Congenital Melanocytic Nevus with Neurocristic Cutaneous Hamartoma: A Case Report
Abstract
:1. Introduction
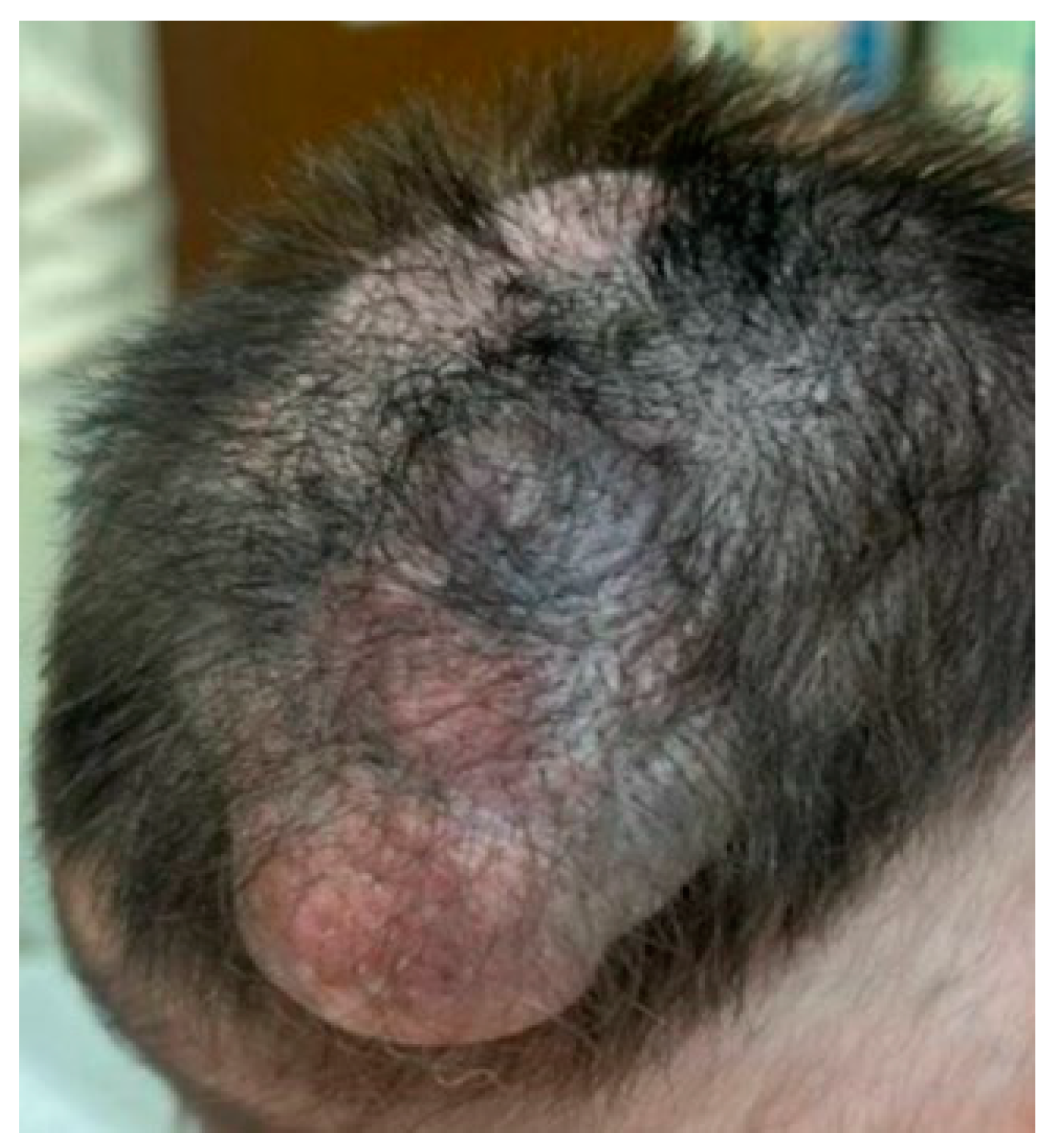
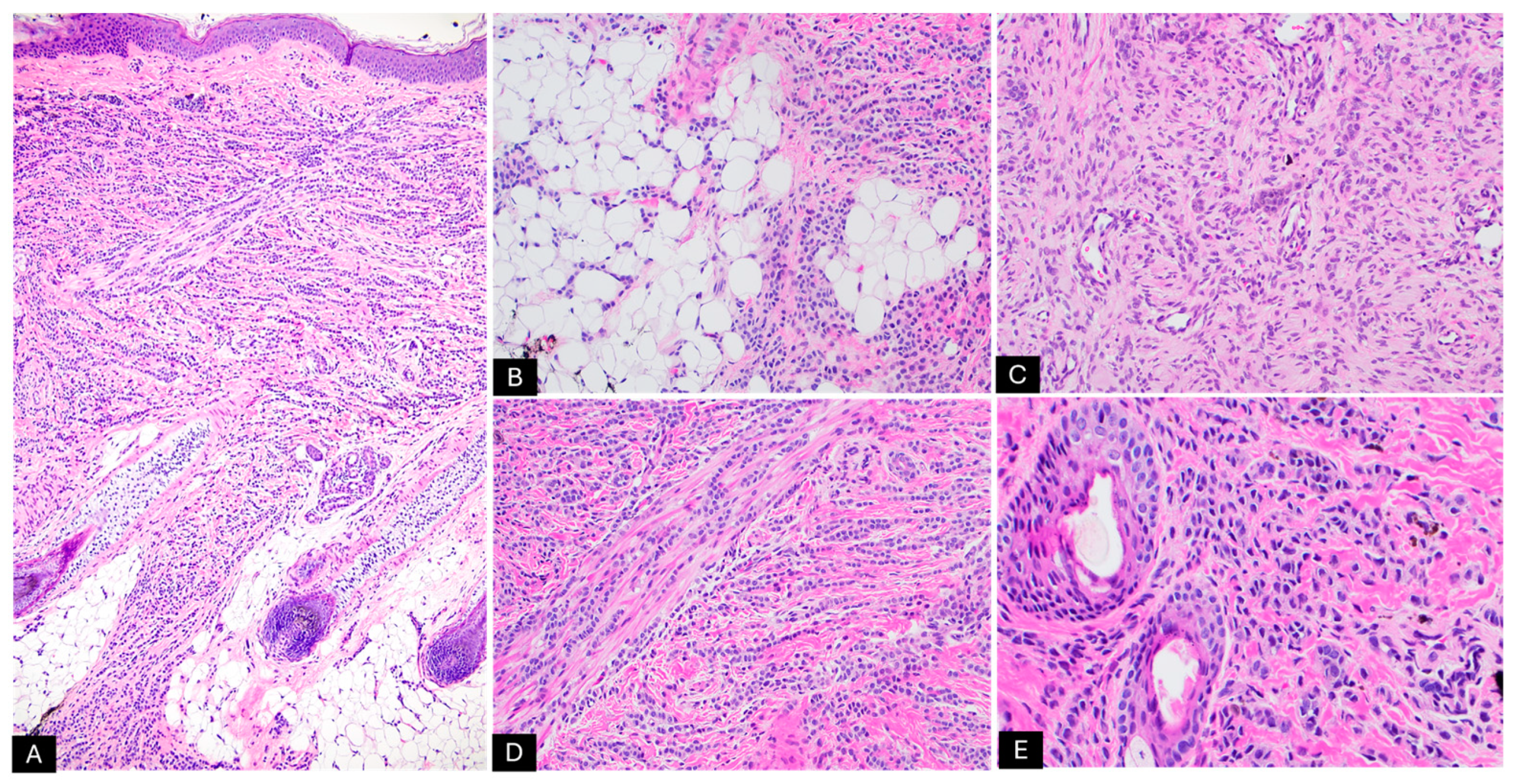
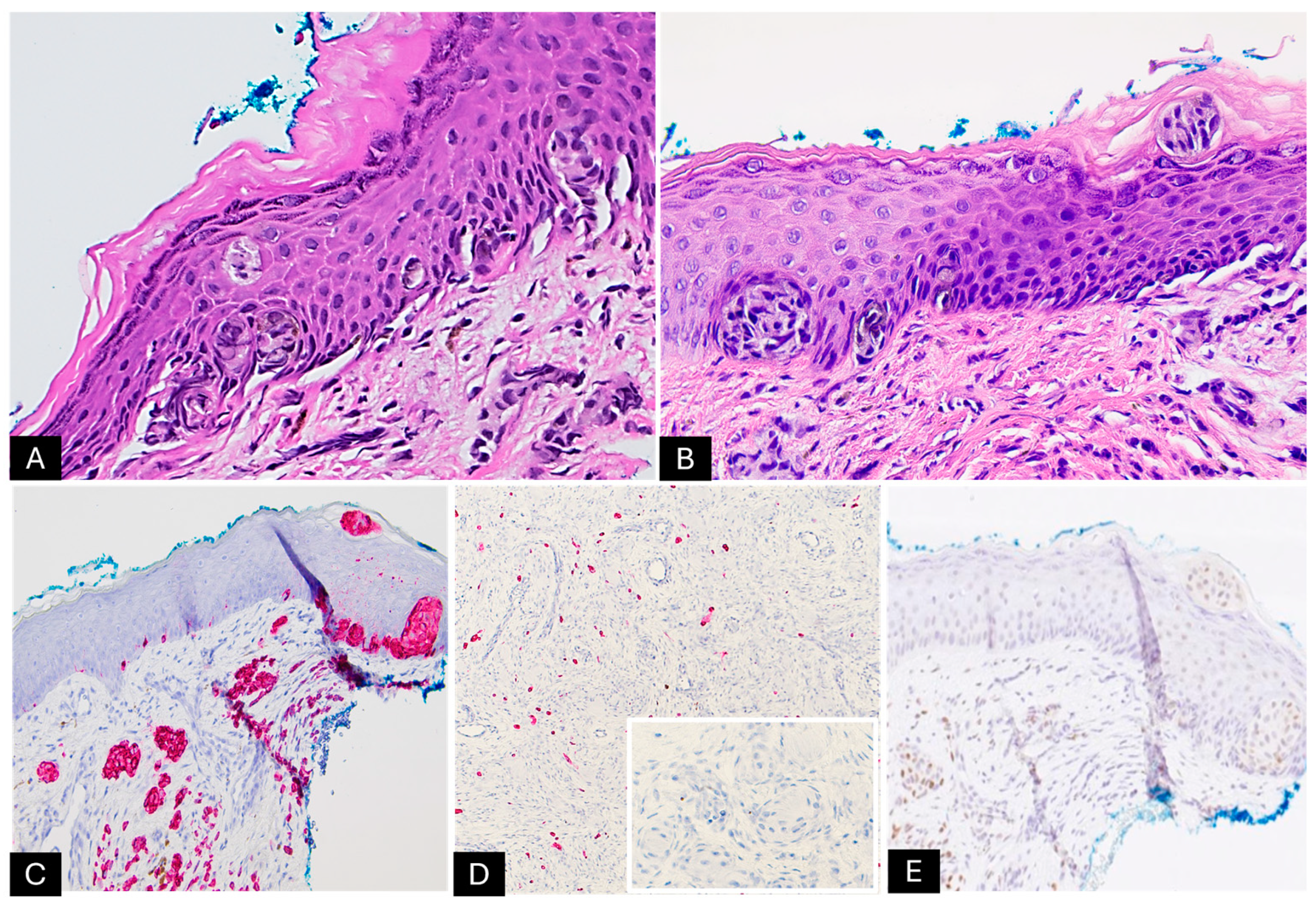
2. Case Presentation
3. Review of Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BN | Blue nevus |
| CMN | Congenital melanocytic nevus |
| GCN | Giant congenital nevus |
| NCH | Nodular proliferative neurocristic cutaneous hamartoma |
| NGS | Next generation sequencing |
References
- Dupin, E. Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells. Biol. Aujourdhui 2011, 205, 53–61. [Google Scholar] [CrossRef]
- Wong, J.; Roy, S.F.; Kokta, V. Neurocristic Cutaneous Hamartoma with Perineuriomatous Differentiation: Can It Be Distinguished From Perineuriomatous Melanocytic Nevi? Am. J. Dermatopathol. 2021, 43, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 1387. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.M.; Tomás-Velázquez, A.; Reyes-Múgica, M. Congenital melanocytic neoplasms: Clinical, histopathological and recent molecular developments. Virchows Arch. 2025, 486, 165–176. [Google Scholar] [CrossRef]
- Kopf, A.W.; Bart, R.S.; Hennessey, P. Congenital nevocytic nevi and malignant melanomas. J. Am. Acad. Dermatol. 1979, 1, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Belysheva, T.S.; Vishnevskaya, Y.V.; Nasedkina, T.V.; Emelyanova, M.A.; Abramov, I.S.; Orlova, K.V.; Lubchenko, L.N.; Utyashev, I.A.; Doroshenko, M.B.; Demidov, L.V.; et al. Melanoma arising in a Giant congenital melanocytic nevus: Two case reports. Diagn. Pathol. 2019, 14, 21. [Google Scholar] [CrossRef]
- Pitjadi, T.M.; Wadee, R.; Grayson, W. Rhabdomyosarcoma Arising in a Giant Congenital Melanocytic Naevus: Case Report and Literature Review. Dermatopathology 2019, 6, 91–98. [Google Scholar] [CrossRef]
