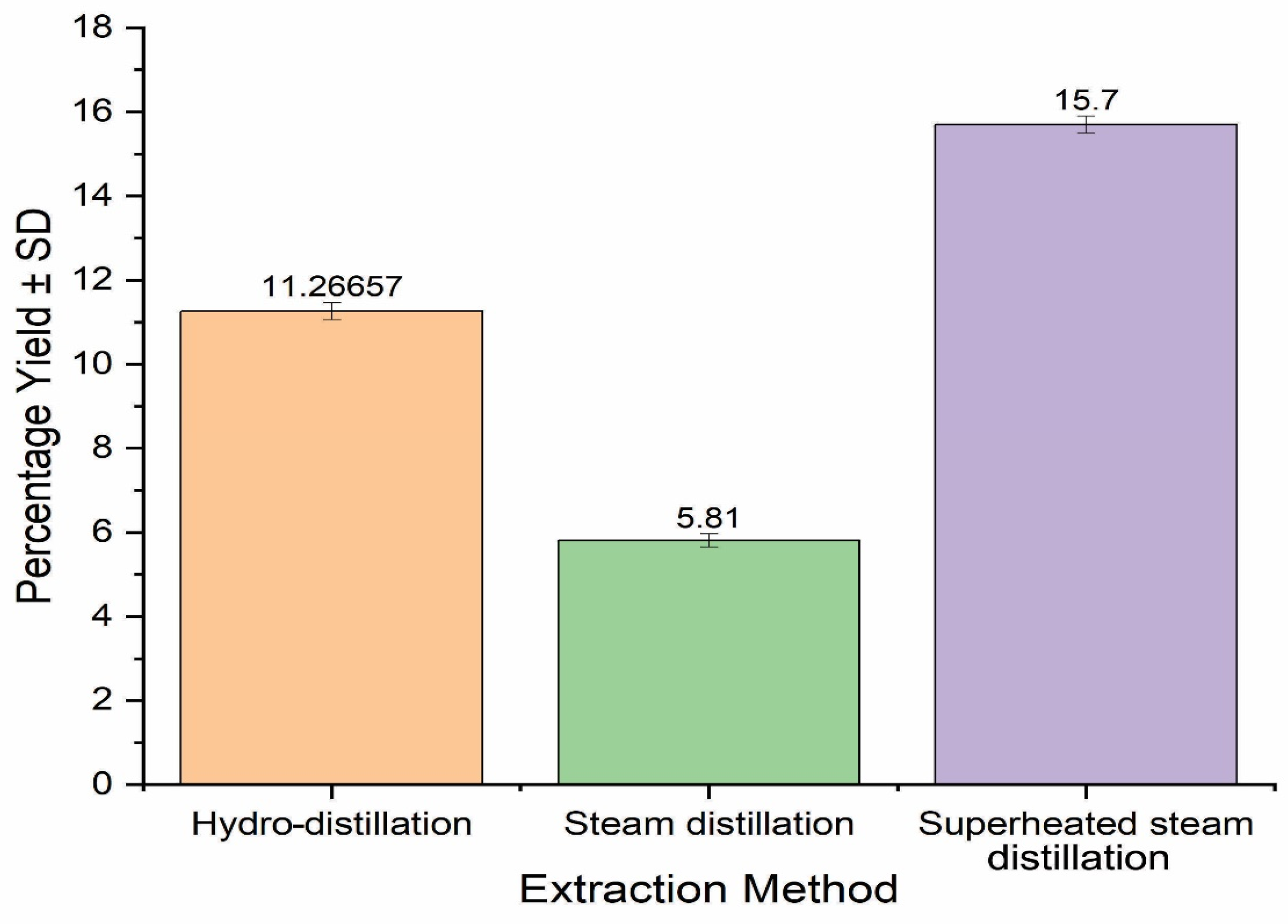
S. aromaticum EO was extracted using hydro-, steam distillation and superheated steam distillation, and the results are provided in Figure 1. It was observed that the extraction techniques significantly affected the yield of EOs. The highest EO yield was obtained using superheated steam distillation (15.70%), while the lowest yield was obtained by steam distillation (5.81%). The steam-distilled EO yield obtained in the present research was much lower (5.81%) than that in previous studies, and may have been due to variations in the seasonal, geological, and climatic conditions [27]. In contrast, the highest yield (15.70%) of S. aromaticum EO was obtained with superheated steam distillation, which may have been due to low viscosity, polarity, higher penetration power, and higher kinetic energy contents of superheated steam. It is evident that superheated steam has higher energy contents and penetration power than normal steam, which could enhance the extraction power [15]. Superheated steam distillation produced a higher percentage yield of S. aromaticum EO than Boswellia serrata oleo gum resin EO [15]. Similarly, extraction of EO from the seeds of S. aromaticum yielded more than that extracted from thyme and black pepper, which was in the range of 1–3%; moreover, it is also evident from the previous literature that extraction of EO increases when in grinded form [26].
