Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pam2Cys
2.2. Isolation of Bovine Tracheal Epithelial Cells
2.3. Culture of Bovine Tracheal Epithelial Monolayers
2.4. Expansion of Basal Progenitors in PneumaCult ExPlus
2.5. Culture of Matrix-Embedded Bovine Tracheal Organoids
2.6. Culture of Bovine Tracheal Epithelial Cells at an Air–Liquid Interface (ALI)
2.7. Live Cell Imaging
2.8. Immunofluorescence and Confocal Microscopy
2.9. Single-Cell RNA Sequencing
2.10. Bioinformatics Analysis
2.11. Measurement of Cytokine Production
2.12. Reverse Transcription Quantitative PCR (RT-qPCR)
2.13. Preparation of BHV-1 Stocks
2.14. Infection of Bovine Tracheal Monolayers and Matrix-Embedded Organoids with BHV-1
2.15. Determination of Viral Titres
2.16. Transmission Electron Microscopy
2.17. Statistical Analysis
3. Results
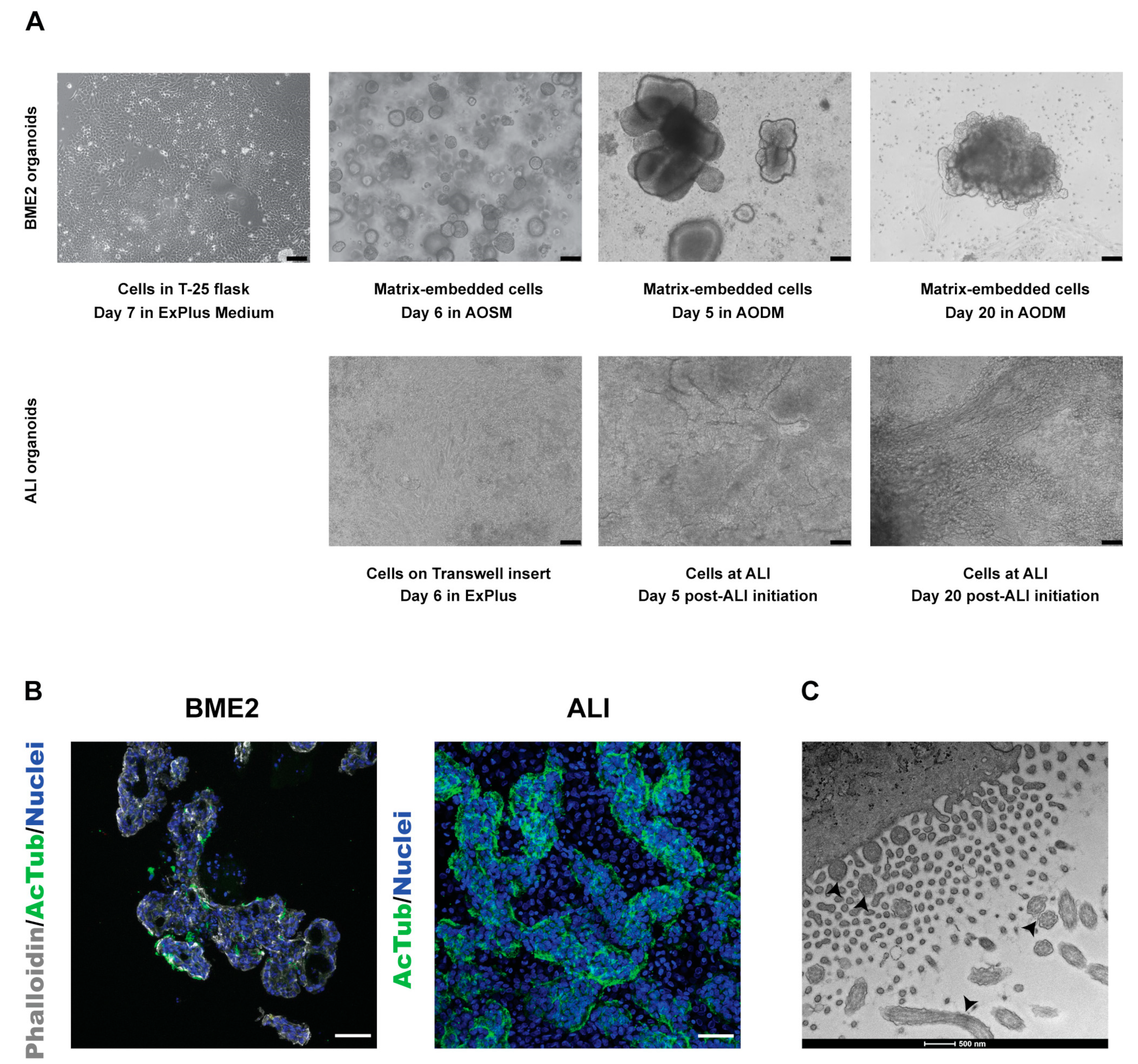
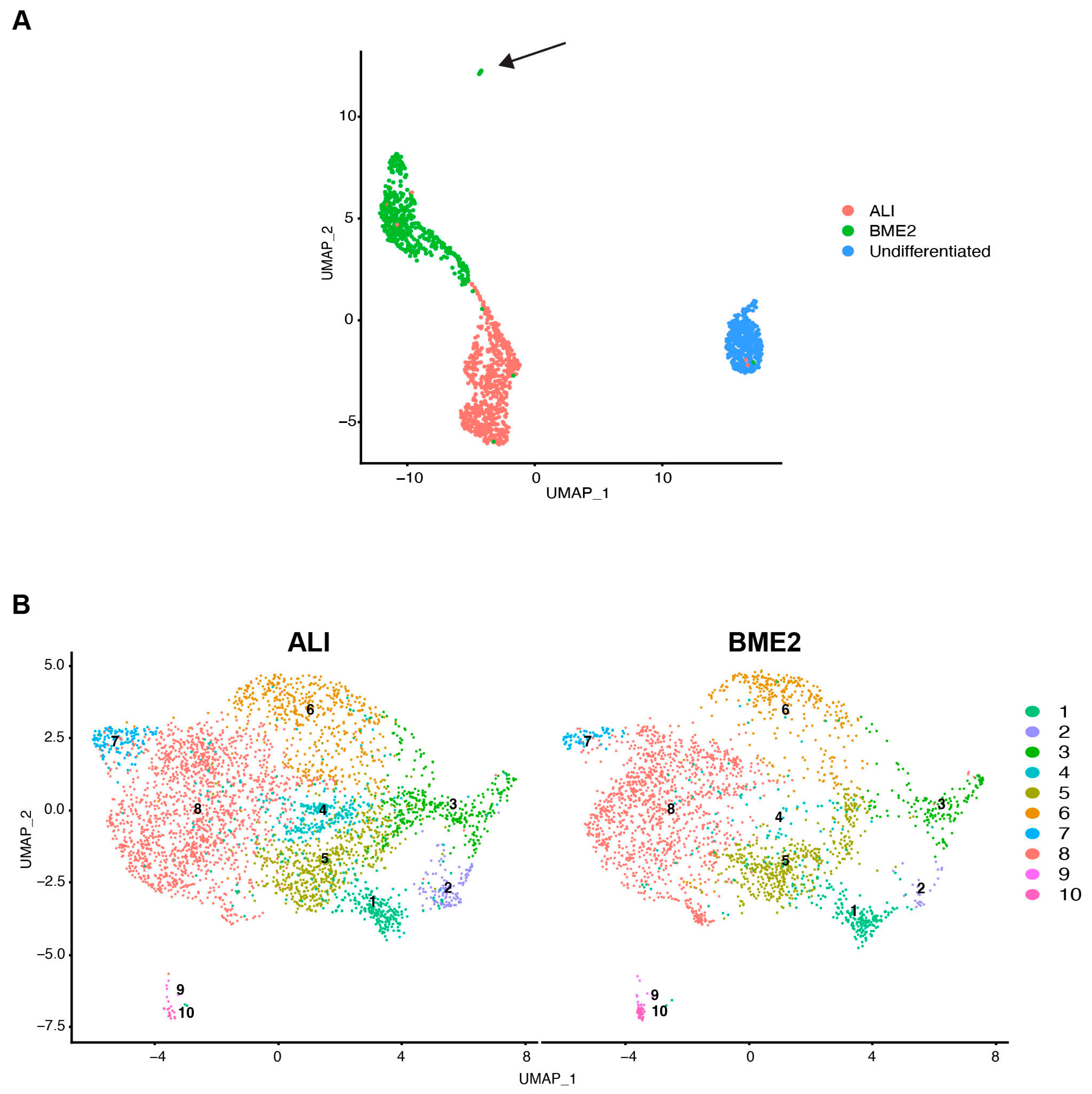
3.1. Establishment and Characterisation of Bovine Tracheal Epithelial Organoids
