Cutaneous Metastases—Histological Particularities of Multifaceted Entities
Abstract
1. Introduction
2. Materials and Methods
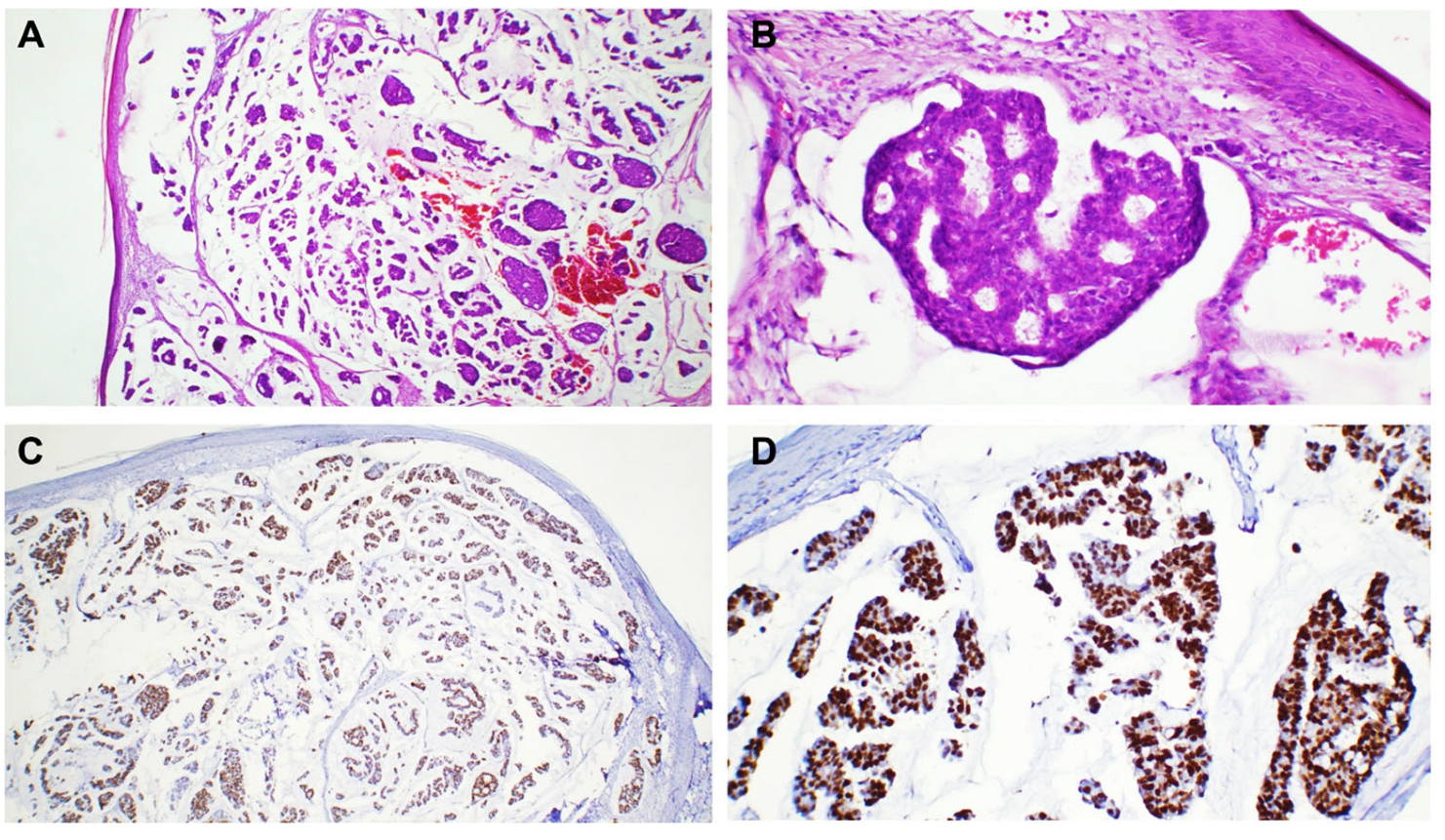
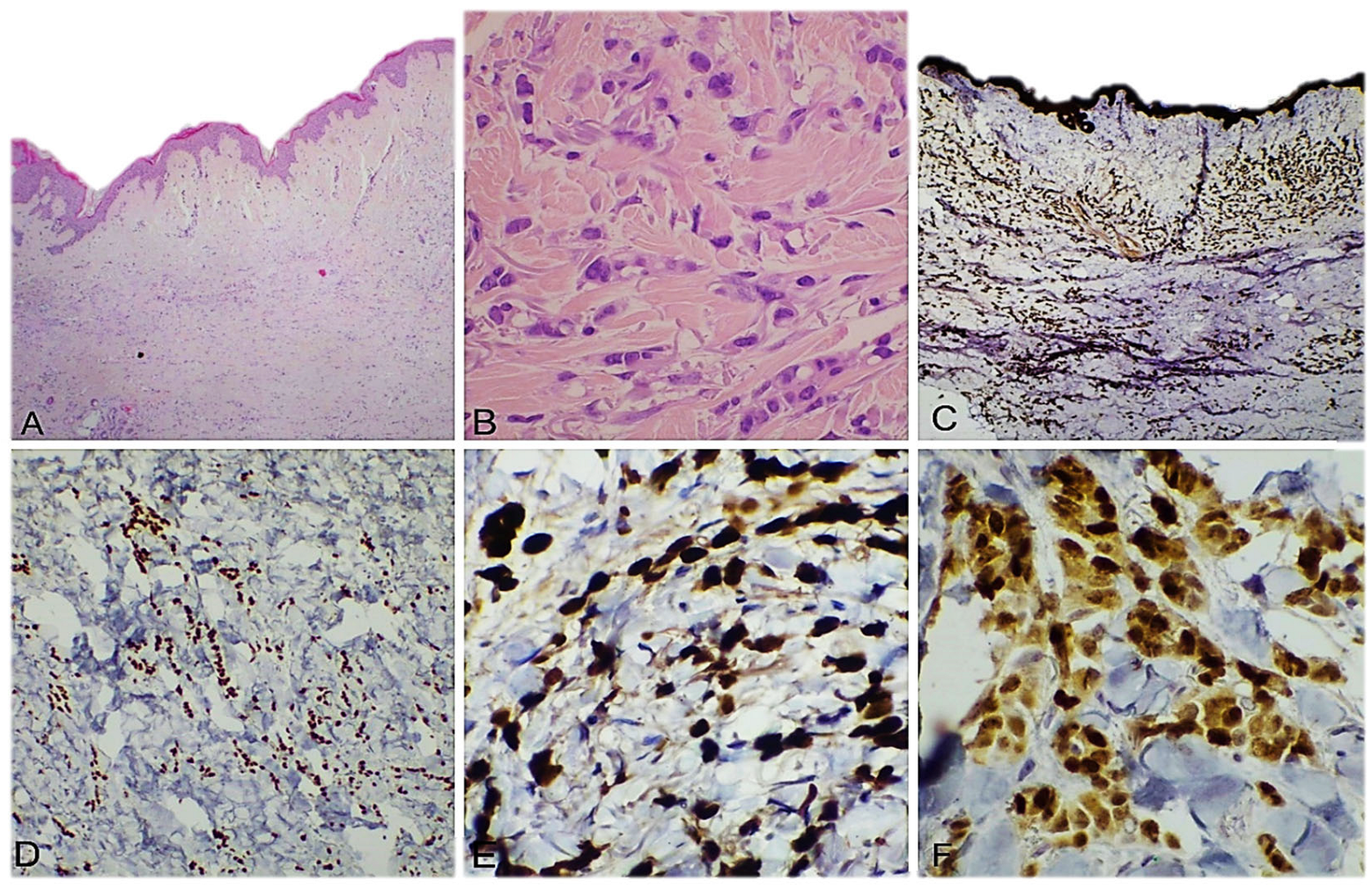
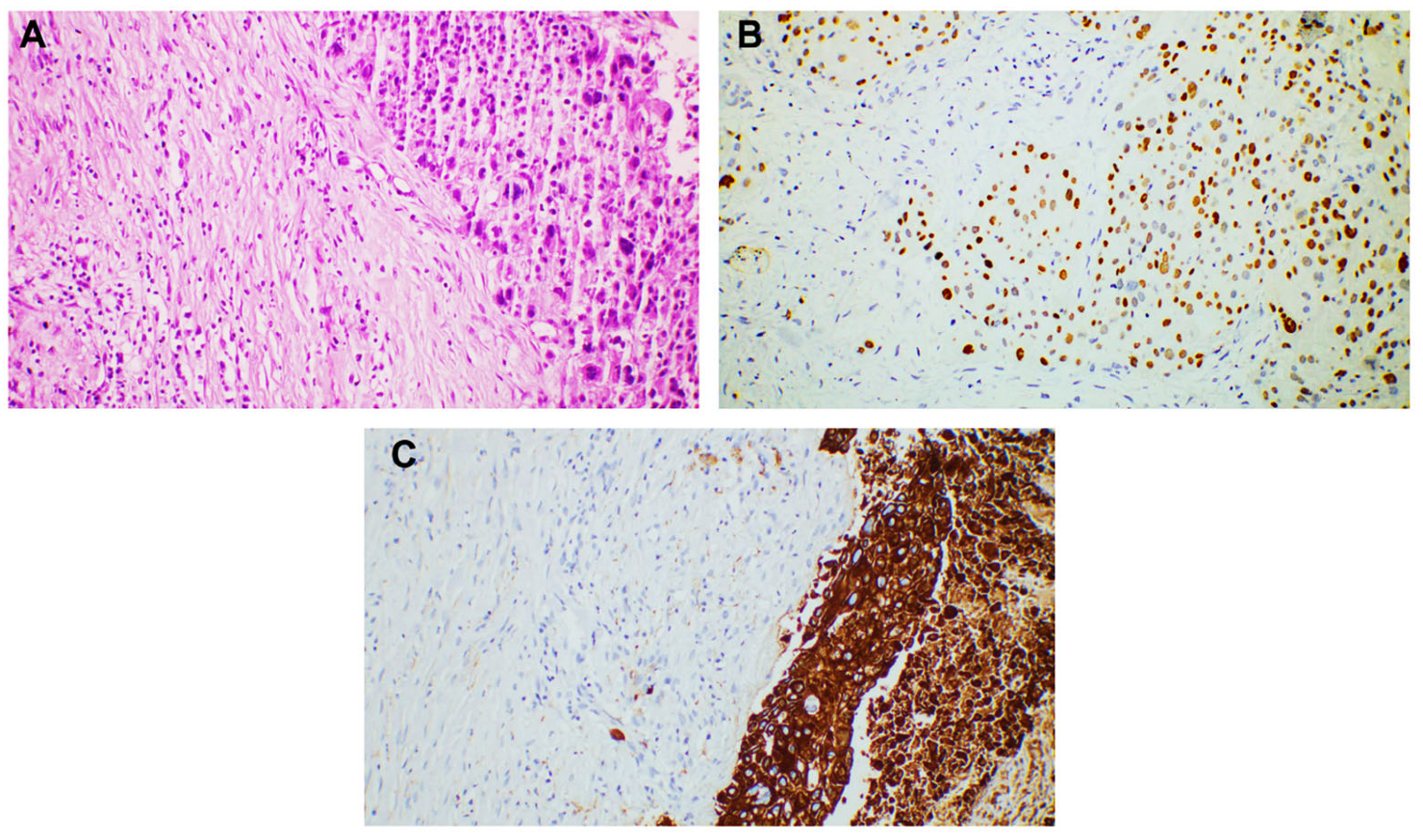
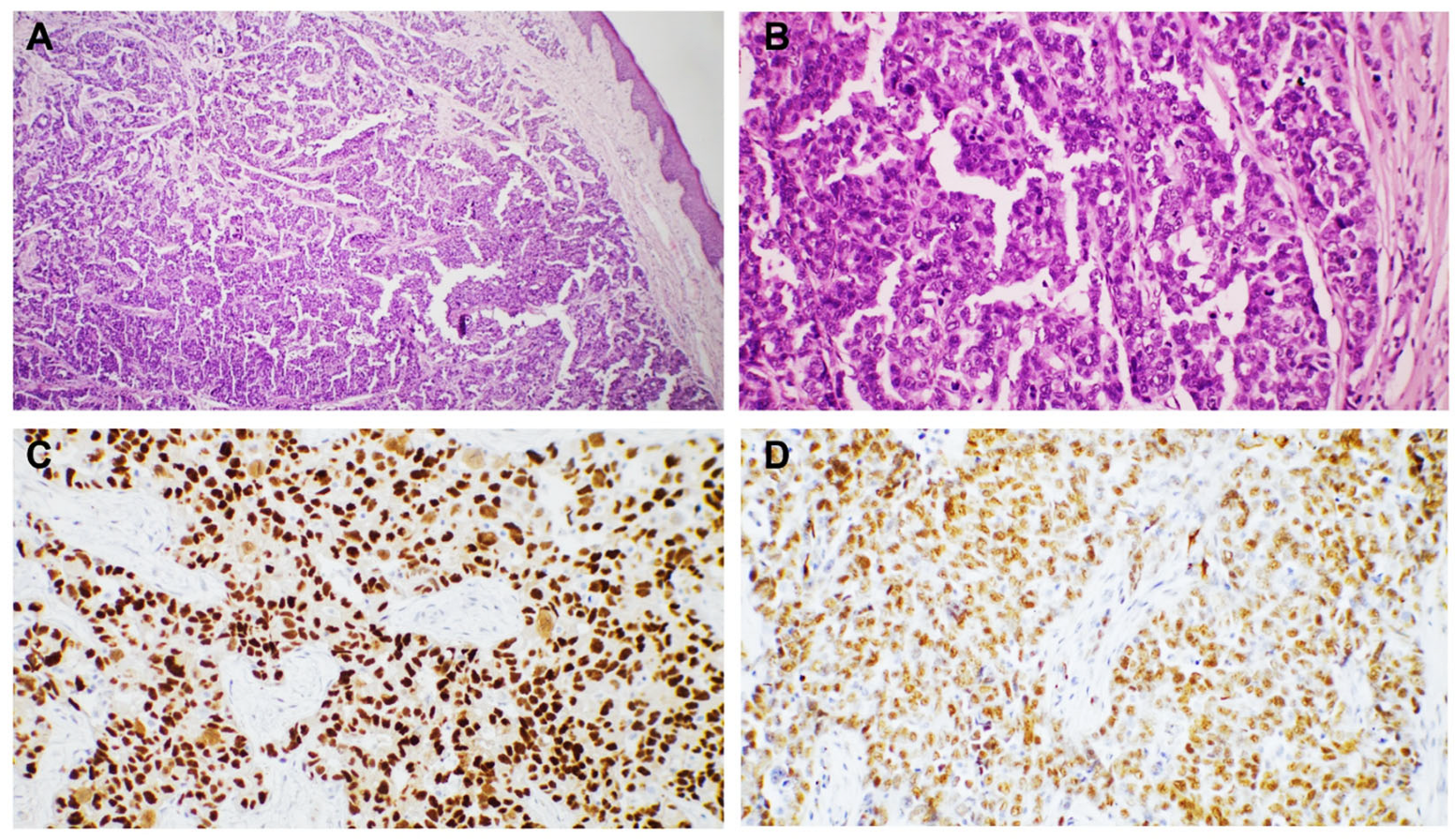
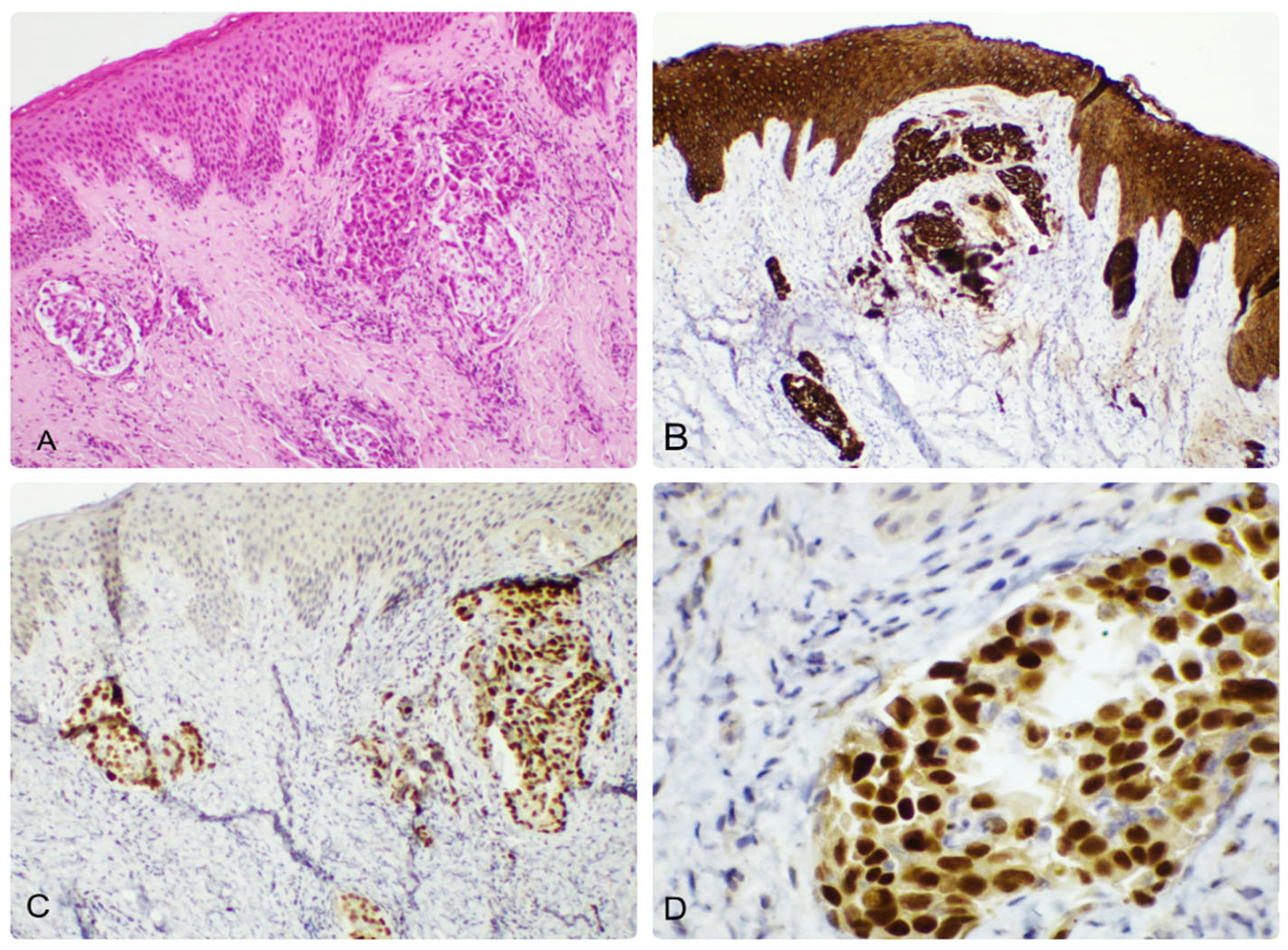
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IHC | Immunohistochemistry |
| HE | Hematoxylin-eosin |
| NST | No special type |
| SCC | Squamous cell carcinoma |
References
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Sampaio, A.L.; Bressan, A.L.; Vasconcelos, B.N.; Gripp, A.C. Skin manifestations associated with systemic diseases—Part I. A Bras. Dermatol. 