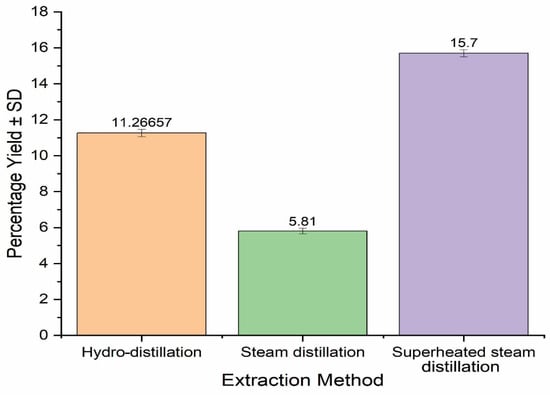
Figure 1.
Percentage yields of S. aromaticum essential oils derived from different extraction methods.
It was observed after a thorough review that there is very limited literature available on extraction using superheated steam distillation; therefore, this method, in comparison with the other extraction methods, has significance. EOs obtained in present research method had greater yields than already reported plant source leaves EO extracted using subcritical water extraction, in which an optimum EO yield of 3.77% was obtained at 175 °C [28].
Moreover, Boswellia (B.) elongata, B. carteri, B. socotrana, and B. dioscorides all had much lower yields (0.44%, 0.42%, 0.23% and 0.28%, respectively) of EOs than S. aromaticum seeds [29]. The EO yield of S. aromaticum was significantly higher than Cinnamomum zeylanicum EO (3.12%) extracted via superheated water from the leaves of plants [30]. Hence, the comparison revealed that superheated steam distillation has a much better efficiency of extracting EOs from S. aromaticum than conventional extraction methods.
2.2. Antimicrobial Activity
Resazurin microtiter plate assay, microdilution broth assay, and well diffusion assay were used to evaluate the antimicrobial activity of S. aromaticum EO extracted by hydro-, steam and superheated steam distillation. The results of the antimicrobial activity of S. aromaticum EO extracted by the above-mentioned techniques are given in Table 1. The values of the minimum inhibitory concentration (MIC) and inhibition zone of S. aromaticum EOs were in the range of 0.04 to 5.00 mg/mL and 12.06 to 26.87 mm, respectively, and the values for positive control were 0.01 to 0.63 mg/mL and 30.21 to 38.52 mm, respectively. It is clear in the observation that the EO of superheated steam distillation had higher antibacterial activity, providing bigger inhibition zones in the range of 26.87 to 18.00 mm, and MIC values ranging from 0.63 to 0.04 mg/mL. The variation in the antibacterial activity of EO of S. aromaticum may be due to differences in the chemical compositions of EOs with extraction methods [31]. It has been determined that different extraction methods show different antimicrobial potential. It may be assumed that the extraction methods may lose the compounds that carry antimicrobial activity in EOs [32]. Literature also supports that steam distillation-extracted EOs possess stronger antibacterial activity against Escherichia coli and Staphylococcus aureus, with inhibition zones of 2.83 and 2.81 cm, respectively, compared to the activity from superheated steam distillation-extracted EOs [2]. In the case of Gram-positive and Gram-negative bacteria, the Gram-negative bacterial strain of Pastrulla multocida showed the highest antibacterial result with an inhibition zone of 26.87 mm, while Escherichia coli showed an inhibition zone of 19.00 mm; meanwhile, in Gram-positive bacteria including Staphylococcus aureus and Bacillus subtilis, the former had lower antibacterial activity with an inhibition zone of 18.00 mm. Our results are in good agreement with already published data, in which S. aromaticum EO exhibited excellent antibacterial activity against Gram-positive as well as Gram-negative bacterial strains [33]. Ayoola et al. [34] determined the antimicrobial activity of S. aromaticum EO against Escherichia coli, Staphylococcus aureus, and Candida albicans, with MIC values of 2.40, 1.63, and 0.067 mg/mL, respectively [25]. Ogunwande et al. [35] evaluated the antibacterial activity of S. aromaticum fruit EO against Staphylococcus aureus. The results of distillation and superheated steam distillation are given in Table 1, representing antifungal activity in the range of 12.06–19.48 mm concerning the inhibition zone. The MIC values of antifungal activity were in the range of 0.08–5.00 µg/mL. Antifungal activity of the EO of S. aromaticum extracted with hydro-distillation, steam distillation, and superheated steam distillation was evaluated, and it was observed that EO from superheated steam distillation yielded better activity against all the tested strains. The highest antifungal activity was observed with superheated steam-extracted EO against Alternaria alternate, with an inhibition zone of 19.48 mm and a MIC value of 0.08 mg/mL; the lowest antifungal activity was noticed against Aspergillus niger, with an inhibition zone of 14.10 mm and a MIC value of 1.25 mg/mL.

Table 1.
Antimicrobial activity of S. aromaticum EOs derived from different extraction methods.
