Erythrodermic Psoriasis in the Context of Emerging Triggers: Insights into Dupilumab-Associated and COVID-19-Induced Psoriatic Disease
Abstract
1. Introduction
2. Case Study
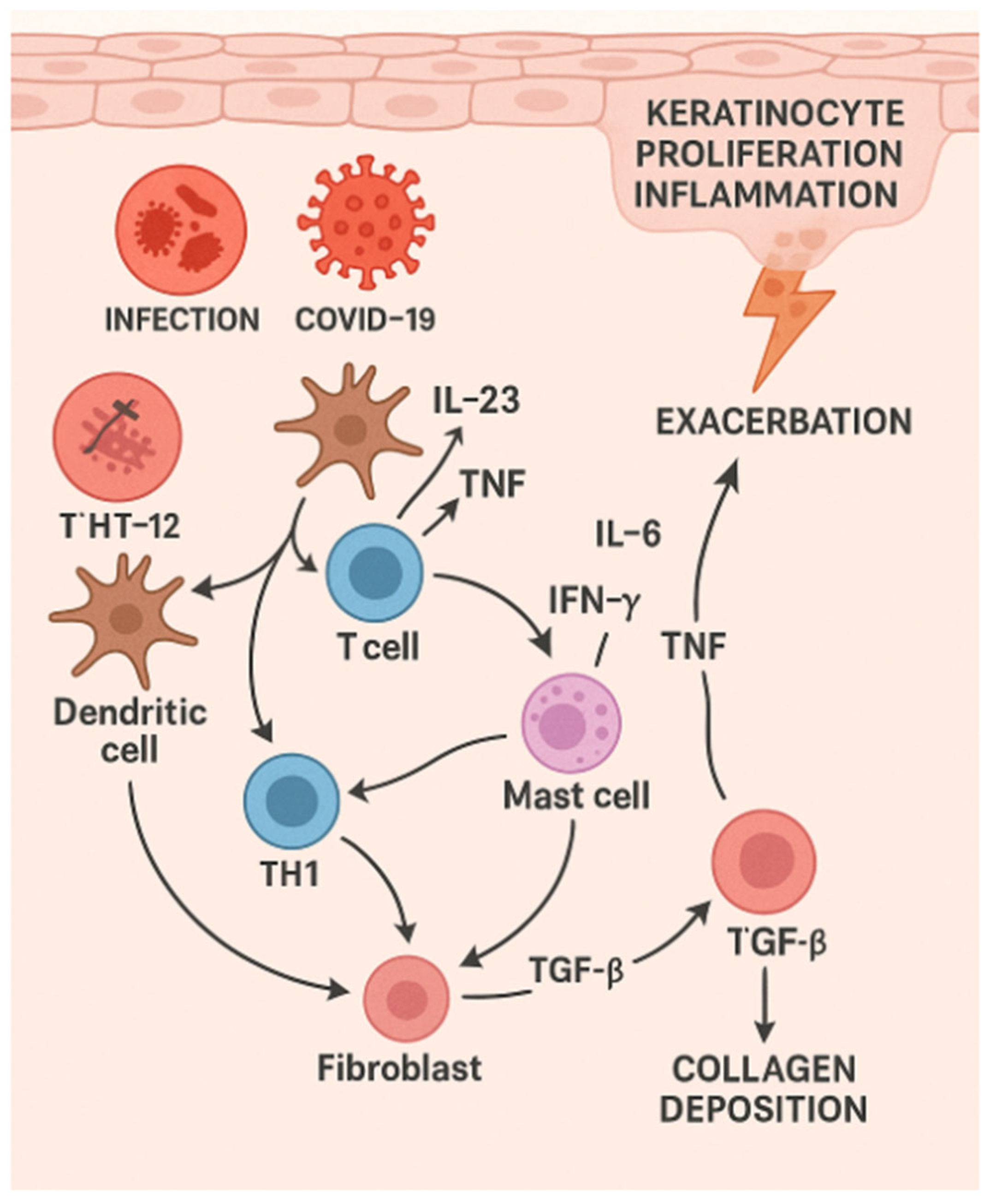
3. SARS-CoV-2 and Psoriasis
4. Psoriasis/Erythrodermic Psoriasis and COVID Vaccines
5. Erythrodermic Psoriasis
6. Treatment Strategies
7. Anticipated Treatments
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TLRs | Toll-like receptors |
| EP | Erythrodermic psoriasis |
| IL | Interleukin |
| TNF | Tumor necrotic factor |
| NF-κB | Nuclear factor kappa B |
| IRFs | Interferon regulatory factors |
| HPA | Hypothalamic–Pituitary–Adrenal |
| CCL2 | Chemokine ligand-2 |
| JAK-STAT | Janus kinases and signal transducer and activators of transcription |
| IFN-γ | Interferon-gamma |
| CRP | C-reactive protein |
| TGF-β | Transforming growth factor-beta |
References
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Boutet, M.A.; Nerviani, A.; Gallo Afflitto, G.; Pitzalis, C. Role of the IL-23/IL-17 Axis in Psoriasis and Psoriatic Arthritis: The Clinical Importance of Its Divergence in Skin and Joints. Int. J. Mol. Sci. 2018, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.P.; Stuart, P.E.; Nistor, I.; Hiremagalore, R.; Chia, N.V.C.; Jenisch, S.; Weichenthal, M.; Abecasis, G.R.; Lim, H.W.; Christophers, E.; et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006, 78, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Bergboer, J.G.; Tjabringa, G.S.; Kamsteeg, M.; van Vlijmen-Willems, I.M.; Rodijk-Olthuis, D.; Jansen, P.A.; Thuret, J.Y.; Narita, M.; Ishida-Yamamoto, A.; Zeeuwen, P.L.; et al. Psoriasis risk genes of the late cornified envelope-3 group are distinctly expressed compared with genes of other LCE groups. Am. J. Pathol. 2011, 178, 1470–1477. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef]