2021, 96, 655–671. [Google Scholar] [CrossRef]
- Leal, J.M.; de Souza, G.H.; Marsillac, P.F.; Gripp, A.C. Skin manifestations associated with systemic diseases—Part II. A Bras. Dermatol. 2021, 96, 672–687. [Google Scholar] [CrossRef]
- Spratt, D.E.; Gordon Spratt, E.A.; Wu, S.; DeRosa, A.; Lee, N.Y.; Lacouture, M.E.; Barker, C.A. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: A meta-analysis. J. Clin. Oncol. 2014, 32, 3144–3155. [Google Scholar] [CrossRef]
- Abrams, H.L.; Spiro, R.; Goldstein, N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950, 3, 74–85. [Google Scholar] [CrossRef]
- Pipkin, C.A.; Lio, P.A. Cutaneous manifestations of internal malignancies: An overview. Dermatol. Clin. 2008, 26, 1–15. [Google Scholar] [CrossRef]
- Krathen, R.A.; Orengo, I.F.; Rosen, T. Cutaneous metastasis: A meta-analysis of data. South. Med. J. 2003, 96, 164–167. [Google Scholar] [CrossRef]
- Jaros, J.; Hunt, S.; Mose, E.; Lai, O.; Tsoukas, M. Cutaneous metastases: A great imitator. Clin. Dermatol. 2020, 38, 216–222. [Google Scholar] [CrossRef]
- Vernemmen, A.I.P.; Li, X.; Roemen, G.M.J.M.; Speel, E.M.; Kubat, B.; Hausen, A.Z.; Winnepenninckx, V.J.L.; Samarska, I.V. Cutaneous