The antifungal activity of S. aromaticum and rosemary EOs as evaluated by micro broth dilution assays depicts that S. aromaticum oil has strong antifungal activity compared to rosemary EO. The S. aromaticum EO exhibited excellent antifungal activity against Candida albicans and Aspergillus Niger. Moreover, the MIC values of S. aromaticum EO were in the range of 0.062 to 0.500%, which was lower than that of rosemary EO, 0.123 to 1.000% [36]. Previously, research showed that eugenol in the EO of S. aromaticum is responsible for strong antifungal activity, and that it has strong antifungal activity against Trichophyton mentagrophytes and Candida albicans [37]. In another previous study, the antifungal activity of S. aromaticum EO was evaluated against Aspergillus sp., Mucor sp., Microsporum gypseum, Trichophyton rubrum, Fusarium moniliforme, and Fusarium oxysporum. It was found that its inhibition zone value was in the range of 12-2 mm, which bears some similarity to results in the present research [38]. The antifungal activity of the EOs of thyme, rosemary, and S. aromaticum were determined against Bacillus subtillis; maximum activity was produced by thyme EO, while S. aromaticum EO possessed intermediate antifungal activity [39]. The antifungal activity of S. aromaticum was determined against dermatophytes, Aspergillus, and Candida, in which the strongest activity was shown against dermatophytes having MIC values in the range of 0.16 µL, while MIC values for Aspergillus and Candida ranged from 0.32 to 0.64 µL [40].
Eugenol and caryophyllene are major components of S. aromaticum EO, so they may be responsible for the strong the antimicrobial activity. It is in accordance with previous research reports on the antimicrobial activity of EOs due to the presence of a high level of eugenol as an antioxidant. Moreover, it has been reported that eugenol denatures proteins and changes the permeability of the cell membrane, hence inhibiting the growth of bacteria and fungi [41]. In another report, it was evident that higher concentrations of phenolic compounds, such as eugenol, carvacrol, and thymol, increased the antimicrobial activity of S. aromaticum EO [39]. Overall, S. aromaticum EOs exhibited excellent antimicrobial activity against tested bacterial and fungal strains when extracted using superheated steam.
2.3. Chemical Composition of S. aromaticum EOs
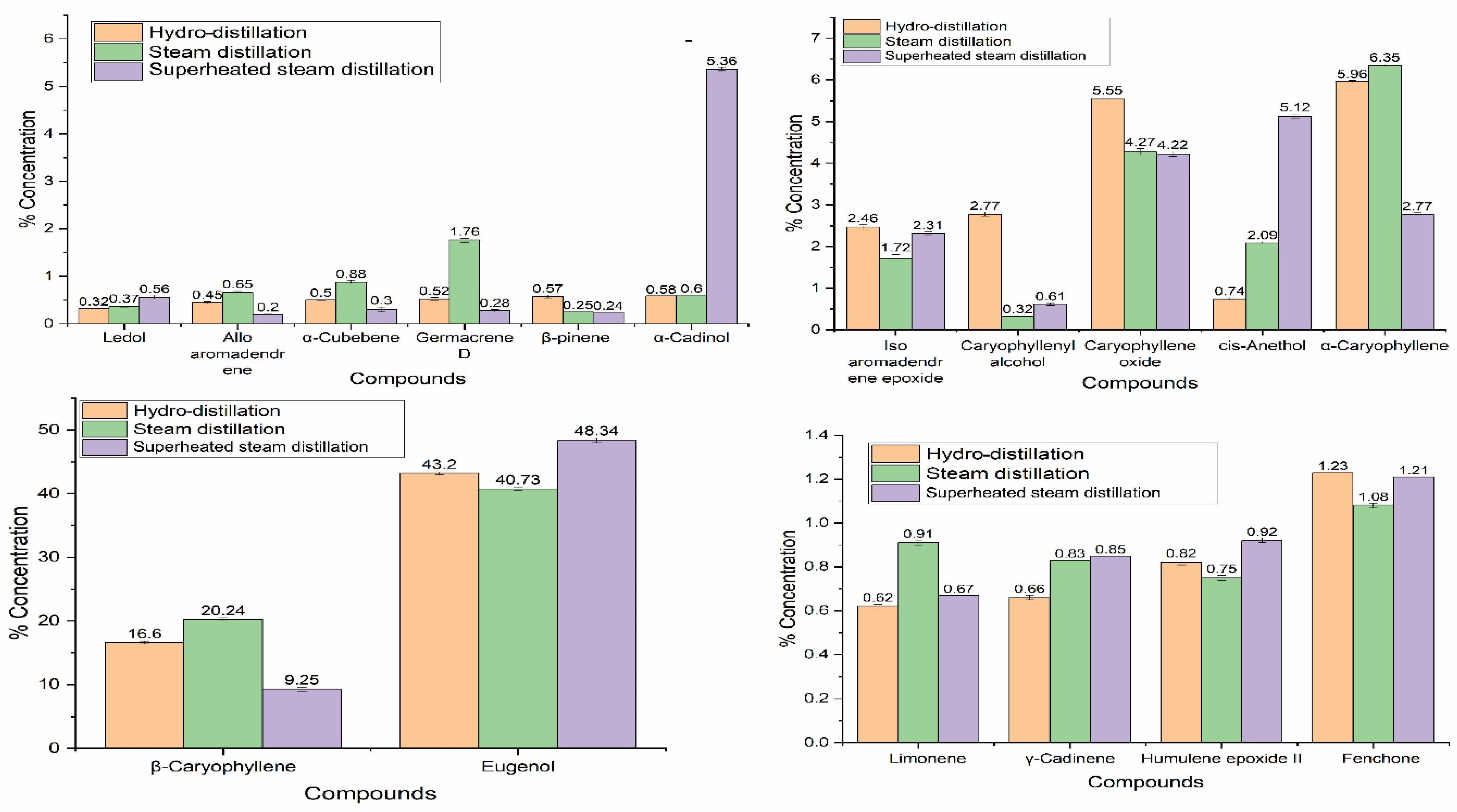
EOs of different extraction regimes were characterized using the state-of-the-art GC-MS technique, and the results of the chemical compositions of S. aromaticum EO extracted by hydro-distillation, steam distillation, and superheated steam distillation are given in Table 2. GC-MS results showed that S. aromaticum EO contained eugenol (47.94–26.5%), caryophyllene (20.24–9.25%), α-amorphene (7.12–1.02%), α-caryophyllene (6.35–2.77%), caryophyllene oxide (5.07–4.22%), and humulol (8.00–0.69%) as major compounds. Sesquiterpenes (45.58–22.82%) and oxygenated monoterpene (60.32–44.45%) and oxygenated sesquiterpenes (20.41–8.7%) were the main classes of compounds present in the EO of S. aromaticum. In oxygenated monoterpenes, eugenol was the major component, which was 47.94–26.5% of the chemical composition of S. aromaticum EO.

