The Multiple Faces of Nodular Trichoblastoma: Review of the Literature with Case Presentation
Abstract
:1. Introduction
2. Materials and Methods
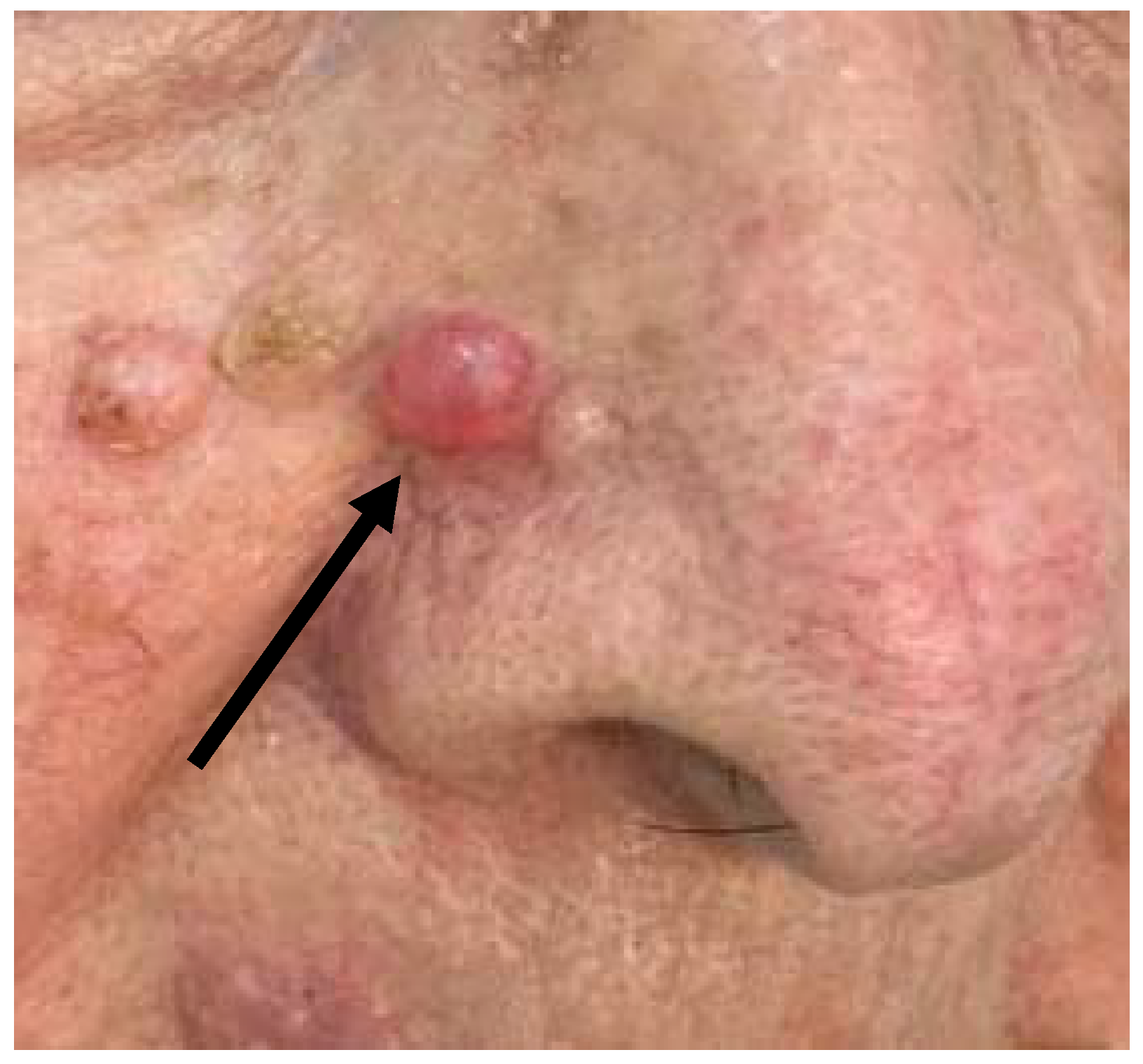
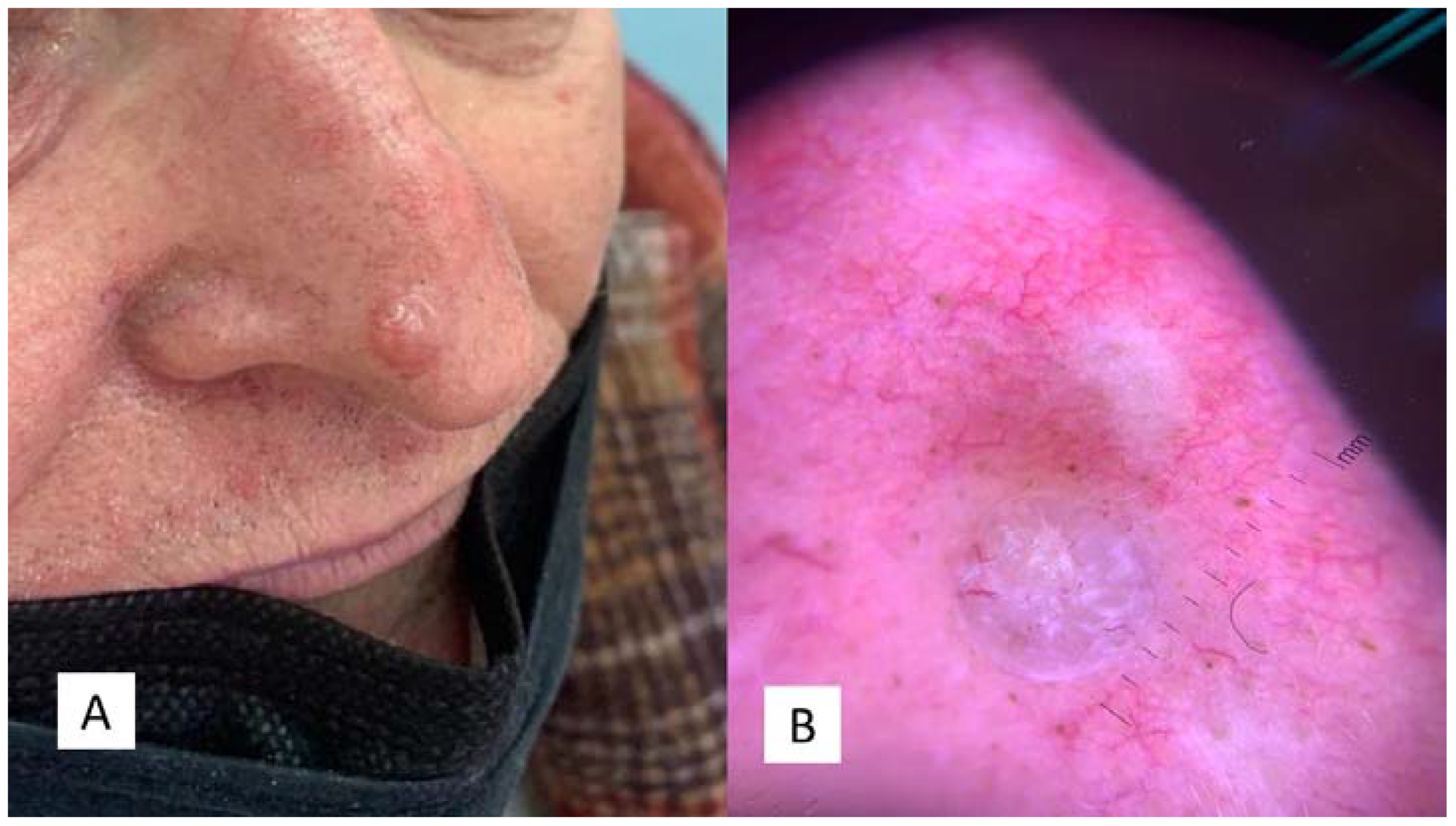
2.1. Case 1
2.2. Case 2
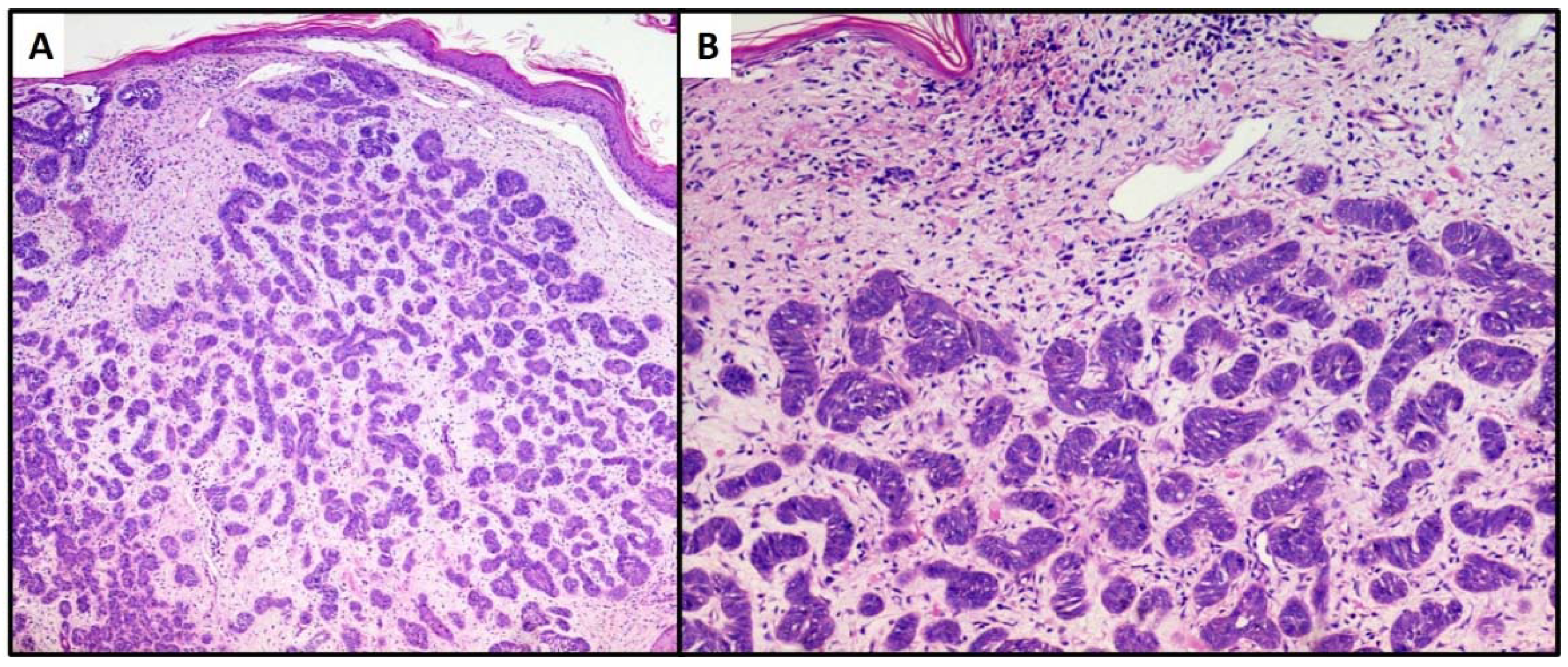
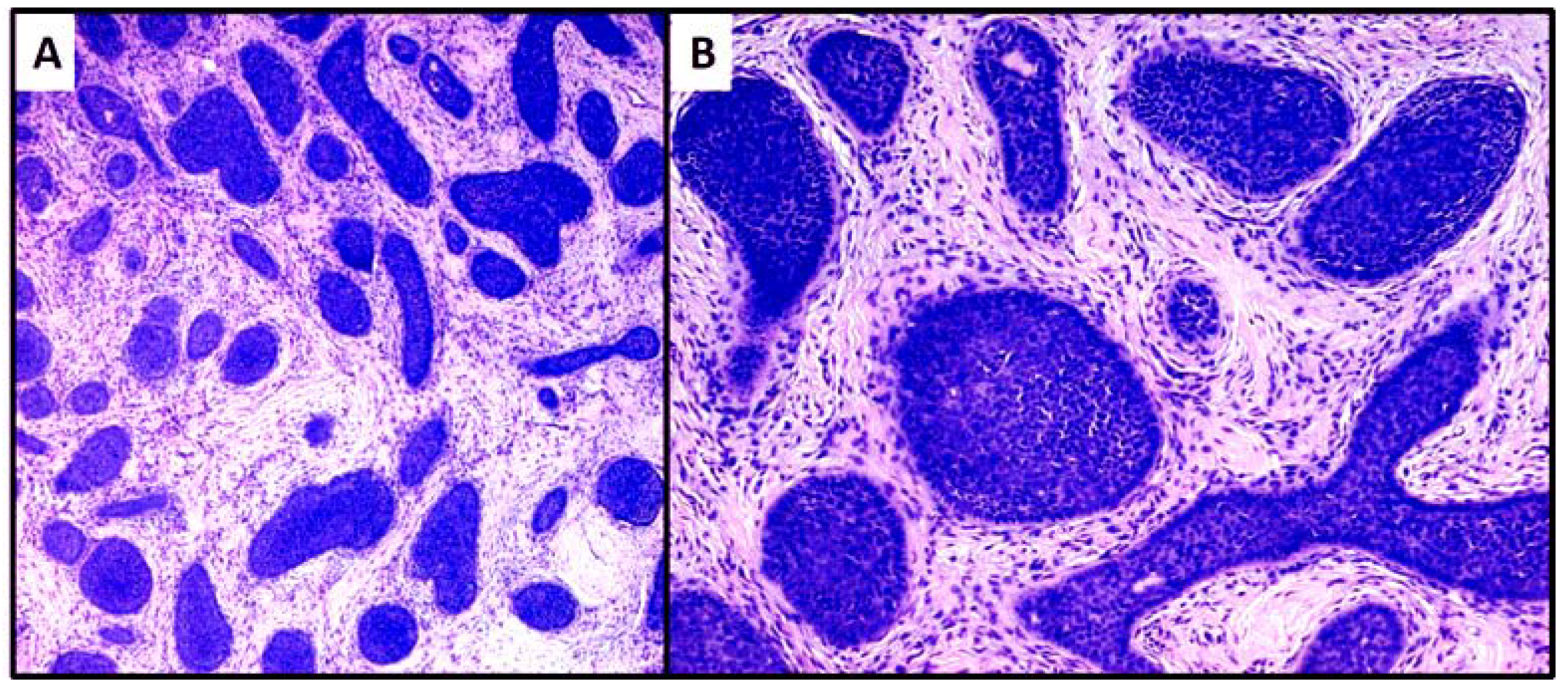
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kamat, G.; Yelikar, B.; Shettar, S.; Karigoudar, M.H. Pigmented trichoblastoma with sebaceous hyperplasia. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 506–508. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours, 4th ed.; WHO: Geneva, Switzerland, 2018; Volume 11. [Google Scholar]
- Fusumae, T.; Tanese, K.; Takeuchi, A.; Takasugi, A.; Kawakita, R.; Shiraishi, J.; Yoshida, T. High-grade trichoblastic carcinoma arising through malignant transformation of trichoblastoma: Immunohistochemical analysis and the expression of p53 and phosphorylated AKT. J. Dermatol. 2019, 46, 57–60. [Google Scholar] [CrossRef]
- Patterson, J. Weedon’s Skin Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Headington, J.T. Differentiating neoplasms of hair germ. J. Clin. Pathol. 1970, 23, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Requena, L.; Sangüeza, O. Cutaneous Adnexal Neoplasms; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Imai, S.; Nitto, H. Trichogenes Trichoblastom [Trichogenic trichoblastoma]. Hautarzt 1982, 33, 609–611. [Google Scholar]
