Influence of Aging and Diabetes on the Mechanical Properties of Mouse Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Diabetes
2.3. HES Staining
2.4. Atomic Force Microscopy Measurements
2.5. Protein Extraction and Immunoblotting
2.6. Statistical Analysis
3. Results
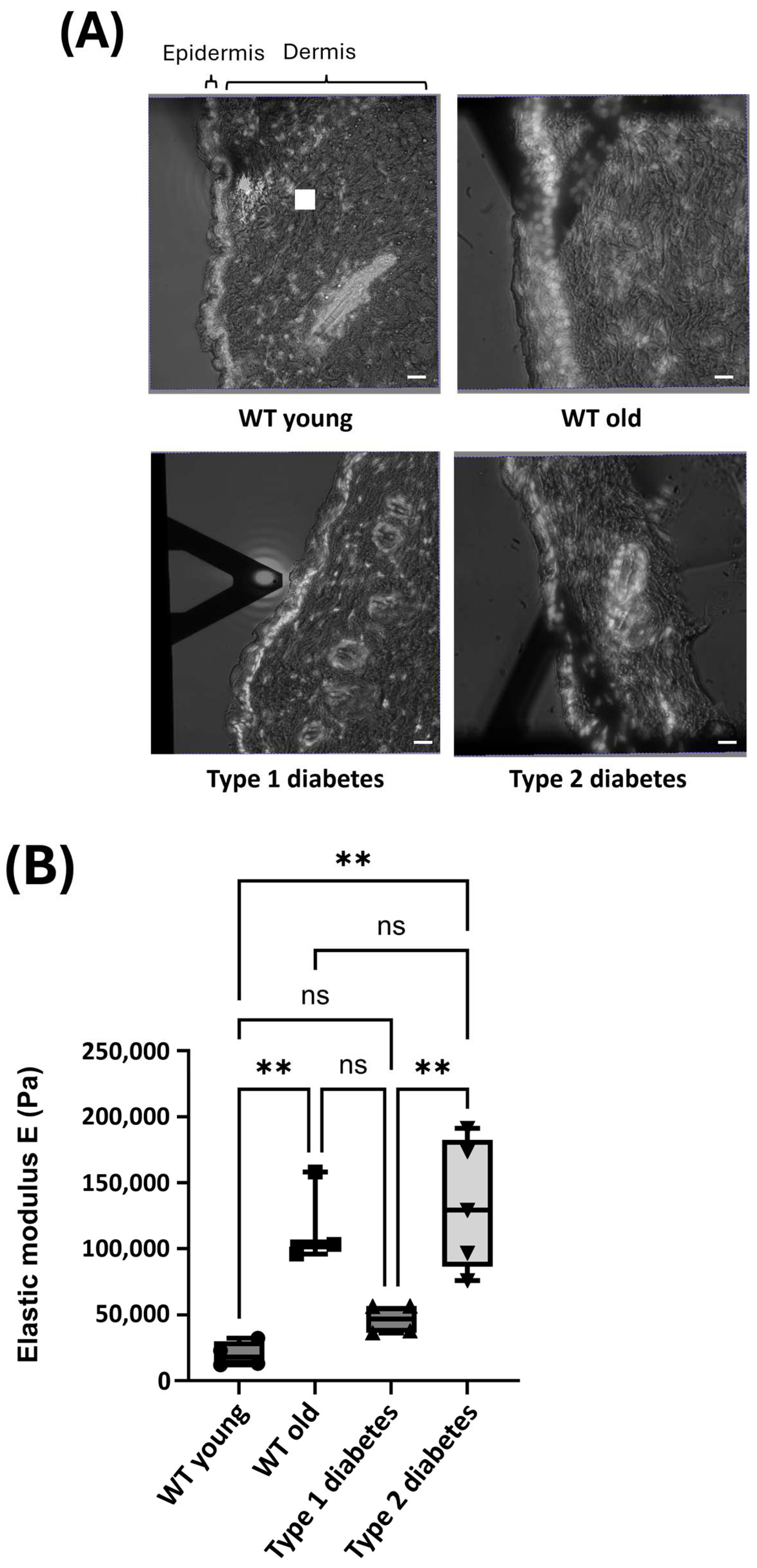
3.1. Mechanical Properties of Skin
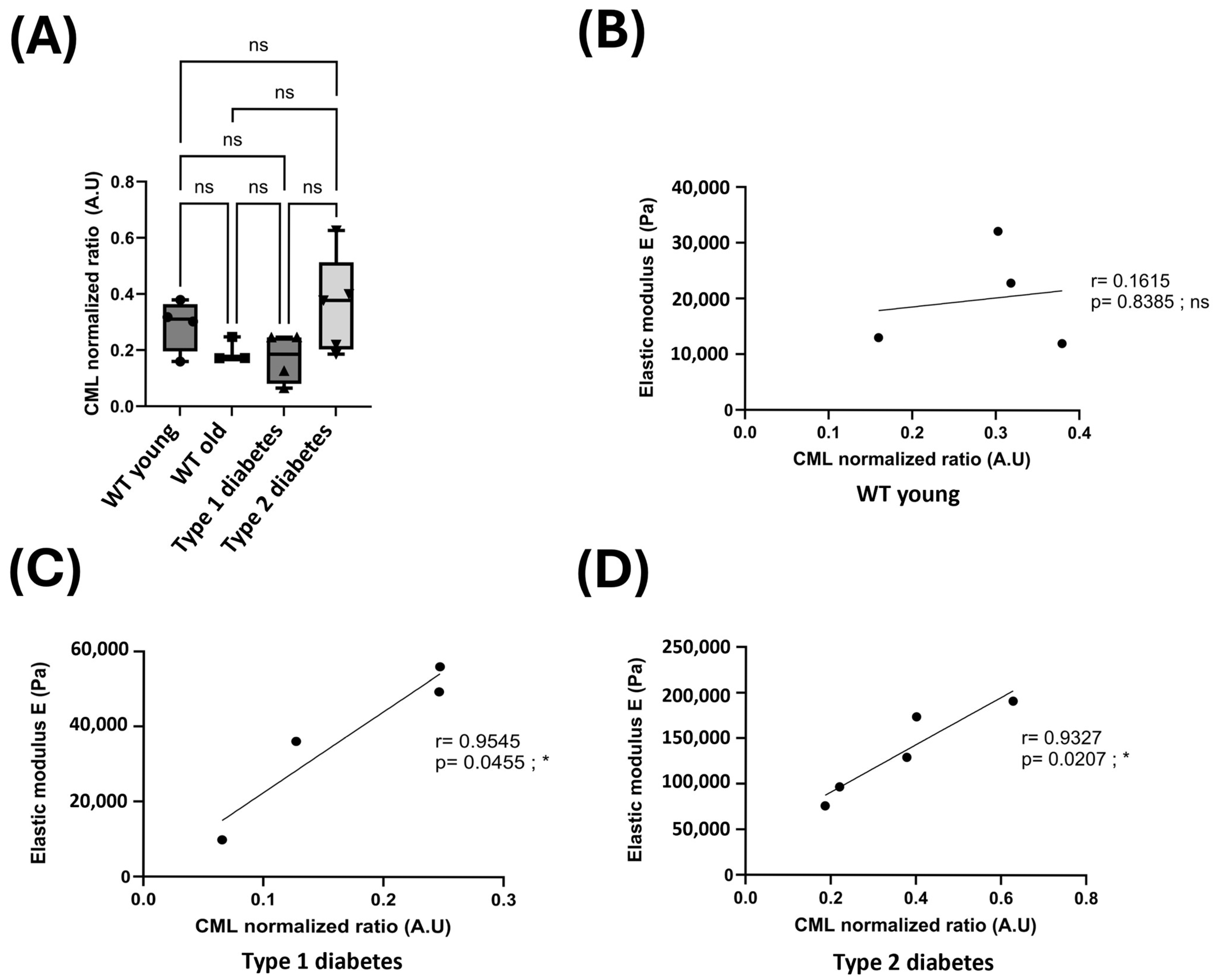
3.2. Glycation and the Mechanical Properties of Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular Matrix |
| AGEs | Advanced Glycation End Products |
| AFM | Atomic Force Microscopy |
References
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Su, W.; Wang, F. Skin Ageing: A Progressive, Multi-Factorial Condition Demanding an Integrated, Multilayer-Targeted Remedy. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- AMendes, L.; Haddad, V.; Miot, H.A. Diabetes mellitus and the skin. An. Bras. Dermatol. 2017, 92, 8–20. [Google Scholar] [CrossRef]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and disease. Drug Metab. Drug Interact. 2008, 23, 125–150. [Google Scholar] [CrossRef]
- Xin, C.; Wang, Y.; Liu, M.; Zhang, B.; Yang, S. Correlation analysis between advanced glycation end products detected noninvasively and skin aging factors. J. Cosmet. Dermatol. 2021, 20, 243–248. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Mera, K.; Takeo, K.; Izumi, M.; Maruyama, T.; Nagai, R.; Otagiri, M. Effect of reactive-aldehydes on the modification and dysfunction of human serum albumin. J. Pharm. Sci. 2010, 99, 1614–1625. [Google Scholar] [CrossRef]
- Kinoshita, S.; Mera, K.; Ichikawa, H.; Shimasaki, S.; Nagai, M.; Taga, Y.; Iijima, K.; Hattori, S.; Fujiwara, Y.; Shirakawa, J.-I. Nω-(Carboxymethyl)arginine is one of the dominant advanced glycation end products in glycated collagens and mouse tissues. Oxidative Med. Cell. Longev. 2019, 2019, 9073451. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Mattos, V.S.; de Oliveira, A.O.; Guimarães, F.E.G.; Bagnato, V.S.; de Castro Neto, J.C. Noninvasive assessments of skin glycated proteins by fluorescence and Raman techniques in diabetics and nondiabetics. J. Biophotonics 2018, 12, e201800162. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard Reaction Products in Skin Collagen in Diabetes and Aging. J. Clin. Investig. 1992, 91, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Mizutari, K.; Ono, T.; Ikeda, K.; Kayashima, K.I.; Horiuchi, S. Photo-enhanced modification of human skin elastin in actinic elastosis by N(ε)-(carboxymethyl)lysine, one of the glycoxidation products of the Maillard reaction. J. Investig. Dermatol. 1997, 108, 797–802. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Fisher, G.J.; Kim, A.J.; Quan, T. Age-related changes in dermal collagen physical properties in human skin. PLoS ONE 2023, 18, e0292791. [Google Scholar] [CrossRef]
- Kamml, J.; Acevedo, C.; Kammer, D.S. Advanced-Glycation Endproducts: How cross-linking properties affect the collagen fibril behavior. J. Mech. Behav. Biomed. Mater. 2023, 148, 106198. [Google Scholar] [CrossRef]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (II): Protein, glycosaminoglycan, water, and lipid content and structure. Ski. Res. Technol. 2006, 12, 145–154. [Google Scholar] [CrossRef]
- Guillon, C.; Ferraro, S.; Clement, S.; Bouschbacher, M.; Sigaudo-Roussel, D.; Bonod, C. Glycation by glyoxal leads to profound changes in the behavior of dermal fibroblasts. BMJ Open Diabetes Res. Care 2021, 9, e002091. [Google Scholar] [CrossRef]
- Laly, A.C.; Sliogeryte, K.; Pundel, O.J.; Ross, R.; Keeling, M.C.; Avisetti, D.; Waseem, A.; Gavara, N.; Connelly, J.T. The keratin network of intermediate filaments regulates keratinocyte rigidity sensing and nuclear mechanotransduction. Sci. Adv. 2021, 7, eabd6187. [Google Scholar] [CrossRef]
- Estrach, S.; Vivier, C.M.; Féral, C.C. ECM and epithelial stem cells: The scaffold of destiny. Front. Cell Dev. Biol. 2024, 12, 1359585. [Google Scholar] [CrossRef]
- Boulter, E.; Estrach, S.; Tissot, F.S.; Hennrich, M.L.; Tosello, L.; Cailleteau, L.; de la Ballina, L.R.; Pisano, S.; Gavin, A.-C.; Féral, C.C. Cell metabolism regulates integrin mechanosensing via an SLC3A2-dependent sphingolipid biosynthesis pathway. Nat. Commun. 2018, 9, 4862. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Peñuela, L.; Negro, C.; Massa, M.; Repaci, E.; Cozzani, E.; Parodi, A.; Scaglione, S.; Quarto, R.; Raiteri, R. Atomic force microscopy for biomechanical and structural analysis of human dermis: A complementary tool for medical diagnosis and therapy monitoring. Exp. Dermatol. 2018, 27, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Prigent, L.; Mercier-Gouy, P.; Bovio, S.; Aubert, A.; Liot, S.; Lambert, E.; Valcourt, U. Analysis of biomechanical properties of mouse skin dermis through atomic force microscopy: Application to demonstrate a sexual dimorphism. Exp. Dermatol. 2023, 32, 1016–1027. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical Atomic Force Microscopy Procedure (SNAP) for Measuring Soft and Biological Samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef]
- Runel, G.; Cario, M.; Lopez-Ramirez, N.; Malbouyres, M.; Ruggiero, F.; Bernard, L.; Puisieux, A.; Caramel, J.; Chlasta, J.; Masse, I. Stiffness measurement is a biomarker of skin ageing in vivo. Exp. Dermatol. 2020, 29, 1233–1237. [Google Scholar] [CrossRef]
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef]
- Sant, S.; Wang, D.; Agarwal, R.; Dillender, S.; Ferrell, N. Glycation alters the mechanical behavior of kidney extracellular matrix. Matrix Biol. Plus 2020, 8, 100035. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef]
- Athmuri, D.N.; Shiekh, P.A. Experimental diabetic animal models to study diabetes and diabetic complications. MethodsX 2023, 11, 102474. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Keyplayers in skin aging? Derm.-Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.J. Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Cao, X.; Song, F.; Xie, T.; Ji, X.; Miao, M.; Dong, J.; Tian, M.; Lin, Y.; Lu, S. Reduced dermis thickness and AGE accumulation in diabetic abdominal skin. Int. J. Low. Extrem. Wounds 2012, 11, 224–230. [Google Scholar] [CrossRef]
- Knoblich, C.; Dunckelmann, K.; Krüger, A.; Küper, T.; Blatt, T.; Weise, J.M. N-acetyl-L-hydroxyproline—A potent skin anti-ageing active preventing advanced glycation end-product formation in vitro and ex vivo. Int. J. Cosmet. Sci. 2023, 46, 297–306. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Jakasa, I.; Riethmüller, C.; Schön, M.P.; Braun, A.; Haftek, M.; Fallon, P.G.; Wróblewski, J.; Jakubowski, H.; Eckhart, L.; et al. Filaggrin Expression and Processing Deficiencies Impair Corneocyte Surface Texture and Stiffness in Mice. J. Investig. Dermatol. 2020, 140, 615–623.e5. [Google Scholar] [CrossRef]