Table 2.
GC-MS results (% composition) of S. aromaticum EOs derived from different extraction methods.
D-cadinene and caryophyllene (alpha, beta, and iso) are the major components found in sesquiterpenes, while in oxygenated sesquiterpenes, humulol, caryophyllene oxide, and isoaromadendrene epoxide are the major constituents. Overall, eugenol is the major component of S. aromaticum EO, and is responsible for its biological activity. The results are in accordance with previous literature in which eugenol was the major component in S. aromaticum EO, followed by caryophyllene [42]. In another study, the antimicrobial activity of S. aromaticum EO was determined, and it was demonstrated that eugenol was the major component in EO responsible for its biological activity [40].
Eugenol was found to be a major component in the S. aromaticum EO extracted from India and Madagascar flora [43,44]. Results of the chemical composition of the EO extracted were in the best agreement with the previous literature, in which eugenol is the major component of the EO, with more than 70% concentration, followed by caryophyllene and caryophyllene oxide [45]. In another study, it was noted that eugenol is a major component found in the EO of S. aromaticum, with 85%, and eugenol was the main component responsible for the antimicrobial activity of S. aromaticum EO [40]. The chemical composition of the EO of dried buds of S. aromaticum contains eugenol as a major component extracted from hydro-distillation [46]. Variations in the chemical components of EOs are given in Figure 2. It was observed that major compounds were significantly affected by the extraction techniques. Eugenol was found in superheated steam extracted EO with the highest concentration of up to 47%. Cinnamaldehyde, β-elemene, β-cubebene, isosativene, and β-bisabolol were only found in superheated steam-extracted EO, in concentrations of 4.56, 1.15, 0.36, 0.31, 0.17, 0.57, and 0.29%, respectively. The extraction of more compounds in superheated steam distillation may have been due to the higher extraction power of superheated steam than normal steam. It has been reported that the polarity of water decreases with increasing temperature, and enhances the extraction of nonpolar compounds [47]. Similarly, there is also the chance of degradation of some heat-sensitive components at high temperatures. It has been reported that high extraction temperatures may cause degradation and artifact formation in EOs [48]. In short, extraction techniques can greatly affect the chemical composition of EOs.
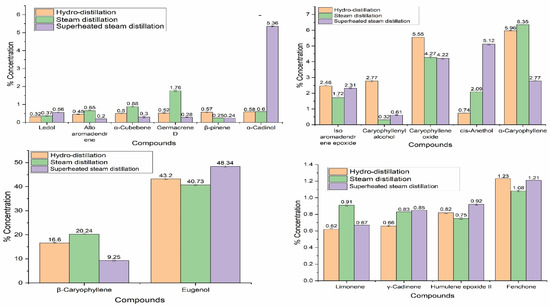
Figure 2.
Variations in chemical components of S. aromaticum essential oils extracted by hydro-distillation, steam distillation, and superheated steam distillation.
2.4. Antioxidant Activity
DPPH, hydrogen peroxide, and reducing power ability (RPA) assays were employed to assess the antioxidant activity of S. aromaticum EOs extracted using various extraction regimes, and their results are given in Table 3. It was noticed in the assessment that antioxidant activities of extracted EOs of S. aromaticum were affected by the extraction technique used; this effect was variable, and depended on the extraction regime. The DPPH assay of antioxidant activity followed the principle of a decrease in the absorbance, whereas hydrogen peroxide increased in the oxidation of H2O2 [49]. The results of the hydrogen peroxide assay showed that the highest FRSA of hydrogen peroxide, 80%, was found in superheated steam-extracted EO, while the lowest FRSA was 73.33% in steam-extracted EO. The reducing power of a compound is measured by the reduction of Fe3+ to Fe2+. This reducing activity of compounds depends on the availability of the presence of hydrogen atoms, which break the free radical chain and possess the antioxidant property through the donation of hydrogen atoms [49]. The total antioxidant activity was determined by FRAP, and the results were in the range of 549.15 to 1483.94 mg/100 g. EO extracted through superheated steam distillation possessed the highest antioxidant content, 1483.94 mg/100 g, while the lowest antioxidant content of 549.15 mg/100 g was found in S. aromaticum EO extracted through steam distillation.