- Schirren, C.G.; Rütten, A.; Sander, C.; McClain, S. Trichoblastoma. A neoplasm with follicular differentiation. Hautarzt 1995, 46, 81–86. [Google Scholar] [CrossRef]
- Karmarkar, P.J.; Mahore, S.D.; Wilkinson, A.R. Solitary trichoblastoma. Indian J. Pathol. Microbiol. 2009, 52, 277–278. [Google Scholar]
- Pina, A.; Sauthier, P.; Rahimi, K. Vulvar trichoblastoma: Case report and literature review. J. Low. Genit. Tract Dis. 2015, 19, e10–e12. [Google Scholar] [CrossRef]
- Cho, J.; Hong, S.A.; Cho, H.D. Composite trichoblastoma and inverted follicular keratosis: A case report. Int. J. Clin. Exp. Pathol. 2018, 11, 2884–2886. [Google Scholar] [PubMed]
- Wang, F.; Wu, Y.; Zheng, Z.; Bai, Y. Syringocystadenoma papilliferum and trichoblastoma arising in the nevus sebaceous. Indian J. Pathol. Microbiol. 2018, 61, 106–108. [Google Scholar] [PubMed]
- Misago, N.; Sada, A.; Narisawa, Y. Trichoblastoma with a dilated pore. J. Am. Acad. Dermatol. 2006, 54, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Nawrocki, S.; Hinther, K.; Khachemoune, A. Trichoblastomas Mimicking Basal Cell Carcinoma: The Importance of Identification and Differentiation. Cureus 2020, 12, e8272. [Google Scholar] [CrossRef]
