Primary Dermal Melanoma: A Rare Clinicopathological Variant Mimicking Metastatic Melanoma
Abstract
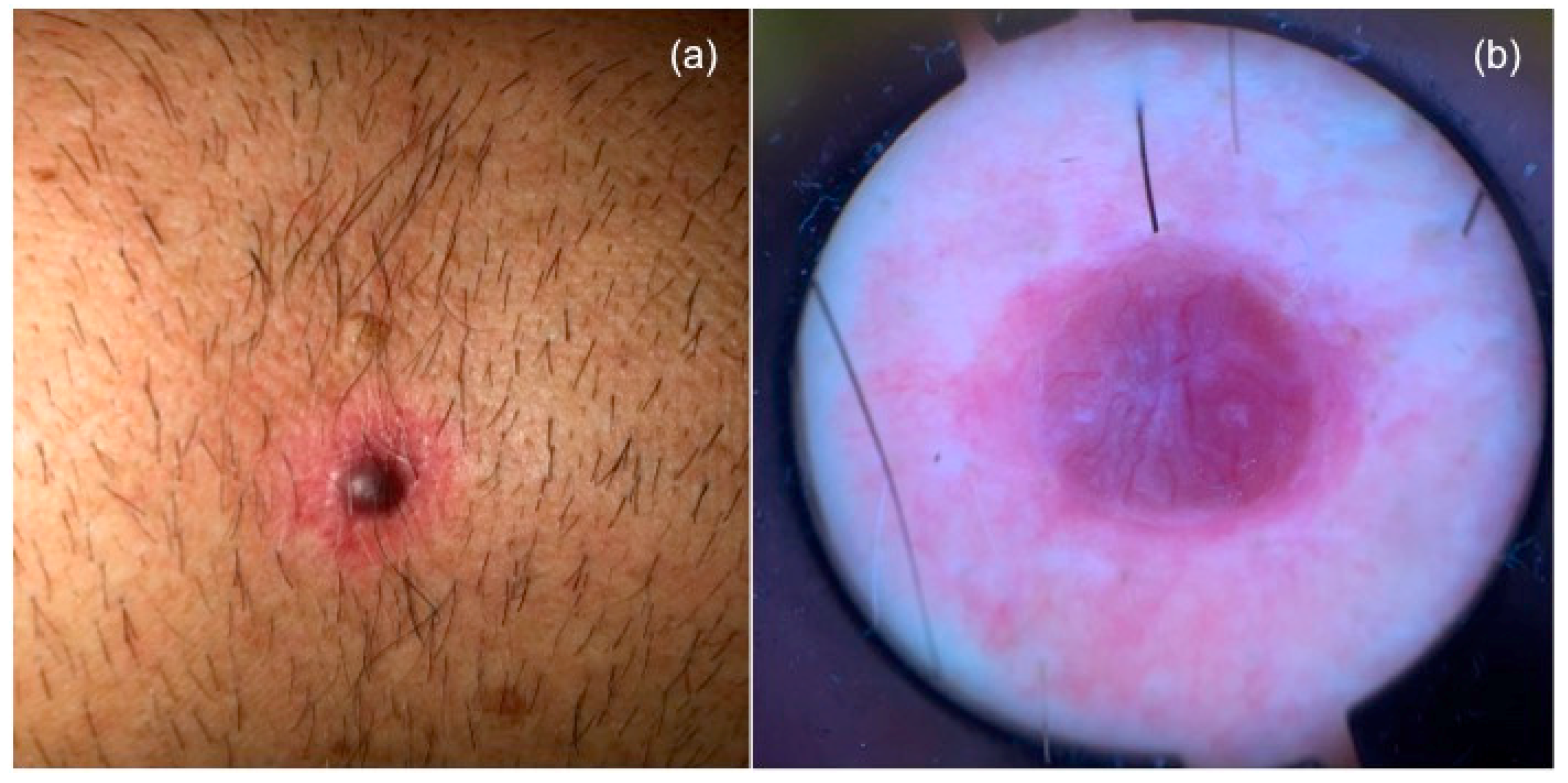
1. Introduction
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Swetter, S.M.; Ecker, P.M.; Johnson, D.L.; Harvell, J.D. Primary dermal melanoma: A distinct subtype of melanoma. Arch. Dermatol. 2004, 140, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Jaros, J.; Hunt, S.; Mose, E.; Lai, O.; Tsoukas, M. Cutaneous metastases: A great imitator. Clin. Dermatol. 2020, 38, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, G.; Sartini, D.; Campanati, A.; Rubini, C.; Molinelli, E.; Brisigotti, V.; Cecati, M.; Pozzi, V.; Campagna, R.; Offidani, A.; et al. Nicotinamide N-methyltransferase: Potential involvement in cutaneous malignant melanoma. Melanoma. Res. 2018, 28, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, M.; Obregon, R.; Cooper, C.; Sholl, L.M.; Guitart, J.; Gerami, P. Primary dermal melanoma: A unique subtype of melanoma to be distinguished from cutaneous metastatic melanoma: A clinical, histologic, and gene expression profiling study. J. Am. Acad. Dermatol. 2014, 71, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, G.; Simonetti, O.; Lazzarini, R.; Giantomassi, F.; Goteri, G.; Offidani, A. Vascular endothelial growth factor/semaphorin-3A ratio and SEMA3A expression in cutaneous malignant melanoma. Melanoma. Res. 2020, 30, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma of the skin. In AJCC Cancer Staging Manual, 8th ed.; Amin, M., Edge, S.B., Greene, F.L., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer: New York, NY, USA, 2017; pp. 563–585. [Google Scholar]
- Pan, Y.; Haydon, A.M.; McLean, C.A.; McDonald, P.B.; Kelly, J.W. Prognosis associated with cutaneous melanoma metastases. Australas. J. Dermatol. 2015, 56, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Faries, M.B.; Ye, X.; Morton, D.L. Solitary dermal melanoma: Beginning or end of the metastatic process? Ann. Surg. Oncol. 2009, 16, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.M.; Chang, A.E.; Lowe, L.; Hamilton, T.; Patel, R.; Johnson, T.M. Solitary melanoma confined to the dermal and/or subcutaneous tissue: Evidence for revisiting the staging classification. Arch. Dermatol. 