Table 3.
DPPH free radical scavenging, hydrogen peroxide scavenging, and total antioxidant/FRAP activities of clove essential oils extracted by different extraction methods.
DPPH is a relatively simple and commonly used assay for the determination of free radical-scavenging activity (FRSA). It is a free radical that reacts with the antioxidants present in the Eos, and changes its color from purple to yellow. DPPH FRSA results showed that the extraction techniques significantly affected the FRSA of EOs. The highest FRSA of 77.47% was observed in superheated steam-distilled EO, and the lowest FRSA, 71.14%, in steam distillation. These results showed that the antioxidant potential of EOs is slightly lower than the standard gallic acid. DPPH FRSA activity of S. aromaticum oils was much higher than those of sage and oregano EOs [50]. The high scavenging activity of S. aromaticum EOs may be due to high concentrations of phenolic compounds, such as the major component eugenol. It has been reported that the scavenging activity of EO increases with increasing eugenol concentration [50]. Earlier research revealed that EO extracted from the floral stage of young plants possesses better antioxidant potential because of their high content of phenolic compounds [51].
2.5. Conclusions
The present research focused on investigating the extraction methodologies used for S. aromaticum EO, and their effect on different aspects after extraction such as yield, activities, and composition. After a detailed study, it may be concluded that the extraction methods greatly affect the EO yield, antimicrobial and antioxidant activities, and the chemical composition of S. aromaticum EO. The highest yield of S. aromaticum EO was produced from superheated steam distillation, followed by hydro-distillation and steam distillation. Overall, superheated steam-distilled EO presented greater antioxidant activity, as evaluated with different antioxidant assays. Similar trends were observed in antimicrobial assays, in which superheated steam-extracted EOs showed optimum antibacterial and antifungal activity against different foodborne disease-causing bacterial and fungal strains. The GC-MS characterization of extracted essential oils confirmed that eugenol and caryophyllene are the main components of S. aromaticum EO, which may be responsible for its antimicrobial and antioxidant activity, as previous literature revealed. Overall, this study concludes that superheated steam distillation is a promising technique that can be used for essential oil extraction, with better efficacy in biological applications.
3. Materials and Methods
3.1. Collection of Plant Materials
Plant material (S. aromaticum L. flower buds) weighing 2.0 kg was collected from the botanical garden and verified by taxonomist Dr. Fahim Arshad, Associate Professor, Department of Botany, University of Okara, Okara, Pakistan. A voucher specimen (OK-881) was deposited at the herbarium Department of Botany, University of Okara, Okara. Plant material was washed thrice with deionized distilled water, dried under the shed until attaining constant weight, ground with a mortar and pestle, and stored in polyethylene zipper bags at 4°C until further processing.
3.2. Extraction Methods
S. aromaticum EO was extracted by hydro-distillation (HD), steam distillation (SD), and superheated steam distillation (SHSE).
3.2.1. Hydro-Distillation
Hydro-distillation was performed with a Clevenger-type apparatus (CTA-001, PAMICO Technologies, Faisalabad, (Punjab), Pakistan), which consists of a heating mantle; a 5000 mL round bottom flask, dean stark, condensers, and a separating funnel. An amount of 300 g of fresh plant material was taken and placed in a 5000 mL round bottom flask and immersed in 3000 mL of distilled water. A volumetric flask containing plant material and water was placed in the heating mantle which boiled the water. The vaporized mixture of water and S. aromaticum oil was condensed. After condensing the vaporized mixture into liquid form, the condensed mixture was flowed into a separating funnel which separated the oil and aqueous layers. After the oil layer separated, its yield was calculated [16].
The following formula was used to compute the EO yield:
3.2.2. Steam Distillation
The steam distillation apparatus consisted of a round bottom flask, biomass flask, condenser, heating mantle, thermometer, and collecting vessel. The S. aromaticum sample in ground form (100 g) was placed in a biomass flask that was adjusted on top of the round bottom flask. Steam was generated by boiling water using a heating mantle. The steam interacted with the plant material after entering the biomass flask. The EO and water vapors were passed through a condenser, which cooled them to liquid droplets. The EO and water layers were separated based on density in the collecting vessel. The EO layer was separated and collected in a glass vial. Steam distillation was carried out for 3 h. Anhydrous sodium sulphate was used for the dehydration of EO. After that, the EO was passed through microfilters. The extraction procedure was repeated five times to ensure that the results were reproducible [52].
