“Animal-Type Melanoma/Pigmented Epithelioid Melanocytoma”: History and Features of a Controversial Entity
Abstract
1. Introduction
2. Materials and Methods
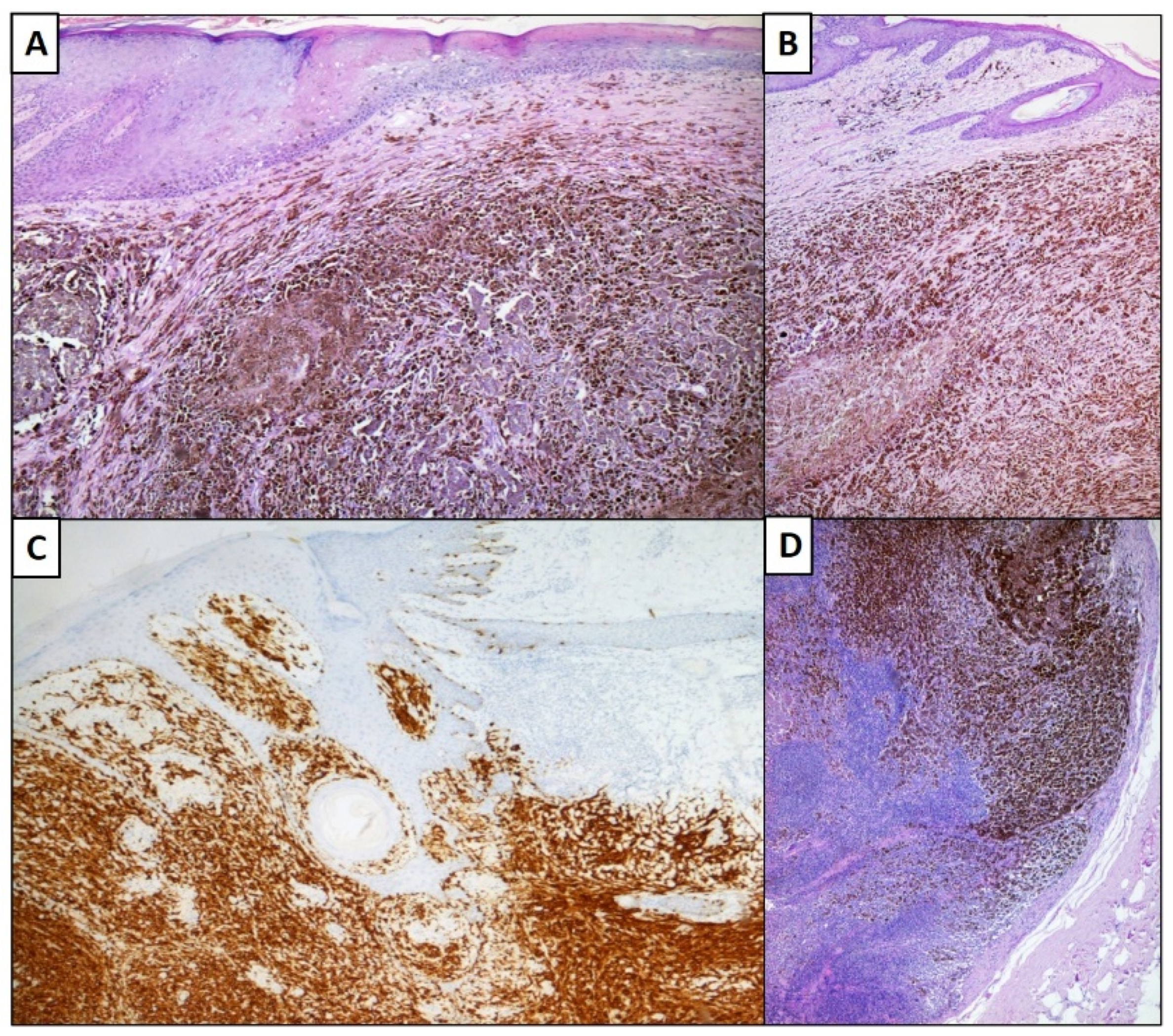
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Levene, A. Disseminated dermal melanocytosis terminating in melanoma. A human condition resembling equine melanotic disease. Br. J. Dermatol. 1979, 101, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Sánchez, A.; Delgado Mucientes, C.; Manchado López, P. Aggressive animal-type melanoma in a 79-year-old man. Rev. Clin. Esp. 2018, 218, 445–446. [Google Scholar] [CrossRef]
- Massi, G.; LeBoit, P. Histological Diagnosis of Nevi and Melanoma, 2nd ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Crowson, A.N.; Magro, C.M.; Mihm, M.C. Malignant melanoma with prominent pigment synthesis: “animal type” melanoma—A clinical and histological study of six cases with a consideration of other melanocytic neoplasms with prominent pigment synthesis. Hum. Pathol. 1999, 30, 543–550. [Google Scholar] [CrossRef]
- Antony, F.C.; Sanclemente, G.; Shaikh, H.; Trelles, A.S.; Calonje, E. Pigment synthesizing melanoma (so-called animal type melanoma): A clinicopathological study of 14 cases of a poorly known distinctive variant of melanoma. Histopathology 2006, 48, 754–762. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Liguori, G.; Lettini, T.; Serio, G.; Ingravallo, G.; Marzullo, A. Atypical Fibroxanthoma-Like Amelanotic Melanoma: A Diagnostic Challenge. Dermatopathology 2021, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Ludgate, M.W.; Fullen, D.R.; Lee, J.; Rees, R.; Sabel, M.S.; Wong, S.L.; Johnson, T.M. Animal-type melanoma: A clinical and histopathological study of 22 cases from a single institution. Br. J. Dermatol. 2010, 162, 129–136. [Google Scholar] [CrossRef][Green Version]
- Roncati, L.; Piscioli, F. Animal-Type Melanoma—A Mini-Review Concerning One of the Rarest Variants of Human Melanoma. Klin. Onkol. 2018, 31, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Posch, C.; Vesely, M.; Monshi, B.; Feichtinger, H.; Cziegler, K.; Rappersberger, K. Animal-type melanoma–tumor cell invasion of dermal lymphatics and molecular identification of lymph node metastasis. J. Dtsch. Dermatol. Ges. 2012, 10, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Mones, J.M.; Ackerman, A.B. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: A critique in historical perspective of three concepts flawed fatally. Am. J. Dermatopathol. 2004, 26, 407–430. [Google Scholar] [CrossRef]