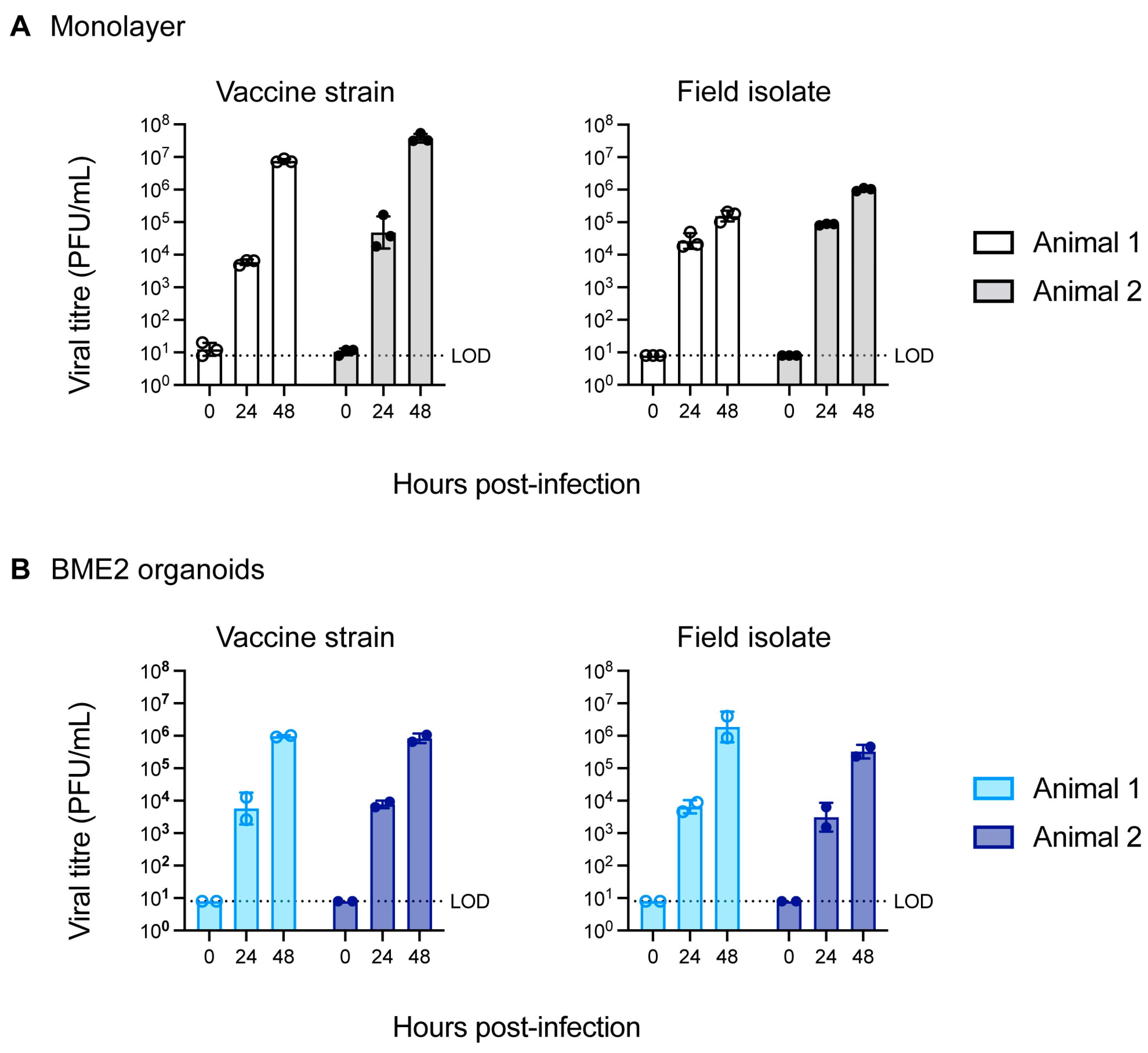
3.2. Infection of Bovine Tracheal Epithelial Organoid with BHV-1
3.3. Innate Immune Response to TLR2 Ligand Pam2Cys in Bovine Tracheal Cell Monolayer and BME2 Organoid
3.4. Effect of Pam2Cys on BHV-1 Infection In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ALFA. Feedlot Qtrly Survey Results. ALFA: Sydney, Australia. Available online: https://www.feedlots.com.au/news/categories/feedlot-qtrly-survey-results (accessed on 1 May 2023).