- Takeshita, J.; Shin, D.B.; Ogdie, A.; Gelfand, J.M. Risk of Serious Infection, Opportunistic Infection, and Herpes Zoster among Patients with Psoriasis in the United Kingdom. J. Investig. Dermatol. 2018, 138, 1726–1735. [Google Scholar] [CrossRef]
- Arifin, A.N.F.; Hengky, A.; Widjaja, M.; Wijaya, L. New-Onset and Exacerbation of Psoriasis following COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series. Indian J. Dermatol. 2023, 68, 724. [Google Scholar] [CrossRef] [PubMed]
- Potestio, L.; Battista, T.; Cacciapuoti, S.; Ruggiero, A.; Martora, F.; Fornaro, L.; Camela, E.; Megna, M. New Onset and Exacerbation of Psoriasis Following COVID-19 Vaccination: A Review of the Current Knowledge. Biomedicines 2023, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: Recent therapeutic perspectives. Chem. Biol. Interact. 2021, 344, 109511. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef]
- Kutlu, Ö.; Metin, A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID-19: Will cases of psoriasis increase after COVID-19 pandemic? Dermatol. Ther. 2020, 33, e13383. [Google Scholar] [CrossRef] [PubMed]
- Agharbi, F.Z.; Basri, G.; Nejjari, S.; Chiheb, S. Erythrodermic Psoriasis Following SARS-CoV-2 Infection. Actas Dermosifiliogr. 2024, 115, 202–203. [Google Scholar] [CrossRef]
- Batubara, I.S.; Budianti, W.K. Erythrodermic psoriasis in post-coronavirus disease 2019 patient. Asia Pac. Allergy 2022, 12, e16. [Google Scholar] [CrossRef]
- Martora, F.; Battista, T.; Fabbrocini, G.; Megna, M. Concomitant Severe Psoriasis and Bullous Pemphigoid Induced by COVID-19. Trop. Med. Infect. Dis. 2023, 8, 107. [Google Scholar] [CrossRef]
- Behrangi, E.; Sadeghzadeh-Bazargan, A.; Salimi, N.; Shaka, Z.; Feyz Kazemi, M.H.; Goodarzi, A. Erythrodermic flare-up of psoriasis with COVID-19 infection: A report of two cases and a comprehensive review of literature focusing on the mutual effect of psoriasis and COVID-19 on each other along with the special challenges of the pandemic. Clin. Case Rep. 2022, 10, e05722. [Google Scholar] [CrossRef]