- Raquena, L. Animal-Type Melanoma” and “Entities” Related to It: Exegesis of a Subject Until Now Incomprehensible. Am. J. Dermatopathol. 2007, 29, 495–496. [Google Scholar] [CrossRef]
- Zembowicz, A.; Knoepp, S.M.; Bei, T.; Stergiopoulos, S.; Eng, C.; Mihm, M.C.; Stratakis, C.A. Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. Am. J. Surg. Pathol. 2007, 31, 1764–1775. [Google Scholar] [CrossRef]
- Punjabi, S.; Wright, C.; Teixeira, F.; Stamp, G.; Chu, A. Intraepidermal animal-type melanoma. Int. J. Dermatol. 2006, 45, 957–959. [Google Scholar] [CrossRef]
- Vyas, R.; Keller, J.J.; Honda, K.; Cooper, K.D.; Gerstenblith, M.R. A systematic review and meta-analysis of animal-type melanoma. J. Am. Acad. Dermatol. 2015, 73, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.J.; Brown, M.D.; Rothberg, P.G.; Laughlin, T.S.; Scott, G.A. Pigmented epithelioid melanocytoma (animal-type melanoma): An institutional experience. J. Am. Acad. Dermatol. 2017, 77, 328–332. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, K.C.; Sweeney, R.W.; Del Piero, F. Metastatic melanoma in horses. J. Vet. Intern. Med. 2002, 16, 452–456. [Google Scholar] [CrossRef]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Demarco, A.; Lospalluti, L.; Arezzo, F.; Resta, L.; Ingravallo, G. The Great Mime: Three Cases of Melanoma with Carcinoid-Like and Paraganglioma-Like Pattern with Emphasis on Differential Diagnosis. Dermatopathology 2021, 8, 130–134. [Google Scholar] [CrossRef]
- Urso, C.; Ginanneschi, C.; Anichini, C.; Paglierani, M.; Saieva, C.; Pimpinelli, N.; Borgognoni, L. Animal-type melanoma: Report of five cases with sentinel node biopsy and fluorescence in-situ hybridization analysis. Melanoma Res. 2014, 24, 47–53. [Google Scholar] [CrossRef]
- Avilés-Izquierdo, J.A.; Leis-Dosil, V.M.; Lázaro-Ochaita, P. Animal-type melanoma: Clinical and dermoscopic features of 3 cases. Actas Dermosifiliogr. 2014, 105, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Vita, G.; Ilardi, G.; Siano, M.; Mascolo, M. Animal type melanoma: An unusual case with aggressive histological features? Pathol. Res. Pract. 2012, 208, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Tarasen, A.; Carlson, J.A.; Leonard, M.K.; Merlino, G.; Kaetzel, D.; Slominski, A.T. Pigmented Epithelioid Melanocytoma (PEM)/Animal Type Melanoma (ATM): Quest for an Origin. Report of One Unusual Case Indicating Follicular Origin and Another Arising in an Intradermal Nevus. Int. J. Mol. Sci. 2017, 18, 1769. [Google Scholar] [CrossRef]