metastases of internal malignancies: A single-institution experience. Histopathology 2022, 81, 329–341. [Google Scholar] [CrossRef]
- Otsuka, I. Cutaneous Metastasis after Surgery, Injury, Lymphadenopathy, and Peritonitis: Possible Mechanisms. Int. J. Mol. Sci. 2019, 20, 3286. [Google Scholar] [CrossRef]
- Sittart, J.A.; Senise, M. Cutaneous metastasis from internal carcinomas: A review of 45 years. Bras. Dermatol. 2013, 88, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A. Cutaneous metastatic disease. J. Am. Acad. Dermatol. 1995, 33, 161–182. [Google Scholar] [CrossRef]
- Brenner, S.; Tamir, E.; Maharshak, N.; Shapira, J. Cutaneous manifestations of internal malignancies. Clin. Dermatol. 2001, 19, 290–297. [Google Scholar] [CrossRef]
- Ruiz, S.J.; Al Salihi, S.; Prieto, V.G.; Nagarajan, P.; Tetzlaff, M.T.; Curry, J.L.; Ivan, D.; Torres-Cabala, C.A.; Aung, P.P. Unusual cutaneous metastatic carcinoma. Ann. Diagn. Pathol. 2019, 43, 151399. [Google Scholar] [CrossRef]
- World Health Organization. Skin Tumours. In Classification of Tumours Editorial Board, 5th ed.; Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2024; Volume 12, Available online: https://publications.iarc.fr (accessed on 23 April 2025).