- Demiri, J.; Abdo, M.; Tsianakas, A. Erythroderme Psoriasis nach COVID-19-Erkrankung [Erythrodermic psoriasis after COVID-19]. Hautarzt 2022, 73, 156–159. (In German) [Google Scholar] [CrossRef] [PubMed]
- Janodia, R.P.; Kupetsky, E.A. Guttate Psoriasis Following COVID-19 Infection. Cutis 2022, 109, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Miladi, R.; Janbakhsh, A.; Babazadeh, A.; Aryanian, Z.; Ebrahimpour, S.; Barary, M.; Sio, T.T.; Wollina, U.; Goldust, M.; Mohseni Afsha, Z. Pustular psoriasis flare-up in a patient with COVID-19. J. Cosmet. Dermatol. 2021, 20, 3364–3368. [Google Scholar] [CrossRef]
- Rouai, M.; Rabhi, F.; Mansouri, N.; Jaber, K.; Dhaoui, R. New-onset guttate psoriasis secondary to COVID-19. Clin. Case Rep. 2021, 9, e04542. [Google Scholar] [CrossRef]
- Öncü, I.N.S.; Güler, D.; Gürel, G. Exacerbation of psoriasis following hydroxychloroquine in a patient with suspected COVID-19. Dermatol. Ther. 2021, 34, e14806. [Google Scholar] [CrossRef] [PubMed]
- Burkauskas, J.; Slabadiene, M.; Podlipskyte, A.; Steibliene, V. Factors associated with worsened clinical symptoms of psoriasis and disease-related quality of life during the COVID-19 lockdown: A cross-sectional study. Front. Med. 2023, 10, 1027853. [Google Scholar] [CrossRef]
- Hussain, H.; Paidas, M.J.; Rajalakshmi, R.; Fadel, A.; Ali, M.; Chen, P.; Jayakumar, A.R. Dermatologic Changes in Experimental Model of Long COVID. Microorganisms 2024, 12, 272. [Google Scholar] [CrossRef]
- Elumalai, N.; Hussain, H.; Sampath, N.; Shamaladevi, N.; Hajjar, R.; Druyan, B.Z.; Rashed, A.B.; Ramamoorthy, R.; Kenyon, N.S.; Jayakumar, A.R.; et al. SPIKENET: An Evidence-Based Therapy for Long COVID. Viruses 2024, 16, 838. [Google Scholar] [CrossRef]