- Homberg, M.; Ramms, L.; Schwarz, N.; Dreissen, G.; Leube, R.E.; Merkel, R.; Hoffmann, B.; Magin, T.M. Distinct Impact of Two Keratin Mutations Causing Epidermolysis Bullosa Simplex on Keratinocyte Adhesion and Stiffness. J. Investig. Dermatol. 2015, 135, 2437–2445. [Google Scholar] [CrossRef]
- Hiermaier, M.; Kugelmann, D.; Radeva, M.Y.; Didona, D.; Ghoreschi, K.; Farzan, S.; Hertl, M.; Waschke, J. Pemphigus Foliaceus Autoantibodies Induce Redistribution Primarily of Extradesmosomal Desmoglein 1 in the Cell Membrane. Front. Immunol. 2022, 13, 882116. [Google Scholar] [CrossRef]
- Krawczyk-Wołoszyn, K.; Żychowska, M.; Reich, A. Evaluation of hair surface structure and morphology of patients with lichen planopilaris (LPP) by atomic force microscopy (AFM). Ski. Res. Technol. 2024, 30, e70030. [Google Scholar] [CrossRef]
- Neshatian, M.; Mittal, N.; Huang, S.; Ali, A.; Khattignavong, E.; Bozec, L. Investigation of dermal collagen nanostructures in Ehlers-Danlos Syndrome (EDS) patients. PLoS ONE 2024, 19, e0307442. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miny, S.; Runel, G.; Chlasta, J.; Bonod, C. Influence of Aging and Diabetes on the Mechanical Properties of Mouse Skin. Dermatopathology 2025, 12, 18. https://doi.org/10.3390/dermatopathology12020018
Miny S, Runel G, Chlasta J, Bonod C. Influence of Aging and Diabetes on the Mechanical Properties of Mouse Skin. Dermatopathology. 2025; 12(2):18. https://doi.org/10.3390/dermatopathology12020018
Chicago/Turabian StyleMiny, Sarah, Gaël Runel, Julien Chlasta, and Christelle Bonod. 2025. "Influence of Aging and Diabetes on the Mechanical Properties of Mouse Skin" Dermatopathology 12, no. 2: 18. https://doi.org/10.3390/dermatopathology12020018
APA StyleMiny, S., Runel, G., Chlasta, J., & Bonod, C. (2025). Influence of Aging and Diabetes on the Mechanical Properties of Mouse Skin. Dermatopathology, 12(2), 18. https://doi.org/10.3390/dermatopathology12020018