- World Health Organization. Urinary and Male Genital Tumours. In Classification of Tumours Editorial Board, 5th ed.; Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 8, Available online: https://publications.iarc.fr/610 (accessed on 23 April 2025).
- World Health Organization. Breast Tumours. In Classification of Tumours Editorial Board, 5th ed.; Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 2, Available online: https://publications.iarc.fr/581 (accessed on 23 April 2025).
- World Health Organization. Thoracic Tumours. In Classification of Tumours Editorial Board, 5th ed.; Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2021; Available online: https://publications.iarc.fr/595 (accessed on 23 April 2025).
- Saeed, S.; Keehn, C.A.; Morgan, M.B. Cutaneous metastasis: A clinical, pathological, and immunohistochemical appraisal. J. Cutan. Pathol. 2004, 31, 419–430. [Google Scholar] [CrossRef]
- Lookingbill, D.P.; Spangler, N.; Sexton, F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990, 22, 19–26. [Google Scholar] [CrossRef]
- Hu, S.C.; Chen, G.S.; Lu, Y.W.; Wu, C.S.; Lan, C.C. Cutaneous metastases from different internal malignancies: A clinical and prognostic appraisal. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 735–740. [Google Scholar] [CrossRef]
- Choi, M.E.; Jung, C.J.; Lee, W.J.; Won, C.H.; Chang, S.E.; Choi, J.H.; Lee, M.W. Clinicopathological study of Korean patients with cutaneous metastasis from internal malignancies. Australas. J. Dermatol. 2020, 61, e139–e142. [Google Scholar] [CrossRef]
- Forest, F.; Laville, D.; Habougit, C.; Da Cruz, V.; Casteillo, F.; Yvorel, V.; Bard-Sorel, S.; Godard, W.; Picot, T.; Tiffet, O.; et al. Histopathological and molecular profiling of lung adenocarcinoma skin metastases reveals specific features. Histopathology 2021, 79, 1051–1060. [Google Scholar] [CrossRef]
- Kwon, H.M.; Kim, G.Y.; Shin, D.H.; Bae, Y.K. Clinicopathologic features of cutaneous metastases from internal malignancies. J. Pathol. Transl. Med. 2021, 55, 289–297. [Google Scholar] [CrossRef]
- Nava, G.; Greer, K.; Patterson, J.; Lin, K.Y. Metastatic cutaneous breast carcinoma: A case report and review of the literature. Can. J. Plast. Surg. 2009, 17, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ronen, S.; Suster, D.; Chen, W.S.; Ronen, N.; Arudra, S.K.C.; Trinidad, C.; Ivan, D.; Prieto, V.G.; Suster, S. Histologic Patterns of Cutaneous Metastases of Breast Carcinoma: A Clinicopathologic Study of 232 Cases. Am. J. Dermatopathol. 2021, 43, 401–411. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous manifestations of breast carcinoma. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef]
- Gerratana, L.; Fanotto, V.; Bonotto, M.; Bolzonello, S.; Minisini, A.M.; Fasola, G.; Puglisi, F. Pattern of metastasis and outcome in patients with breast cancer. Clin. Exp. Metastasis 2015, 32, 125–133. [Google Scholar] [CrossRef]
- Pipal, V.R.; Singh, P.; Pipal, D.K.; Elhence, P. Skin Metastases in Ovarian Malignancy: A Case Report with Literature Review. J. Midlife Health 2023, 14, 49–52. [Google Scholar] [CrossRef]
- Otsuka, I. Cutaneous Metastases in Ovarian Cancer. Cancers 2019, 11, 1292. [Google Scholar] [CrossRef]