- Hussain, H.; Elumalai, N.; Sampath, N.; Shamaladevi, N.; Hajjar, R.; Druyan, B.Z.; Rashed, A.B.; Ramamoorthy, R.; Kenyon, N.S.; Jayakumar, A.R.; et al. Acute and Long COVID Intestinal Changes in an Experimental Model of Coronavirus in Mice. Viruses 2024, 16, 832. [Google Scholar] [CrossRef]
- Paidas, M.J.; Mohamed, A.B.; Norenberg, M.D.; Saad, A.; Barry, A.F.; Colon, C.; Kenyon, N.S.; Jayakumar, A.R. Multi-Organ Histopathological Changes in a Mouse Hepatitis Virus Model of COVID-19. Viruses 2021, 13, 1703. [Google Scholar] [CrossRef]
- Paidas, M.J.; Cosio, D.S.; Ali, S.; Kenyon, N.S.; Jayakumar, A.R. Long-Term Sequelae of COVID-19 in Experimental Mice. Mol. Neurobiol. 2022, 59, 5970–5986. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, R.; Hussain, H.; Ravelo, N.; Sriramajayam, K.; Di Gregorio, D.M.; Paulrasu, K.; Chen, P.; Young, K.; Masciarella, A.D.; Jayakumar, A.R.; et al. Kidney Damage in Long COVID: Studies in Experimental Mice. Biology 2023, 30, 1070. [Google Scholar] [CrossRef] [PubMed]
- Hasannejad, H.; Takahashi, R.; Kimishima, M.; Hayakawa, K.; Shiohara, T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J. Allergy Clin. Immunol. 2007, 120, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Paidas, M.J.; Fadel, A.; Ramamoorthy, R.; Garcia, E.; Saadoon, Z.F.; Casmartino, E.; Mendez, L.; Williams, E.A.; Ruiz-Cordero, R.; et al. Prior viral infection determines the mode and severity of monkeypox virus. Int. J. Infect. Dis. 2023, 131, 95–99. [Google Scholar] [CrossRef]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and intrinsic antiviral immunity in skin. J. Dermatol. Sci. 2014, 75, 159–166. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Carbone, F.; Geijtenbeek, T.B. Langerhans cells and viral immunity. Eur. J. Immunol. 2008, 38, 2377–2385. [Google Scholar] [CrossRef]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 16, 273–279. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Trepanowski, N.; Coleman, E.L.; Melson, G.; Brem, C.E.; Lam, C.S. Erythrodermic psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022, 28, 123–126. [Google Scholar] [CrossRef]