- Leonard, M.K.; Pamidimukkala, N.; Puts, G.S.; Snyder, D.E.; Slominski, A.T.; Kaetzel, D.M. The HGF/SF Mouse Model of UV-Induced Melanoma as an In Vivo Sensor for Metastasis-Regulating Gene. Int. J. Mol. Sci. 2017, 18, 1647. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Yeh, I.; Mully, T.W.; LeBoit, P.E.; McCalmont, T.H. Genomic and Clinicopathologic Characteristics of PRKAR1A-inactivated Melanomas: Toward Genetic Distinctions of Animal-type Melanoma/Pigment Synthesizing Melanoma. Am. J. Surg. Pathol. 2020, 44, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.P.; McGinniss, M.J.; Esmay, P.; Velazquez, E.F.; Reimann, J.D. Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi. Mod. Pathol. 2013, 26, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Arezzo, F.; Colagrande, A.; Cimmino, A.; Lettini, T.; Sablone, S.; Resta, L.; Ingravallo, G. “Animal-Type Melanoma/Pigmented Epithelioid Melanocytoma”: History and Features of a Controversial Entity. Dermatopathology 2021, 8, 271-276. https://doi.org/10.3390/dermatopathology8030033
Cazzato G, Arezzo F, Colagrande A, Cimmino A, Lettini T, Sablone S, Resta L, Ingravallo G. “Animal-Type Melanoma/Pigmented Epithelioid Melanocytoma”: History and Features of a Controversial Entity. Dermatopathology. 2021; 8(3):271-276. https://doi.org/10.3390/dermatopathology8030033
Chicago/Turabian StyleCazzato, Gerardo, Francesca Arezzo, Anna Colagrande, Antonietta Cimmino, Teresa Lettini, Sara Sablone, Leonardo Resta, and Giuseppe Ingravallo. 2021. "“Animal-Type Melanoma/Pigmented Epithelioid Melanocytoma”: History and Features of a Controversial Entity" Dermatopathology 8, no. 3: 271-276. https://doi.org/10.3390/dermatopathology8030033
APA StyleCazzato, G., Arezzo, F., Colagrande, A., Cimmino, A., Lettini, T., Sablone, S., Resta, L., & Ingravallo, G. (2021). “Animal-Type Melanoma/Pigmented Epithelioid Melanocytoma”: History and Features of a Controversial Entity. Dermatopathology, 8(3), 271-276. https://doi.org/10.3390/dermatopathology8030033