- Gaudino, M.; Nagamine, B.; Ducatez, M.F.; Meyer, G. Understanding the mechanisms of viral and bacterial coinfections in bovine respiratory disease: A comprehensive literature review of experimental evidence. Vet. Res. 2022, 53, 70. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the evidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Hay, K.; Barnes, T.; Morton, J.; Gravel, J.; Commins, M.; Horwood, P.; Ambrose, R.; Clements, A.; Mahony, T. Associations between exposure to viruses and bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016, 127, 121–133. [Google Scholar] [CrossRef]
- Biswas, S.; Bandyopadhyay, S.; Dimri, U.; Patra, P.H. Bovine herpesvirus-1 (BHV-1)—A re-emerging concern in livestock: A revisit to its biology, epidemiology, diagnosis, and prophylaxis. Vet. Q. 2013, 33, 68–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastenkos, G.; Lee, B.; Pritchard, S.M.; Nicola, A.V. Bovine Herpesvirus 1 Entry by a Low-pH Endosomal Pathway. J. Virol. 2018, 92, e00839-18. [Google Scholar] [CrossRef] [Green Version]
- Cusack, P.M. Bovine Respiratory Disease Preventive Practices Handbook; Meat & Livestock Australia: North Sydney, Australia, 2022. [Google Scholar]
- Srikumaran, S.; Kelling, C.L.; Ambagala, A. Immune evasion by pathogens of bovine respiratory disease complex. Anim. Health Res. Rev. 2007, 8, 215–229. [Google Scholar] [CrossRef]
- Beutler, B.A. TLRs and Innate Immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Berghuis, L.; Abdelaziz, K.T.; Bierworth, J.; Wyer, L.; Jacob, G.; Karrow, N.A.; Sharif, S.; Clark, M.E.; Caswell, J.L. Comparison of innate immune agonists for induction of tracheal antimicrobial peptide gene expression in tracheal epithelial cells of cattle. Vet. Res. 2014, 45, 105. [Google Scholar] [CrossRef]
- Mühlradt, P.F.; Kiess, M.; Meyer, H.; Süssmuth, R.; Jung, G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fer-mentans acting at picomolar concentration. J. Exp. Med. 1997, 185, 1951–1958. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, O.; Kawai, T.; Mühlradt, P.F.; Morr, M.; Radolf, J.D.; Zychlinsky, A.; Takeda, K.; Akira, S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001, 13, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.L.; Mifsud, E.J.; Zeng, W.; Edenborough, K.; McVernon, J.; Brown, L.; Jackson, D.C. Intranasal Administration of the TLR2 Agonist Pam2Cys Provides Rapid Protection against Influenza in Mice. Mol. Pharm. 2012, 9, 2710–2718. [Google Scholar] [CrossRef]
- Deliyannis, G.; Wong, C.Y.; McQuilten, H.A.; Bachem, A.; Clarke, M.; Jia, X.; Horrocks, K.; Zeng, W.; Girkin, J.; Scott, N.E.; et al. TLR2-mediated activation of innate responses in the upper airways confers antiviral protection of the lungs. J. Clin. Investig. 2021, 6, e140267. [Google Scholar] [CrossRef]
- Burucua, M.M.; Quintana, S.; Lendez, P.; Cobo, E.R.; Ceriani, M.C.; Dolcini, G.; Odeón, A.C.; Pérez, S.E.; Marin, M.S. Modulation of cathelicidins, IFNbeta and TNFalpha by bovine alpha-herpesviruses is dependent on the stage of the infectious cycle. Mol. Immunol. 2019, 111, 136–144. [Google Scholar] [CrossRef]
- Kirchhoff, J.; Uhlenbruck, S.; Goris, K.; Keil, G.M.; Herrler, G. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Vet. Res. 2014, 45, 20. [Google Scholar] [CrossRef] [Green Version]