- Karampinis, E.; Papadopoulou, M.M.; Chaidaki, K.; Georgopoulou, K.E.; Magaliou, S.; Roussaki Schulze, A.V.; Bogdanos, D.P.; Zafiriou, E. Plaque Psoriasis Exacerbation and COVID-19 Vaccination: Assessing the Characteristics of the Flare and the Exposome Parameters. Vaccines 2024, 12, 178. [Google Scholar] [CrossRef]
- Nia, A.M.; Silva, M.M.; Spaude, J.; Gonzalez-Fraga, J.D. Erythrodermic psoriasis eruption associated with SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15380. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Pham, N.T.U.; Phan, H.N.; Nguyen, H.T. Generalized erythrodermic psoriasis triggered by vaccination against severe acute respiratory syndrome Coronavirus 2. Dermatol. Ther. 2022, 35, e15464. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.D.; Javed, N.; Upadhyay, S.; Shekhar, R.; Sheikh, A.B. Acute exacerbation of psoriasis after COVID-19 Pfizer vaccination. Proc. (Bayl. Univ. Med. Cent.) 2021, 35, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Onsun, N.; Kaya, G.; Işık, B.G.; Güneş, B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Health Promot. Perspect. 2021, 11, 261–262. [Google Scholar] [CrossRef]
- Patel, H.A.; Revankar, R.R.; Pedroza, S.T.; Graham, S.; Feldman, S.R. The Genetic Susceptibility to Psoriasis and the Relationship of Linked Genes to Our Treatment Options. Int. J. Mol. Sci. 2023, 15, 12310. [Google Scholar] [CrossRef]
- Linh, N.T.T.; Giang, N.H.; Lien, N.T.K.; Trang, B.K.; Trang, D.T.; Ngoc, N.T.; Nghia, V.X.; My, L.T.; Mao, C.V.; Hoang, N.H.; et al. Association of PSORS1C3, CARD14 and TLR4 genotypes and haplotypes with psoriasis susceptibility. Genet. Mol. Biol. 2022, 45, e20220099. [Google Scholar] [CrossRef]