- Ghigliotti, G.; De Col, E.; Parodi, A.; Bombonato, C.; Argenziano, G. Trichoblastoma: Is a clinical or dermoscopic diagnosis possible? J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1978–1980. [Google Scholar] [CrossRef]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]
- Betti, R.; Cerri, A.; Moneghini, L.; Inselvini, E.; Crosti, C. Adenoid-cystic cribriform trichoblastoma. Hautarzt 1997, 48, 417–419. [Google Scholar] [CrossRef]
- Kazakov, D.V.; Mentzel, T.; Erlandson, R.A.; Mukensnabl, P.; Michal, M. Clear cell trichoblastoma: A clinicopathological and ultrastructural study of two cases. Am. J. Dermatopathol. 2006, 28, 197–201. [Google Scholar] [CrossRef]
- Al Omoush, T.M.; Michal, M.; Konstantinova, A.M.; Michal, M.; Kutzner, H.; Kazakov, D.V. Melanocytic Hyperplasia in the Epidermis Overlying Trichoblastomas in 100 Randomly Selected Cases. Am. J. Dermatopathol. 2016, 38, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Quist, E.E.; DiMaio, D.J. Melanotrichoblastoma: A rare pigmented variant of trichoblastoma. Cutis 2017, 100, 243–246. [Google Scholar]