- Leech, S.N.; Bell, H.; Leonard, N.; Jones, S.L.; Geurin, D.; McKee, P.H.; Lawrence, C.M. Neonatal giant congenital nevi with proliferative nodules: A clinicopathologic study and literature review of neonatal melanoma. Arch. Dermatol. 2004, 140, 83–88. [Google Scholar] [CrossRef]
- Kinsler, V.A.; O’Hare, P.; Bulstrode, N.; Calonje, J.E.; Chong, W.K.; Hargrave, D.; Jacques, T.; Lomas, D.; Sebire, N.J.; Slater, O. Melanoma in congenital melanocytic naevi. Br. J. Dermatol. 2017, 176, 1131–1143. [Google Scholar] [CrossRef]
- Salgado, C.M.; Basu, D.; Nikiforova, M.; Bauer, B.S.; Johnson, D.; Rundell, V.; Grunwaldt, L.J.; Reyes-Múgica, M. BRAF mutations are also associated with neurocutaneous melanocytosis and large/giant congenital melanocytic nevi. Pediatr. Dev. Pathol. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Martin, S.B.; Polubothu, S.; Bruzos, A.L.; Kelly, G.; Horswell, S.; Sauvadet, A.; Bryant, D.; Zecchin, D.; Riachi, M.; Michailidis, F.; et al. Mosaic BRAF Fusions Are a Recurrent Cause of Congenital Melanocytic Nevi Targetable by MAPK Pathway Inhibition. J. Investig. Dermatol. 2024, 144, 593–600.e597. [Google Scholar] [CrossRef]
- Garrido, M.C.; Maroñas-Jiménez, L.; Ruano, Y.; Rodriguez-Peralto, J.L. Proliferating Neurocristic Hamartoma Arising in a Giant Congenital Nevus: Comparative Genomic Hybridization Findings. Am. J. Dermatopathol. 2019, 41, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Terry, J. Gene Expression Patterns in a Congenital Neurocristic Hamartoma With Multiple Proliferative Nodules. J. Cutan. Pathol. 2025, 52, 85–91. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Liu, X.; Tu, P. The association of BRAF V600E gene mutation with proliferative activity and histopathological characteristics of congenital melanocytic nevi in children. An. Bras. Dermatol. 2023, 98, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, R.J.; Clark, W.H.; Levene, A. Pilar neurocristic hamartoma: Its relationship to blue nevus and equine melanotic disease. Arch. Dermatol. 