2000, 136, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, G.; Rubini, C.; Campanati, A.; Zizzi, A.; Molinelli, E.; Rosa, L.; Simonacci, F.; Offidani, A. IL-17, IL-23, and p73 expression in cutaneous melanoma: A pilot study. Melanoma. Res. 2015, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Cabral, E.S.; Kartha, R.V.; Swetter, S.M. Primary dermal melanoma: Distinct immunohistochemical findings and clinical outcome compared with nodular and metastatic melanoma. Arch. Dermatol. 2008, 144, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Shaigany, S.; Tessier-Cloutier, B.; Busam, K.J.; Horst, B.A. Lack of distinct molecular profile of Primary Dermal Melanoma. Hum. Pathol. 2020, 106, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Teow, J.; Chin, O.; Hanikeri, M.; Wood, B.A. Primary dermal melanoma: A West Australian cohort. ANZ J. Surg. 2015, 85, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Zamir, H.; Feinmesser, M.; Gutman, H. Primary Dermal Melanoma (PDM): Histological and Clinical Interdependence to Guide Therapy. J. Cutan. Med. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Doepker, M.P.; Thompson, Z.J.; Harb, J.N.; Messina, J.L.; Puleo, C.A.; Egan, K.M.; Sarnaik, A.A.; Gonzalez, R.J.; Sondak, V.K.; Zager, J.S. Dermal melanoma: A report on prognosis, outcomes, and the utility of sentinel lymph node biopsy. J. Surg. Oncol. 2016, 113, 98–102. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, O.; Molinelli, E.; Brisigotti, V.; Brancorsini, D.; Talevi, D.; Offidani, A. Primary Dermal Melanoma: A Rare Clinicopathological Variant Mimicking Metastatic Melanoma. Dermatopathology 2021, 8, 29-32. https://doi.org/10.3390/dermatopathology8010005
Simonetti O, Molinelli E, Brisigotti V, Brancorsini D, Talevi D, Offidani A. Primary Dermal Melanoma: A Rare Clinicopathological Variant Mimicking Metastatic Melanoma. Dermatopathology. 2021; 8(1):29-32. https://doi.org/10.3390/dermatopathology8010005
Chicago/Turabian StyleSimonetti, Oriana, Elisa Molinelli, Valerio Brisigotti, Donatella Brancorsini, Davide Talevi, and Annamaria Offidani. 2021. "Primary Dermal Melanoma: A Rare Clinicopathological Variant Mimicking Metastatic Melanoma" Dermatopathology 8, no. 1: 29-32. https://doi.org/10.3390/dermatopathology8010005
APA StyleSimonetti, O., Molinelli, E., Brisigotti, V., Brancorsini, D., Talevi, D., & Offidani, A. (2021). Primary Dermal Melanoma: A Rare Clinicopathological Variant Mimicking Metastatic Melanoma. Dermatopathology, 8(1), 29-32. https://doi.org/10.3390/dermatopathology8010005