3.2.3. Superheated Steam Distillation
Superheated steam distillation was carried out in a custom-sized, superheated steam extractor apparatus consisting of a stainless steel extraction vessel connected to the superheated steam generator, condenser, and hydrosol collection vessel. The temperature of superheated steam was continuously monitored via thermocouples attached at the entry and exit of the stainless steel extraction vessel. The ground (60 mesh) plant biomass (100 g) was packed in the extraction chamber and subjected to superheated steam at 150 °C and 75 PSI pressure for 1 h. Moisture from EOs (10 g) was removed by adding sodium sulphate anhydrous was filtered using a micro filter, and was stored in amber glass vials until further analysis. The extraction procedure was repeated five times to ensure that the results were reproducible [15].
3.3. Antimicrobial Activity
3.3.1. Microbial Strains
EOs were examined against Aspergillus flavus, E. coli, Staphylococcus aureus, Bacillus subtilis, Alternaria alternate, Fusarium solani, Aspergillus niger, and Pasteurella multocida, collected from the University of Agriculture Faisalabad, Pakistan.
3.3.2. Agar Well Diffusion Method
The antibacterial and antifungal properties of EOs were tested using the agar well diffusion method [53]. The microbial strains were moved to 25 mL of growth medium solution after an overnight culture containing 108 colony-forming units per milliliter. The flask contents were then transported to medium-sized Petri dishes. Sterilized cork borer was used for well formation when the contents solidified at room temperature. These wells were supplied with 10 mL of EOs in pure form extracted using superheated steam at various temperatures, as well as a standard drug to test antibacterial activity. For bacteria, the Petri plates were then incubated at 37 °C for 24 h, and fungal strains were incubated at 30 °C for 40 h. The width of inhibitory zones (mm) was evaluated using a digital Vernier caliper after the incubation time.
3.3.3. Resazurin Micro-Titer Plate Assay
The esazurin micro-titer plate assay is a modified form of assay used for the measurement of a minimum inhibitory concentration of EOs for various microbial strains [54]. A volume of 10 mL of EO was dissolved in 1 mL of 10% DMSO to prepare the sample solution. In 4 mL of deionized water, 27 mg of resazurin was dissolved to prepare the resazurin indicator. Into the first row of ninety-six well plates, sample solution and antibiotic ampicillin were pipetted up to about 100 µL, excluding the first row where 50 µL of nutrient broth was added in all of the wells; dilutions of two-fold serials were made in such a way that each of the wells consisted of 50 µL of the sample. After this, 30 µL of isosensitized broth of 3.3× strength, and 10 µL of the resazurin solution were introduced into all of the wells. Incubation of plates was carried out for 24 h at 37 °C. The MIC values were evaluated visually after the incubation. Bacterial growth was indicated if the color changed from purple to pink or colorless. The MIC value was taken at the lowest concentration where a change in color took place.
3.3.4. Micro-Dilution Broth Susceptibility Assay
The micro-dilution broth susceptibility assay was used to measure the MIC of EOs derived from different extraction methods against various fungal strains [55]. Firstly, 10 mg of EO was dissolved in 1 mL of 10% DMSO to prepare sample solutions of EOs from different extraction methods, and 1 mL of standard antibiotic fluconazole in 1 mL of 10% DMSO solution was dissolved to prepare the standard antibiotic fluconazole solution. Into the first of the well plates, 100 µL of sample and standard were pipetted, except for the first row, where 50 µL of sabouraud was added.
Dextrose broth was added to all of the well plates. After this, two-fold serial dilutions were made in such a way that each well consisted of 50 µL of the sample solution. Then, 130 µL of sabouraud solution and 20 µL of microorganism suspension were added to all of the wells. Well plates were incubated for 48 h at 30 ℃. The MIC value with the lowest concentration at which whole inhibition of fungal growth took place was analyzed visually.