1982, 118, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Kletskaya, I.; Belousova, I.; Makarova, O.; Narbutov, A.; Oganesyan, R.; Donati, M.; Říčař, J.; Salgado, C.M.; Reyes-Múgica, M.; Kazakov, D.V. Schwannian and Perineuriomatous Differentiation in a Series of Giant Congenital Melanocytic Nevi. Am. J. Dermatopathol. 2024, 46, 483–491. [Google Scholar] [CrossRef]
- Clements, S.A.; Kelley, B.F.; Rivera, L.; Greenway, H.T. Neurotropic melanoma arising from a neurocristic hamartoma. J. Cutan. Pathol. 2023, 50, 197–200. [Google Scholar] [CrossRef]
- Finberg, A.; Martin, S.; Gradecki, S.; Zlotoff, B.; Raghavan, S.S. Cutaneous Neurocristic Hamartoma Mimicking Basal Cell Carcinoma in a Patient With Xeroderma Pigmentosum. Am. J. Dermatopathol. 2022, 44, e54–e56. [Google Scholar] [CrossRef]
- Wilson, L.M.; Beasley, K.J.; Sorrells, T.C.; Johnson, V.V. Congenital neurocristic cutaneous hamartoma with poliosis: A case report. J. Cutan. Pathol. 2017, 44, 974–977. [Google Scholar] [CrossRef]
- Zembowicz, A.; Mihm, M.C. Dermal dendritic melanocytic proliferations: An update. Histopathology 2004, 45, 433–451. [Google Scholar] [CrossRef]
- Bevona, C.; Tannous, Z.; Tsao, H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J. Am. Acad. Dermatol. 2003, 49, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Scolyer, R.; Taylor, K. Melanocytic Tumors of the Skin; AFIP, Ed.; American Registry of Pathology: Rockville, MD, USA, 2024; Volume 19, p. 272. [Google Scholar]
- Pearson, J.P.; Weiss, S.W.; Headington, J.T. Cutaneous malignant melanotic neurocristic tumors arising in neurocristic hamartomas. A melanocytic tumor morphologically and biologically distinct from common melanoma. Am. J. Surg. Pathol. 1996, 20, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Turel, M.K.; Chacko, G.; Raja, A.; Scheithauer, B.W. Neurocristic cutaneous hamartoma of the scalp. J. Pediatr. Neurosci. 2012, 7, 181–184. [Google Scholar] [CrossRef]