- Coco, D.; Leanza, S. Cutaneous Metastasis in Ovarian Cancer: Case Report and Literature Review. Maedica 2020, 15, 552–555. [Google Scholar] [CrossRef]
- Thomakos, N.; Diakosavvas, M.; Machairiotis, N.; Fasoulakis, Z.; Zarogoulidis, P.; Rodolakis, A. Rare Distant Metastatic Disease of Ovarian and Peritoneal Carcinomatosis: A Review of the Literature. Cancers 2019, 11, 1044. [Google Scholar] [CrossRef]
- Cheng, H.; Gao, C.; Zhang, R.; Yang, Z.; Zhang, G. Two independent incidences of skin metastases in the umbilicus and abdominal wall in ovarian serous adenocarcinoma: A case report and review of the literature. Medicine 2017, 96, e9118. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Bese, T.; Demirkiran, F.; Ilvan, S.; Sanioglu, C.; Arvas, M.; Kosebay, D. Skin metastasis in ovarian carcinoma. Int. J. Gynecol. Cancer. 2006, 16 (Suppl. S1), 414–418. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.H.; Lee, Y.Y.; Choi, C.H.; Kim, T.J.; Lee, J.W.; Lee, J.H.; Bae, D.S.; Kim, B.G. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: A case report and literature review. Cancer Res. Treat. 2012, 44, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, C.; Liu, P.; Yan, Z.; Xing, D.; Liu, A. Cutaneous metastasis of carcinomatous component of ovarian carcinosarcoma: A case report and review of the literature. Diagn. Pathol. 2022, 17, 76. [Google Scholar] [CrossRef]
- Köbel, M.; Kang, E.Y. The Many Uses of p53 Immunohistochemistry in Gynecological Pathology: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual9 Meeting. Int. J. Gynecol. Pathol. 2021, 40, 32–40. [Google Scholar] [CrossRef]
- Bates, M.; Mohamed, B.M.; Lewis, F.; O’Toole, S.; O’Leary, J.J. Biomarkers in high grade serous ovarian cancer. Biochim. Biophys. Acta Rev. Cancer. 2024, 1879, 189224. [Google Scholar] [CrossRef]
- Sajitha, K.; Arumugam, M.; Shetty, J.; Shetty, R.A.; Asani, R.; Shetty, P.D. Breast Carcinoma—A Comparative Study of Immunohistochemistry and Fluorescence in Situ Hybridization for Her-2 Assessment and Association of ER, PR, HER-2 and Ki-67 Expression with Clinico-Pathological Parameters. Iran. J. Pathol. 2022, 17, 435–442. [Google Scholar] [CrossRef]
- Mueller, T.J.; Wu, H.; Greenberg, R.E.; Hudes, G.; Topham, N.; Lessin, S.R.; Uzzo, R.G. Cutaneous metastases from genitourinary malignancies. Urology 2004, 63, 1021–1026. [Google Scholar] [CrossRef]
- Hayoune, Z.; Ramdani, M.; Tahri, Y.; Barki, A. Late Cutaneous Metastases of Bladder Urothelial Carcinoma: A Case Report. Cureus 2023, 15, e38038. [Google Scholar] [CrossRef]
- Wong, S.Y.; Hynes, R.O. Lymphatic or hematogenous dissemination: How does a metastatic tumor cell decide? Cell Cycle 2006, 5, 812–817. [Google Scholar] [CrossRef]
- Brownstein, M.H.; Helwig, E.B. Metastatic tumors of the skin. Cancer 1972, 29, 1298–1307. [Google Scholar] [CrossRef]
- Erdemır, A.T.; Atılganoglu, U.; Onsun, N.; Somay, A. Cutaneous metastases from gastric adenocarcinoma. Indian J. Dermatol. 2011, 56, 236–237. [Google Scholar] [CrossRef]
- Kemal, Y.; Odabaşı, E.A.; Kemal, Ö.; Bakırtaş, M. Cutaneous metastasis of colon adenocarcinoma. Turk. J. Surg. 2018, 34, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, R.; Zhang, R. Cutaneous metastasis of a colon adenocarcinoma presenting as an unusual manifestation: A report of one case. Int. J. Clin. Exp. Pathol. 2020, 13, 1897–1901. [Google Scholar] [PubMed]