- Betti, R.; Alessi, E. Nodular trichoblastoma with adamantinoid features. Am. J. Dermatopathol. 1996, 18, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Keesecker, S.; Saab, J.; Magro, C.M.; Dokania, V.; Sclafani, A.P. Cutaneous Lymphadenoma: A Trichoblastoma with Regressive Inflammatory Changes. Facial Plast. Surg. 2017, 33, 109–111. [Google Scholar] [CrossRef] [PubMed]
- McNiff, J.M.; Eisen, R.N.; Glusac, E.J. Immunohistochemical comparison of cutaneous lymphadenoma, trichoblastoma, and basal cell carcinoma: Support for classification of lymphadenoma as a variant of trichoblastoma. J. Cutan. Pathol. 1999, 26, 119–124. [Google Scholar] [CrossRef]
- Vega Memije, M.E.; Luna, E.M.; de Almeida, O.P.; Taylor, A.M.; Cuevas González, J.C. Immunohistochemistry panel for differential diagnosis of Basal cell carcinoma and trichoblastoma. Int. J. Trichology 2014, 6, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchmann, T.T.; Prieto, V.G.; Smoller, B.R. CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch. Dermatol. 1994, 130, 589–592. [Google Scholar] [CrossRef]
- Kirchmann, T.T.; Prieto, V.G.; Smoller, B.R. Use of CD34 in assessing the relationship between stroma and tumor in desmoplastic keratinocytic neoplasms. J. Cutan. Pathol. 1995, 22, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Penneys, N.S. Immunostaining for CD34 to determine trichoepithelioma. Arch. Dermatol. 1995, 131, 616–617. [Google Scholar] [CrossRef]