3.4. Gas Chromatography–Mass Spectrometry Analysis
The volatile fraction of EO from each extraction technique was characterized through GC-MS (GCMS-QP 2010, Shimadzu, Japan) equipped with a mass detector and capillary column, namely DB-5, which was 50m x 0.25 mm, and having 0.25 µm of film thickness). Then, 1 µL of EO diluted to a 1:10 ratio with n-hexane was used as the injection volume, and it was injected with the help of a syringe into the injection port. The GC column was heated for 3 min at 60 °C. After this, the temperature of the column was increased slowly up to 240 °C with a heating rate of 24 °C/min, and was kept constant for 10 min. The carrier gas used in this system was helium (He), and its flow rate was 1.5 mL/min. The temperature of the mass spectrometer transfer line was kept at 240 °C. MS detection used 70 eV in electron ionization mode. Standards of n-alkanes were run for the determination of the retention indices of the compounds which were detected. This mass spectrum data and retention indices were compared with the already present mass spectral data base and the NIST library [16]. Co-injection of the authentic standards was conducted for the confirmation of the compounds. The quantitative analysis of the components of the EOs was performed [56]. RF values were calculated using the following equation:
RF represents the response factor of the component of the essential oil. Ac represents the peak area of the component of the EO, and Ais is the peak area of the internal standard. Cc and Cis are the concentrations of the components of the EO and internal standard, respectively. The % of each of the components of the EO was calculated with the RFs as follows:
RFC = (Ac/Airs)/(Cc/Cis)
Corrected area = peak area for the component/response factor for that component
% = (corrected area for the component/total of the corrected areas) × 100
3.5. Antioxidant Assays
3.5.1. DPPH Free Radical-Scavenging Activity
The DPPH free radical-scavenging activity was performed by adding 1 mL of 0.3 M DPPH solution into a 2.5 mL solution of the plant extract. Incubation of samples and standard took place in the dark at room temperature for 20 min. The absorbance of the samples was taken at 518 nm. For the preparation of control absorbance, DPPH was not added; however, 1.0 mL of methanol was added in the 2.5 mL of plant extract. Positive control was prepared by the addition of 1 mL of DPPH solution up to the 2.5 mL of gallic acid [57]. The % DPPH scavenging activity was calculated with the following formula:
DPPH scavenging activity (%) = 100 − [(Abs sample/Abs control) × 100]
3.5.2. Reducing Power Ability (RPA)
Reducing power ability was determined by mixing 1 mL of sample/standard solution gallic acid (whose concentrations were 25, 50, 75, and 100 mg/L) into 2.3 mL of phosphate buffer with a molarity of 0.2 M, and 2.5 mL of 1% potassium ferricyanide. Incubation of the sample took place at 37 °C for 20 min. After that, 2.5 mL of 10% trichloroacetic acid and the mixture were centrifuged at 1000 rpm for 10 min. Then, 2.5 mL of the above sample was mixed with 0.5 mL of 0.1% FeCl3 and 2.5 mL of distilled water. The solution was kept for 10 min, and the absorbance was taken at 700 nm [58].
3.5.3. Hydrogen Peroxide Scavenging Activity
The hydrogen peroxide scavenging activity of EOs was determined spectrophotometrically [59]. A 2 mM solution of hydrogen peroxide was prepared in a phosphate buffer of 0.17 M having a pH of 7.4. Various concentrations of 600 µL of solution and 0–10 mg/mL of ascorbic acid was added to the 2 mM hydrogen peroxide solution. The solution was incubated for 10 min, and the absorbance was taken at 230 nm. The following formula was used to calculate the percentage scavenging activity of hydrogen peroxide:
%age of hydrogen peroxide scavenged = (A0 − A1) × 100
Here, A0 represents the absorbance of the control, and A1 represents the absorbance of the sample.
3.6. Statistical Analysis
All of the experiments were performed in triplicate, and the data were analyzed with STATISTICA 5.5 (Stat Soft Inc., Tulsa, OK, USA) software and analysis of variance (ANOVA) with the post hoc Tukey’s HSD test. Statistical significance was defined as a probability value of p ≤ 0.05. Based on triplicate measurements, the data were presented as mean values with standard deviations.
Author Contributions
Conceptualization, M.A.A. (Muhammad Adnan Ayub); investigation, A.F.; validation M.A.A. (Muhammad Amin Abid); project administration and methodology, M.A.A. (Muhammad Adnan Ayub); resources, M.Z.; review and editing, G.G. and M.S.; writing and draft preparation, A.F. and G.G.; supervision, M.A.A. (Muhammad Adnan Ayub); funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of a research project (Grant No. 20-15988/NRPU/R&D/HEC/2021) funded by the Higher Education Commission (HEC), Islamabad, Pakistan.
Data Availability Statement
All data generated or analyzed during this study are available on request through the corresponding author at adnanayub@uosahiwal.edu.pk.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Otunola, G.A. Culinary spices in food and medicine: An overview of Syzygium aromaticum (L.) Merr. and LM Perry [Myrtaceae]. Front. Pharmacol. 2021, 12, 793200. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Boughendjioua, H. Essential oil composition of Syzygium aromaticum (L.). IRJPMS 2018, 11, 26–28. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Antibacterial activity and action mechanism of the essential oil from Enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Barron, U.; Thébault, A.; Kooh, P.; Watier, L.; Sanaa, M.; Cadavez, V. Strategy for systematic review of observational studies and meta-analysis modelling of risk factors for sporadic foodborne diseases. Microb. Risk Anal. 2021, 17, 100082. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Sobiyi, O.; Sadgrove, N.; Magee, A.; Van Wyk, B.-E. The ethnobotany and major essential oil compounds of anise root (Annesorhiza species, Apiaceae). S. Afr. J. Bot. 2019, 126, 309–316. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Jr, R.N.; Moura, L.S.; Rosa, P.T.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Dawra, M.; Nehme, N.; El Rayess, Y.; El Beyrouthy, M.; Taillandier, P.; Bouajila, J. Folk medicinal applications, phytochemical composition and biological activities of some Lebanese endemic plants. S. Afr. J. Bot. 2022, 150, 511–527. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Abidin, Z.Z.; Siajam, S.I.; Hean, C.G.; Harun, M.R. Optimization studies and compositional analysis of subcritical water extraction of essential oil from Citrus hystrix DC. leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Ayub, M.A.; Hanif, M.A.; Blanchfield, J.; Zubair, M.; Abid, M.A.; Saleh, M.T. Chemical composition and antimicrobial activity of Boswellia serrata oleo-gum-resin essential oil extracted by superheated steam. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.A.; Hanif, M.A.; Sarfraz, R.A.; Shahid, M. Biological activity of Boswellia serrata Roxb. oleo gum resin essential oil: Effects of extraction by supercritical carbon dioxide and traditional methods. Int. J. Food Prop. 2018, 21, 808–820. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Sadeghmousavi, S. Subcritical water extraction of fennel seeds essential oil and comparison with hydrodistillation. J. Food Sci. Technol. 2019, 16, 119–128. [Google Scholar]
- Masondo, N.; Makunga, N. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J. Bot. 2019, 126, 40–57. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- de Vasconcelos Silva, M.G.; de Abreu Matos, F.J.; Roberto, P.; Lopes, O.; Silva, F.O.; Holanda, M.T. Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction. Arkivoc 2004, 6, 66–71. [Google Scholar] [CrossRef]
- Jin, C.; Guo, J.; Zhu, H.; Wen, J. Optimization of superheated steam treatment conditions for wheat aleurone layer flour. Food Sci. Technol. 2021, 42, e71920. [Google Scholar] [CrossRef]
- Rouatbi, M.; Duquenoy, A.; Giampaoli, P. Extraction of the essential oil of thyme and black pepper by superheated steam. J. Food Eng. 2007, 78, 708–714. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Kaymaz, H. Superheated water extraction, steam distillation and Soxhlet extraction of essential oils of Origanum onites. Anal. Bioanal. Chem. 2004, 379, 1127–1133. [Google Scholar] [CrossRef]
- Prakash, B.; Mishra, P.K.; Kedia, A.; Dubey, N. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT-Food Sci. Technol. 2014, 56, 240–247. [Google Scholar] [CrossRef]
- Jayawardena, B.; Smith, R.M. Superheated water extraction of essential oils from Cinnamomum zeylanicum (L.). Phytochem. Anal. 2010, 21, 470–472. [Google Scholar] [CrossRef]
- Glišić, S.B.; Milojević, S.Ž.; Dimitrijević, S.I.; Orlović, A.M.; Skala, D.U. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007, 72, 311–320. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, G.; Lawore, F.; Adelowotan, T.; Aibinu, I.; Adenipekun, E.; Coker, H.; Odugbemi, T. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). Afr. J. Microbiol. Res. 2008, 2, 162–166. [Google Scholar]
- Ogunwande, I.; Olawore, N.; Ekundayo, O.; Walker, T.; Schmidt, J.; Setzer, W. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Turhan, S. Antimicrobial activity and antioxidant capacity of thyme, rosemary and clove essential oils and their mixtures. Int. J. Innov. Sci. Eng. Technol. 2018, 2, 25–33. [Google Scholar]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Nuñez, L.; D’Aquino, M. Microbicide activity of clove essential oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.N.I.; Begum, J.; Nandi, N.C.; Akter, F. Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston). Afr. J. Plant Sci. 2010, 4, 451–454. [Google Scholar]
- Raina, V.; Srivastava, S.; Aggarwal, K.; Syamasundar, K.; Kumar, S. Essential oil composition of Syzygium aromaticum leaf from Little Andaman, India. Flavour Fragr. J. 2001, 16, 334–336. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, S.; Syamsundar, K. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour Fragr. J. 2005, 20, 51–53. [Google Scholar] [CrossRef]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Insuan, W. Superheated water extraction of lime peel: A comparison with conventional methods. Anal. Lett. 2004, 37, 2393–2409. [Google Scholar] [CrossRef]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2020, 34, 1014–1031. [Google Scholar] [CrossRef]
- Özkan, A.; Erdoğan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Keramat, J.; Goli, S. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L.) and sage (Salvia officinalis L.) essential oils in various model systems. Int. Food Res. J. 2017, 24, 1628. [Google Scholar]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) bud oil at the different phenological stages in young and mature trees. Scientifica 2020, 9701701. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Abbasi, A.M.; Abid, M.; Mozūratis, R.; Alwahibi, M.S.; Elshikh, M.S. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab. J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Ali, M.; Singh, H.; Gupta, J.; Sharma, G. A novel antifungal pyrrole derivative from Datura metel leaves. Int. J. Pharm. Sci. Res. 2004, 59, 568–570. [Google Scholar]
- Das, A.; Dey, S.; Sahoo, R.K.; Sahoo, S.; Subudhi, E. Antibiofilm and antibacterial activity of essential oil bearing Zingiber officinale Rosc.(Ginger) Rhizome against multi-drug resistant isolates. J. Essent. Oil-Bear. Plants 2019, 22, 1163–1171. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.d.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Habila, J.; Bello, I.; Dzikwi, A.; Musa, H.; Abubakar, N. Total phenolics and antioxidant activity of Tridax procumbens Linn. Afr. J. Pharm. Pharmacol. 2010, 4, 123–126. [Google Scholar]
- Bittencourt, V.; Bahiense, T.; Fernandes, E.; Souza, E.; Botelho, M.; Nogueira, N.; Bastos, G.; Fonseca, S.; Lemos, T.; Cavalcanti, S. essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).