- Smith, K.J.; Mezebish, D.; Williams, J.; Elgart, M.L.; Skelton, H.G. The spectrum of neurocristic cutaneous hamartoma: Clinicopathologic and immunohistochemical study of three cases. Ann. Diagn. Pathol. 1998, 2, 213–223. [Google Scholar] [CrossRef]
- Sleiman, R.; Kurban, M.; Succaria, F.; Abbas, O. Poliosis circumscripta: Overview and underlying causes. J. Am. Acad. Dermatol. 2013, 69, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Kini, U.A.; Kini, H. Cutaneous neurocristic hamartoma presenting as cutis verticis gyrata. Am. J. Dermatopathol. 2014, 36, e66–e69. [Google Scholar] [CrossRef]
- Linskey, K.R.; Dias-Santagata, D.; Nazarian, R.M.; Le, L.P.; Lam, Q.; Bellucci, K.S.; Robinson-Bostom, L.; Mihm, M.C.; Hoang, M.P. Malignant neurocristic hamartoma: A tumor distinct from conventional melanoma and malignant blue nevus. Am. J. Surg. Pathol. 2011, 35, 1570–1577. [Google Scholar] [CrossRef]
- Bastian, B.C.; Xiong, J.; Frieden, I.J.; Williams, M.L.; Chou, P.; Busam, K.; Pinkel, D.; LeBoit, P.E. Genetic changes in neoplasms arising in congenital melanocytic nevi: Differences between nodular proliferations and melanomas. Am. J. Pathol. 2002, 161, 1163–1169. [Google Scholar] [CrossRef]
- Gill, P.; Prieto, V.G.; Austin, M.T.; Giubellino, A.; Torres-Cabala, C.A. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J. Cutan. Pathol. 2021, 48, 1410–1415. [Google Scholar] [CrossRef]
- Conrad, D.M.; Chaplin, A.; Walsh, N.M.; Pasternak, S. Extensive neurocristic hamartoma with bone marrow involvement. Am. J. Dermatopathol. 2010, 32, 486–488. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.C. Neurocristic cutaneous hamartoma of the scalp. Ann. Dermatol. 2009, 21, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Kuryan, P.; Gupta, A.K.; Thomas, M. Dermoscopy of Cutaneous Neurocristic Hamartoma and Report of its Rare Clinical Presentation. Dermatol. Pract. Concept. 2022, 12, e2022138. [Google Scholar] [CrossRef] [PubMed]