- Liu, P.; Tan, F.; Liu, H.; Ge, J.; Liu, S.; Lei, T.; Zhao, X. Skin Metastasis of Gastrointestinal Stromal Tumors: A Case Series and Literature Review. Cancer Manag. Res. 2020, 12, 7681–7690. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef]





| Estrogen (ER) | anti-Estrogen Receptor (ER) (SP1) Rabbit Monclonal Primary Antibody, VENTANA |
| Progesteron (PR) | anti-Progesterone Receptor (PR) (1E2) Rabbit Monoclonal Primary Antibody),VENTANA |
| GATA3 | GATA3 (L50-823) Mouse Monoclonal Primary Antibody, VENTANA |
| E-Cadherin | E-Cadherin (EP700Y), Rabbit Monoclonal Antibody, CELL MARQUE |
| CTK AE1/AE3 | (PCK26),Anti-Pan Keratin (AE1/AE3/PCK26) Primary Antibody), VENTANA |
| Cytokeratin 7 (CK7) | (SP52), Rabbit Monclonal Antibody) |
| Cytokeratin 20 (CK20) | (SP33), Rabbit Monclonal Primary Antibody), VENTANA |
| CDX2 | EPR2764Y)) Rabbit Monoclonal Antibody),VENTANA |
| TTF1 | SP141, Rabbit Monoclonal Antibody) VENTANA |
| p40 | anti- p40 (BC28) Mouse Monoclonal Primary Antibody,), VENTANA |
| CD56 | anti-CD56 (123C3) Mouse Monoclonal Primary Antibody, VENTANA |
| CD10 | anti-CD10 (SP67) Rabbit Monclonal Primary Antibody, VENTANA |
| CD 117 | PATHWAY Anti-c-KIT (9.7) Primary Antibody, VENTANA |
| PAX-8 | Anti-PAX 8, anti-PAX 8 (MRQ-50) Mouse Monoclonal Antibody, CELL MARQUE |
| Site of Primary Malignancy | Number of Cutaneous Metastases | ||||||
|---|---|---|---|---|---|---|---|
| Chest | Trunk | Scalp | Shoulder | Abdomen | Upper Extremity | Total | |
| Breast | 4 | 0 | 0 | 0 | 0 | 2 | 6 |
| Lung | 3 | 1 | 0 | 1 | 0 | 2 | 7 |
| Gastrointestinal tract | 0 | 1 | 0 | 1 | 2 | 1 | 5 |
| Urinary tract | 1 | 0 | 2 | 0 | 1 | 1 | 5 |
| Reproductive tract | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Total | 8 | 3 | 2 | 2 | 4 | 6 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinca, A.C.; Lazar, B.A.; Cozac-Szőke, A.R.; Radu, G.N.; Simion, S.P.; Chiorean, D.M.; Kosovski, I.B.; Sabău, A.H.; Niculescu, R.; Cocuz, I.G.; et al. Cutaneous Metastases—Histological Particularities of Multifaceted Entities. Dermatopathology 2025, 12, 14. https://doi.org/10.3390/dermatopathology12020014
Tinca AC, Lazar BA, Cozac-Szőke AR, Radu GN, Simion SP, Chiorean DM, Kosovski IB, Sabău AH, Niculescu R, Cocuz IG, et al. Cutaneous Metastases—Histological Particularities of Multifaceted Entities. Dermatopathology. 2025; 12(2):14. https://doi.org/10.3390/dermatopathology12020014
Chicago/Turabian StyleTinca, Andreea Cătălina, Bianca Andreea Lazar, Andreea Raluca Cozac-Szőke, Georgian Nicolae Radu, Simina Petra Simion, Diana Maria Chiorean, Irina Bianca Kosovski, Adrian Horațiu Sabău, Raluca Niculescu, Iuliu Gabriel Cocuz, and et al. 2025. "Cutaneous Metastases—Histological Particularities of Multifaceted Entities" Dermatopathology 12, no. 2: 14. https://doi.org/10.3390/dermatopathology12020014
APA StyleTinca, A. C., Lazar, B. A., Cozac-Szőke, A. R., Radu, G. N., Simion, S. P., Chiorean, D. M., Kosovski, I. B., Sabău, A. H., Niculescu, R., Cocuz, I. G., Hagău, R.-D., Szasz, E. A., Turdean, S. G., & Cotoi, O. S. (2025). Cutaneous Metastases—Histological Particularities of Multifaceted Entities. Dermatopathology, 12(2), 14. https://doi.org/10.3390/dermatopathology12020014