- Rosales, J.J.; Verna, A.; Marin, M.; Pérez, S. Bovine alphaherpesvirus type 5 replicates more efficiently than bovine alphaherpesvirus type 1 in undifferentiated human neural cells. Virus Res. 2020, 286, 198037. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Wyer, L.; Berghuis, L.; Bassel, L.L.; Clark, M.E.; Caswell, J.L. Regulation of tracheal antimicrobial peptide gene expression in airway epithelial cells of cattle. Vet. Res. 2016, 47, 44. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.M.; Deliyannis, G.; Hachani, A.; Earnest, L.; Torresi, J.; Vincan, E. Organoid Models of SARS-CoV-2 Infection: What Have We Learned about COVID-19? Organoids 2022, 1, 2–27. [Google Scholar] [CrossRef]
- Alling, C.R.; Liu, C.-C.; Langohr, I.M.; Haque, M.; Carter, R.T.; Baker, R.E.; Lewin, A.C. Assessment of Cidofovir for Treatment of Ocular Bovine Herpesvirus-1 Infection in Cattle Using an Ex-Vivo Model. Viruses 2021, 13, 2102. [Google Scholar] [CrossRef]
- Niesalla, H.; Dale, A.; Slater, J.; Scholes, S.; Archer, J.; Maskell, D.; Tucker, A. Critical assessment of an in vitro bovine respiratory organ culture system: A model of bovine herpesvirus-1 infection. J. Virol. Methods 2009, 158, 123–129. [Google Scholar] [CrossRef]
- Cozens, D.; Grahame, E.; Sutherland, E.; Taylor, G.; Berry, C.C.; Davies, R.L. Development and optimization of a differentiated airway epithelial cell model of the bovine respiratory tract. Sci. Rep. 2018, 8, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozens, D.; Sutherland, E.; Marchesi, F.; Taylor, G.; Berry, C.C.; Davies, R.L. Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface. Sci. Rep. 2018, 8, 14893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef]
- Collett, S.; Torresi, J.; Silveira, L.E.; Truong, V.K.; Christiansen, D.; Tran, B.M.; Vincan, E.; Ramsland, P.A.; Elbourne, A. Investigating virus–host cell interactions: Comparative binding forces between hepatitis C virus-like particles and host cell receptors in 2D and 3D cell culture models. J. Colloid Interface Sci. 2021, 592, 371–384. [Google Scholar] [CrossRef]
- Quintana, A.M.; Landolt, G.A.; Annis, K.M.; Hussey, G.S. Immunological characterization of the equine airway epithelium and of a primary equine airway epithelial cell culture model. Vet. Immunol. Immunopathol. 2011, 140, 226–236. [Google Scholar] [CrossRef]
- Hussey, G.S.; Ashton, L.V.; Quintana, A.M.; Lunn, D.; Goehring, L.S.; Annis, K.; Landolt, G. Innate immune responses of airway epithelial cells to infection with Equine herpesvirus-1. Vet. Microbiol. 2014, 170, 28–38. [Google Scholar] [CrossRef]
- Mitchell, G.B.; Al-Haddawi, M.H.; Clark, M.E.; Beveridge, J.D.; Caswell, J.L. Effect of Corticosteroids and Neuropeptides on the Expression of Defensins in Bovine Tracheal Epithelial Cells. Infect. Immun. 2007, 75, 1325–1334. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.M.; Grimley, S.L.; McAuley, J.L.; Hachani, A.; Earnest, L.; Wong, S.L.; Caly, L.; Druce, J.; Purcell, D.F.J.; Jackson, D.C.; et al. Air-Liquid-Interface Differentiated Human Nose Epithelium: A Robust Primary Tissue Culture Model of SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 835. [Google Scholar] [CrossRef]
- Deprez, M.; Zaragosi, L.-E.; Truchi, M.; Becavin, C.; García, S.R.; Arguel, M.-J.; Plaisant, M.; Magnone, V.; Lebrigand, K.; Abelanet, S.; et al. A Single-Cell Atlas of the Human Healthy Airways. Am. J. Respir. Crit. Care Med. 2020, 202, 1636–1645. [Google Scholar] [CrossRef]
- Ravindra, N.G.; Alfajaro, M.M.; Gasque, V.; Huston, N.C.; Wan, H.; Szigeti-Buck, K.; Yasumoto, Y.; Greaney, A.M.; Habet, V.; Chow, R.D.; et al. Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes. PLoS Biol. 2021, 19, e3001143. [Google Scholar] [CrossRef]