- Swanson, P.E.; Fitzpatrick, M.M.; Ritter, J.H.; Glusac, E.J.; Wick, M.R. Immunohistologic differential diagnosis of basal cell carcinoma, squamous cell carcinoma, and trichoepithelioma in small cutaneous biopsy specimens. J. Cutan. Pathol. 1998, 25, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Colagrande, A.; Romita, P.; Foti, C.; Resta, L.; Ingravallo, G. Pilomatrixcarcinoma of the Foot: A New Localization of an Extremely Rare Adnexal Tumour. Diagnostics 2021, 11, 793. [Google Scholar] [CrossRef]
- Izikson, L.; Bhan, A.; Zembowicz, A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am. J. Dermatopathol. 2005, 27, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Battistella, M.; Peltre, B.; Cribier, B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: A case-control study, with first description of granular-cell trichoblastoma. Am. J. Dermatopathol. 2014, 36, 643–650. [Google Scholar] [CrossRef]
- Leblebici, C.; Bambul Sığırcı, B.; Kelten Talu, C.; Koca, S.B.; Huq, G.E. CD10, TDAG51, CK20, AR, INSM1, and Nestin Expression in the Differential Diagnosis of Trichoblastoma and Basal Cell Carcinoma. Int. J. Surg. Pathol. 