- Mezebish, D.; Smith, K.; Williams, J.; Menon, P.; Crittenden, J.; Skelton, H. Neurocristic cutaneous hamartoma: A distinctive dermal melanocytosis with an unknown malignant potential. Mod. Pathol. 1998, 11, 573–578. [Google Scholar] [PubMed]
- Tahir, M.; Samman, A.; Shalin, S.; Knowles, K.; Herrera, G.A.; Liles, J.S.; Phung, T. Malignant Cutaneous Neurocristic Hamartoma With Features of Melanoma: A Rare Entity. Cureus 2024, 16, e69594. [Google Scholar] [CrossRef]
- Denlinger, C.E.; Slingluff, C.L.; Mihm, M.C.; Patterson, J.W. Extensive neurocristic hamartoma with skeletal muscle involvement. J. Cutan. Pathol. 2007, 34, 634–639. [Google Scholar] [CrossRef]
- Karamitopoulou-Diamantis, E.; Paredes, B.; Vajtai, I. Cutaneous neurocristic hamartoma with blue naevus-like features and plexiform dermal hyperneury. Histopathology 2006, 49, 326–328. [Google Scholar] [CrossRef]
- Fernández-Eire, P.; Betancor-Santos, M.A.; Zulaica Garate, A.; Concheiro Guisan, A. Neurocristic Cutaneous Hamartoma and Mohs Surgery in Pediatric Patients. Actas Dermosifiliogr. 2023, 114, 740–742. [Google Scholar] [CrossRef]
- Kumar, V.; Vashishta, M.; Kong, L.; Wu, X.; Lu, J.J.; Guha, C.; Dwarakanath, B.S. The Role of Notch, Hedgehog, and Wnt Signaling Pathways in the Resistance of Tumors to Anticancer Therapies. Front. Cell Dev. Biol. 2021, 9, 650772. [Google Scholar] [CrossRef]
- Petronic-Rosic, V.; Shea, C.R.; Krausz, T. Pagetoid melanocytosis: When is it significant? Pathology 2004, 36, 435–444. [Google Scholar] [CrossRef]
- de la Fouchardiere, A. Blue naevi and the blue tumour spectrum. Pathology 2023, 55, 187–195. [Google Scholar] [CrossRef]
- Pathy, A.L.; Helm, T.N.; Elston, D.; Bergfeld, W.F.; Tuthill, R.J. Malignant melanoma arising in a blue nevus with features of pilar neurocristic hamartoma. J. Cutan. Pathol. 1993, 20, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Stieler, K.M.; Röwert-Huber, J.; Vogt, A.; Meyer, L.; Ulrike, B.P. Cutaneous Cephalic Neurocristic Hamartoma on the Head With Melanocytic, Cartilage, Blood Vessel, Neural, and Bony Tissue. Am. J. Dermatopathol. 