- Carrier, Y.; Ma, H.L.; Ramon, H.E. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: Implications in psoriasis pathogenesis. J. Investig. Dermatol. 2011, 131, 2428–2437. [Google Scholar] [CrossRef]
- Kopp, T. The IL-23/IL-17 axis in inflammatory skin diseases. J. Dermatol. Sci. 2015, 79, 165–172. [Google Scholar]
- Weller, R.B. Psoriasis as a systemic inflammatory disease: The role of cytokines and implications for therapy. Clin. Exp. Dermatol. 2014, 39, 661–668. [Google Scholar]
- Bowcock, A.M.; Krueger, J.G. Getting under the skin: The immunogenetics of psoriasis. Nat. Rev. Immunol. 2005, 5, 699–711. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef]
- Johnson-Huang, L.M.; Lowes, M.A.; Krueger, J.G. Putting together the psoriasis puzzle: An update on developing targeted therapies. Dis. Models Mech. 2012, 5, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Mehta, H. Biological treatment for erythrodermic psoriasis. Expert Opin. Biol. Ther. 2022, 12, 1531–1543. [Google Scholar] [CrossRef]
- Mumoli, N.; Vitale, J.; Gambaccini, L.; Sabatini, S.; Brondi, B.; Cei, M. Erythrodermic psoriasis. QJM 2014, 107, 315. [Google Scholar] [CrossRef]
- Olbrich, H.; Sadik, C.D.; Ludwig, R.J.; Thaçi, D.; Boch, K. Dupilumab in Inflammatory Skin Diseases: A Systematic Review. Biomolecules 2023, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of Psoriasis: A Comprehensive Review of Entire Therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef]
- Papp, K.A.; Menter, M.A.; Abe, M.; Elewski, B.; Feldman, S.R.; Gottlieb, A.B.; Langley, R.; Luger, T.; Thaci, D.; Buonanno, M.; et al. OPT Pivotal 1 and OPT Pivotal 2 investigators. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Results from two randomized, placebo-controlled, phase III trials. Br. J. Dermatol. 2015, 173, 949–961. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaçi, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Wan, T.; Pan, Q.; Duan, J.; Zhang, S.; Wang, R.; Gao, P.; Lv, J.; Wang, H.; Li, D.; et al. Dual-responsive nanocarriers for efficient cytosolic protein delivery and CRISPR-Cas9 gene therapy of inflammatory skin disorders. Sci. Adv. 2024, 10, eadl4336. [Google Scholar] [CrossRef] [PubMed]

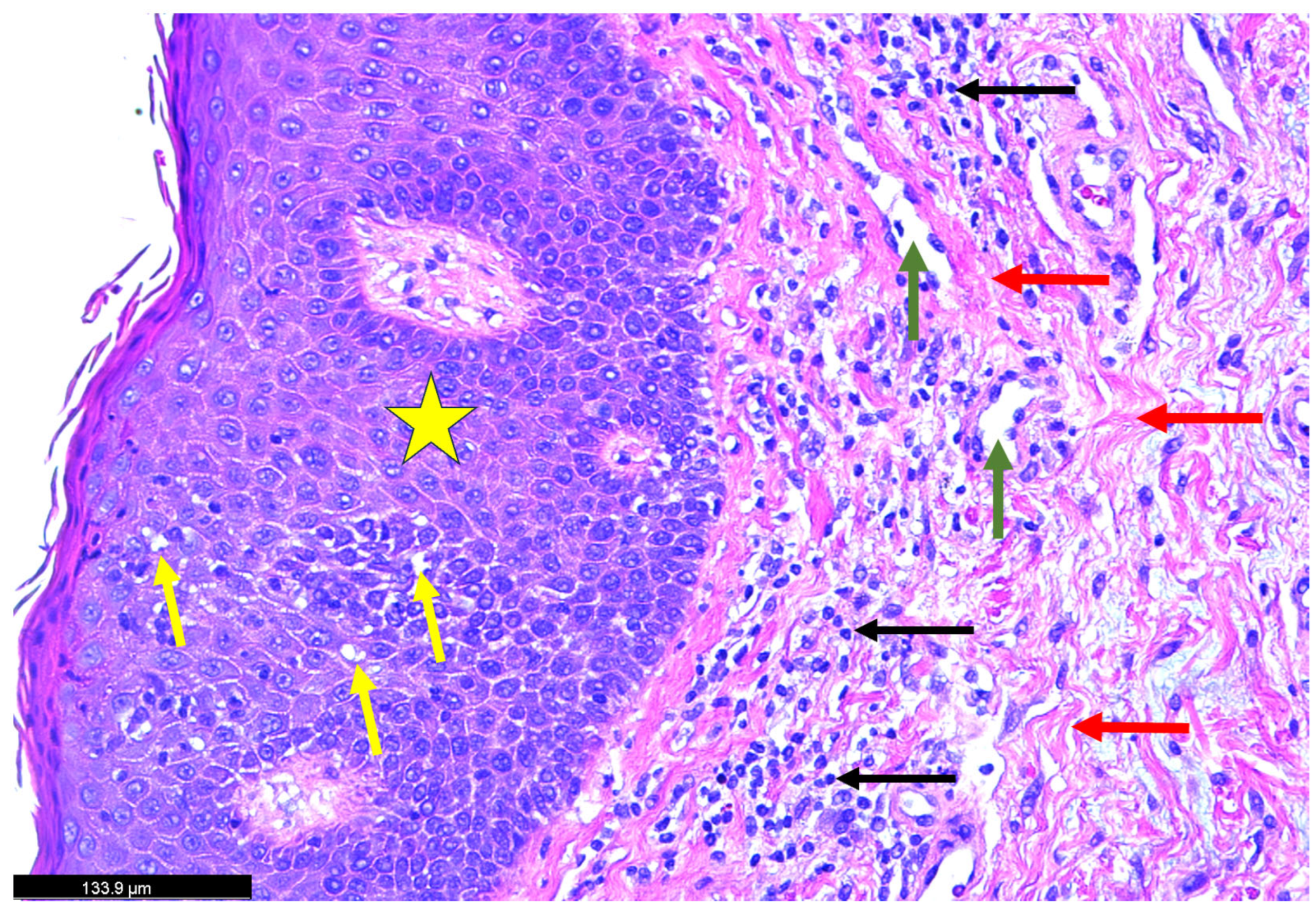
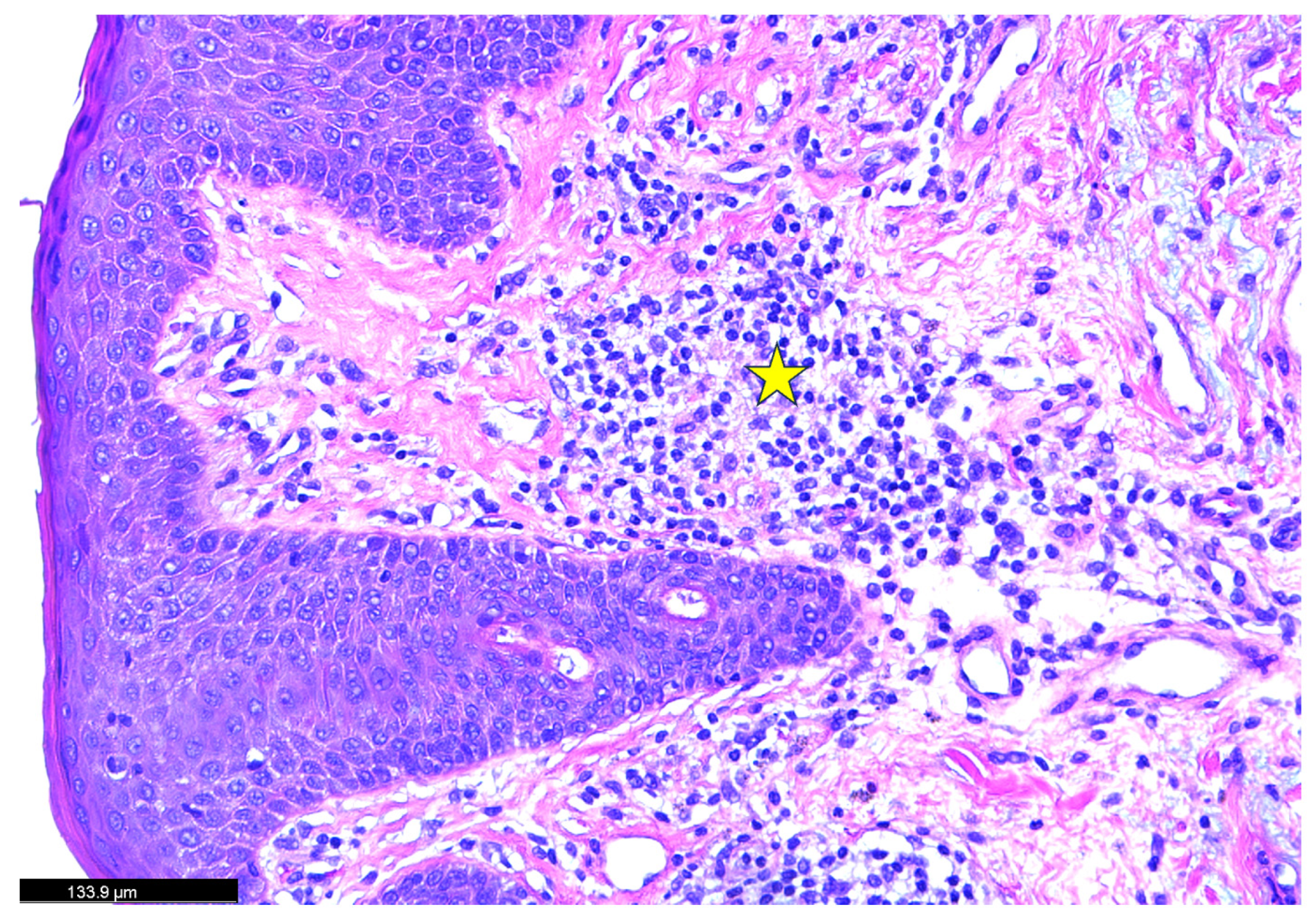
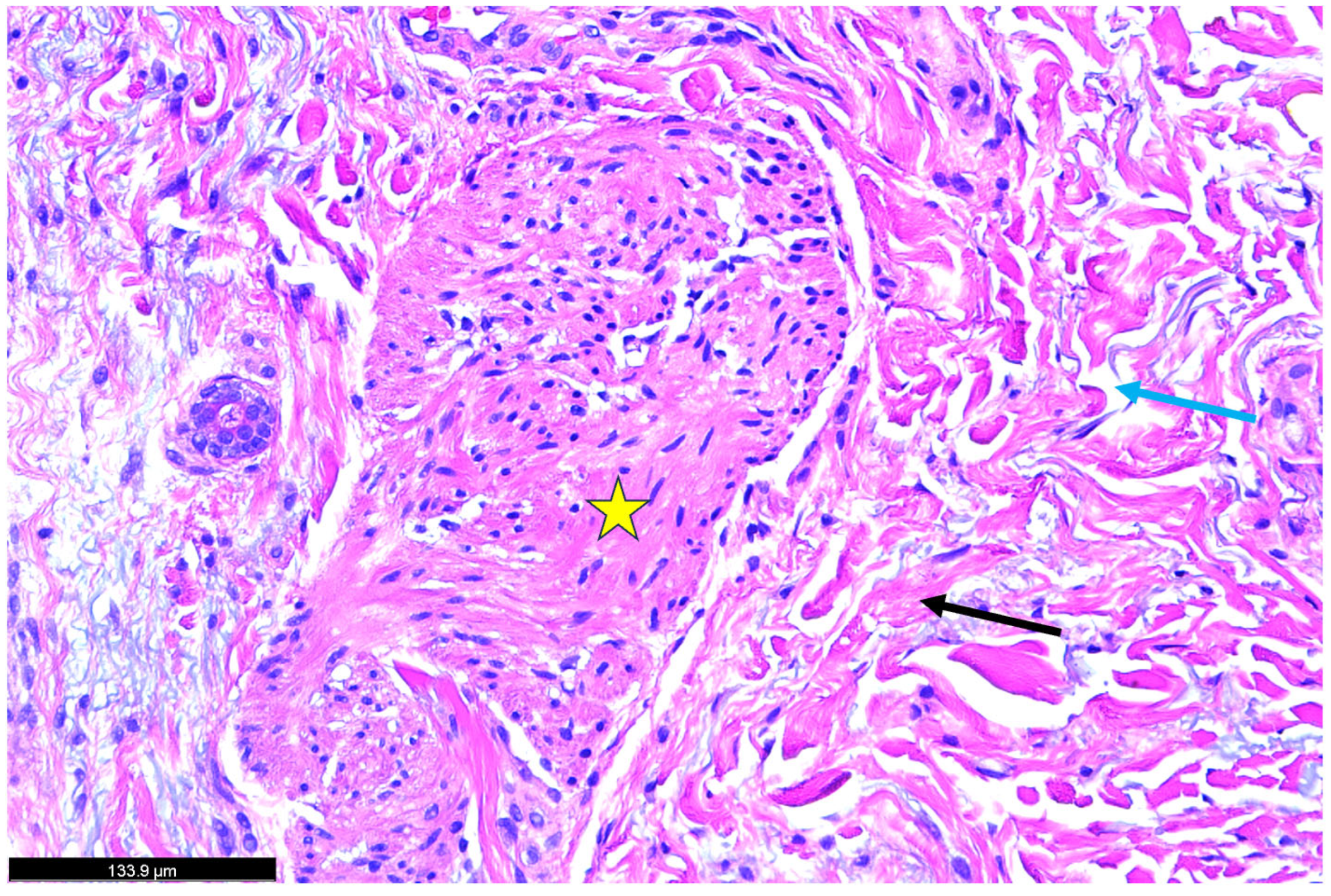

| Authors | Sex | Age | Type of Psoriasis | Concomitant Conditions and Medications | History of Psoriasis |
|---|---|---|---|---|---|
| Kutlu et al. [16] | Female | 71 | Psoriasis vulgaris | Oseltamivir and hydroxychloroquine | Yes |
| Agharbi et al. [17] | Male | 55 | Erythrodermic psoriasis | Dermocorticoids | Yes |
| Batubara et al. [18] | Male | 46 | Erythrodermic psoriasis | Narrowband ultraviolet B phototherapy | Yes |
| Martora et al. [19] | Male | 61 | Psoriasis exacerbation | Topical calcipotriol and betamethasone | Yes |
| Behrangi et al. [20] | 2 cases: Male and Female | Male 50, Female 57 | Male and Female: Erythrodermic psoriasis | Male: None Female: Diabetes, hepatic cirrhosis, insulin, methotrexate | Yes |
| Demiri et al. [21] | Male | 60 | Erythrodermic psoriasis | None | Yes |
| Janodia et al. [22] | Female | 55 | Guttate psoriasis | None | Yes |
| Authors | Sex | Age | Type of Psoriasis | Concomitant Conditions and Medications | History of Psoriasis |
|---|---|---|---|---|---|
| Trepanowski N et al. [39] | Male | 53 | Erythrodermic psoriasis | None | Yes |
| Karampinis E et al. [40] | Male | 56 | Plaque psoriasis exacerbation | Dyslipidemia and diabetes mellitus | No |
| Nila AM et al. [41] | Male | 58 | Erythrodermic psoriasis | Topical corticosteroids | Yes |
| Tran TB et al. [42] | Female | 30 | Erythrodermic psoriasis | None | No |