2019, 27, 19–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Cimmino, A.; Colagrande, A.; Arezzo, F.; Lospalluti, L.; Sablone, S.; Lettini, T.; Resta, L.; Ingravallo, G. The Multiple Faces of Nodular Trichoblastoma: Review of the Literature with Case Presentation. Dermatopathology 2021, 8, 265-270. https://doi.org/10.3390/dermatopathology8030032
Cazzato G, Cimmino A, Colagrande A, Arezzo F, Lospalluti L, Sablone S, Lettini T, Resta L, Ingravallo G. The Multiple Faces of Nodular Trichoblastoma: Review of the Literature with Case Presentation. Dermatopathology. 2021; 8(3):265-270. https://doi.org/10.3390/dermatopathology8030032
Chicago/Turabian StyleCazzato, Gerardo, Antonietta Cimmino, Anna Colagrande, Francesca Arezzo, Lucia Lospalluti, Sara Sablone, Teresa Lettini, Leonardo Resta, and Giuseppe Ingravallo. 2021. "The Multiple Faces of Nodular Trichoblastoma: Review of the Literature with Case Presentation" Dermatopathology 8, no. 3: 265-270. https://doi.org/10.3390/dermatopathology8030032
APA StyleCazzato, G., Cimmino, A., Colagrande, A., Arezzo, F., Lospalluti, L., Sablone, S., Lettini, T., Resta, L., & Ingravallo, G. (2021). The Multiple Faces of Nodular Trichoblastoma: Review of the Literature with Case Presentation. Dermatopathology, 8(3), 265-270. https://doi.org/10.3390/dermatopathology8030032