2021, 43, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C. Cartilaginous differentiation in a giant congenital melanocytic naevus. Pathology 2008, 40, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Chen, Y.T.; Old, L.J.; Stockert, E.; Iversen, K.; Coplan, K.A.; Rosai, J.; Barnhill, R.L.; Jungbluth, A.A. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am. J. Surg. Pathol. 1998, 22, 976–982. [Google Scholar] [CrossRef]
- Chen, Y.; Klonowski, P.W.; Lind, A.C.; Lu, D. Differentiating neurotized melanocytic nevi from neurofibromas using Melan-A (MART-1) immunohistochemical stain. Arch. Pathol. Lab. Med. 2012, 136, 810–815. [Google Scholar] [CrossRef]
- Singh, N.; Chandrashekar, L.; Kar, R.; Sylvia, M.T.; Thappa, D.M. Neurotized congenital melanocytic nevus resembling a pigmented neurofibroma. Indian J. Dermatol. 2015, 60, 46–50. [Google Scholar] [CrossRef]

| Category | Summarized Findings |
|---|---|
| Clinical Findings | - Usually congenital or early-onset - Variable pigmentation (often less pigmented or hypopigmented than conventional CMN) - Variable size (often > 3 cm) - Commonly scalp and head involvement, but can involve trunk and limbs - Associated findings in some cases: poliosis, alopecia, cutis verticis gyrata-like appearance, or skeletal involvement |
| Histologic Findings | - Epidermal and dermal proliferation of melanocytes - Schwannian differentiation - Lesions can be either predominantly melanocytic or neuromesenchymal - Myxoid or collagenous background - Increased vascular proliferation - Generally low mitotic rate unless malignant transformation occurs - Usually extends deep into the subcutaneous tissue |
| Immunohistochemistry | - Positive: S100, SOX10, Melan-A, and HMB-45 - Negative: PRAME - Areas with Schwannian differentiation: positive for S100 and EMA, while negative for Melan-A and HMB-45 - Stromal cells: positive for CD34 |