| Lopez ED et al. [43] | Male | 58 | Erythrodermic psoriasis | Hypertension, psoriasis, prior intravenous drug use with heroine, L4-5 methicillin-resistant Staphylococcus aureus osteomyelitis, untreated hepatitis C, and tobacco | Yes |
| Onsun N et al. [44] | Male | 72 | Erythrodermic psoriasis | Hypertension and psoriasis | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, A.; Nithura, J.; Saadoon, Z.F.; Naseer, L.; Lopez-Lacayo, A.; Rojas Solano, L.E.; Palau Morales, C.; Hernandez, R.J.; Hussain, H. Erythrodermic Psoriasis in the Context of Emerging Triggers: Insights into Dupilumab-Associated and COVID-19-Induced Psoriatic Disease. Dermatopathology 2025, 12, 17. https://doi.org/10.3390/dermatopathology12020017
Fadel A, Nithura J, Saadoon ZF, Naseer L, Lopez-Lacayo A, Rojas Solano LE, Palau Morales C, Hernandez RJ, Hussain H. Erythrodermic Psoriasis in the Context of Emerging Triggers: Insights into Dupilumab-Associated and COVID-19-Induced Psoriatic Disease. Dermatopathology. 2025; 12(2):17. https://doi.org/10.3390/dermatopathology12020017
Chicago/Turabian StyleFadel, Aya, Jayakumar Nithura, Zahraa F. Saadoon, Lamia Naseer, Angelo Lopez-Lacayo, Ligia Elena Rojas Solano, Chaveli Palau Morales, Robert J. Hernandez, and Hussain Hussain. 2025. "Erythrodermic Psoriasis in the Context of Emerging Triggers: Insights into Dupilumab-Associated and COVID-19-Induced Psoriatic Disease" Dermatopathology 12, no. 2: 17. https://doi.org/10.3390/dermatopathology12020017
APA StyleFadel, A., Nithura, J., Saadoon, Z. F., Naseer, L., Lopez-Lacayo, A., Rojas Solano, L. E., Palau Morales, C., Hernandez, R. J., & Hussain, H. (2025). Erythrodermic Psoriasis in the Context of Emerging Triggers: Insights into Dupilumab-Associated and COVID-19-Induced Psoriatic Disease. Dermatopathology, 12(2), 17. https://doi.org/10.3390/dermatopathology12020017