| Molecular Features | - Multiple chromosomal gains and losses by comparative genomic hybridization and chromosomal microarray analysis - No mutations identified by molecular analyses |
| Prognosis | - Generally benign behavior, particularly in smaller lesions - Rare malignant transformations reported; prognosis guarded in these cases - Larger lesions warrant closer follow-up |
| Malignant Transformation Findings | - Clinical signs: rapid growth - Histology: increased cytologic atypia, mitotic figures, invasion of deeper tissue structures - Molecular changes: acquisition of additional genetic alterations |
| Management | - Conservative observation for small lesions - A wide local excision is considered for cosmetic or symptomatic relief, especially with lesion growth or concerning clinical/histologic features - Close long-term follow-up recommended - Mohs surgery for poorly defined margins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Rayes, D.; Wilson, K.; Maguiness, S.; Miller, D.; Cazzato, G.; Giubellino, A. Congenital Melanocytic Nevus with Neurocristic Cutaneous Hamartoma: A Case Report. Dermatopathology 2025, 12, 12. https://doi.org/10.3390/dermatopathology12020012
El-Rayes D, Wilson K, Maguiness S, Miller D, Cazzato G, Giubellino A. Congenital Melanocytic Nevus with Neurocristic Cutaneous Hamartoma: A Case Report. Dermatopathology. 2025; 12(2):12. https://doi.org/10.3390/dermatopathology12020012
Chicago/Turabian StyleEl-Rayes, Dina, Katlin Wilson, Sheilagh Maguiness, Daniel Miller, Gerardo Cazzato, and Alessio Giubellino. 2025. "Congenital Melanocytic Nevus with Neurocristic Cutaneous Hamartoma: A Case Report" Dermatopathology 12, no. 2: 12. https://doi.org/10.3390/dermatopathology12020012
APA StyleEl-Rayes, D., Wilson, K., Maguiness, S., Miller, D., Cazzato, G., & Giubellino, A. (2025). Congenital Melanocytic Nevus with Neurocristic Cutaneous Hamartoma: A Case Report. Dermatopathology, 12(2), 12. https://doi.org/10.3390/dermatopathology12020012