- García, S.R.; Deprez, M.; Lebrigand, K.; Cavard, A.; Paquet, A.; Arguel, M.-J.; Magnone, V.; Truchi, M.; Caballero, I.; Leroy, S.; et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 2019, 146, dev.177428. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, D.J.; Phesse, T.J.; Barker, N.; Schwab, R.H.M.; Amin, N.; Malaterre, J.; Stange, D.E.; Nowell, C.J.; Currie, S.A.; Saw, J.T.S.; et al. Frizzled7 Functions as a Wnt Receptor in Intestinal Epithelial Lgr5+ Stem Cells. Stem Cell Rep. 2015, 4, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Brake, F.; Studdert, M.I. Molecular epidemiology and pathogenesis of ruminant herpesviruses including bovine, buffalo and caprine herpesviruses 1 and bovine encephalitis herpesvirus. Aust. Vet. J. 1985, 62, 331–334. [Google Scholar] [CrossRef]
- Hanssen, E.; Dekiwadia, C.; Riglar, D.T.; Rug, M.; Lemgruber, L.; Cowman, A.F.; Cyrklaff, M.; Kudryashev, M.; Frischknecht, F.; Baum, J.; et al. Electron tomography of Plasmodium falciparum merozoites reveals core cellular events that underpin erythrocyte invasion. Cell Microbiol. 2013, 15, 1457–1472. [Google Scholar] [CrossRef]
- Snowdon, W.A. Infectious bovine rhinotracheitis and infectious pustular vulvovaginitis in aus-tralian cattle. Aust. Vet. J. 1964, 40, 277–288. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Mukaida, N.; Harada, A.; Matsushima, K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998, 9, 9–23. [Google Scholar] [CrossRef]
- Zaidman, N.A.; Panoskaltsis-Mortari, A.; O’Grady, S.M. Differentiation of human bronchial epithelial cells: Role of hydrocortisone in development of ion transport pathways involved in mucociliary clearance. Am. J. Physiol. Physiol. 2016, 311, C225–C236. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, J.K.; Kumar, S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Vet. Sci. 2020, 7, 206. [Google Scholar] [CrossRef]
- Barnes, T.; Hay, K.; Morton, J.; Mahony, T. Epidemiology and Management of Bovine Respiratory Disease in Feedlot Cattle: Final Report for Project B.FLT.0225; Meat & Livestock Australia: North Sydney, Australia, 2015. [Google Scholar]
- Zaidman, N.A.; Panoskaltsis-Mortari, A.; O’Grady, S.M. Large-conductance Ca2+-activated K+channel activation by apical P2Y receptor agonists requires hydrocortisone in differentiated airway epithelium. J. Physiol. 2017, 595, 4631–4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Rivas, J.J.; Kisiela, D.; Czuprynski, C.J. Bovine herpesvirus type 1 infection of bovine bronchial epithelial cells increases neutrophil adhesion and activation. Vet. Immunol. Immunopathol. 2009, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primer | Sequence |
|---|---|---|
| GAPDH | Forward | 5′-GGCGTGAACCACGAGAAGTATAA-3′ |
| Reverse | 5′-CCCTCCACGATGCCAAAGT-3′ | |
| TAP | Forward | 5′-TCTTCCTGGTCCTGTCTGCT-3′ |
| Reverse | 5′-GCTGTGTCTTGGCCTTCTTT-3′ |
| Cluster | Cell Type | Marker Genes |
|---|---|---|
| 1 | Cycling basal | MKI67 |
| Neuroendocrine | SYT1 | |
| 2 | Basal | TP63, KRT5, S100A2, PTMA |
| Ciliated | TUBB4B | |
| Cycling basal | MKI67, HMGB2 | |
| Deuterosomal | PLK4 | |
| Suprabasal | KRT5 | |
| Neuroendocrine | SYT1 | |
| 3 | Basal | TP63, KRT5, DAPL1, PTMA, S100A2 |
| Cycling basal | MT2A, GAPDH, PTTG1 | |
| Suprabasal | KRT5 | |
| Neuroendocrine | SYT1 | |
| 4 | Basal | KRT5 |
| Suprabasal | KRT5 | |
| Mucous | AGR2 | |
| 5 | Basal | S100A2, PTMA |
| Mucous | AGR2 | |
| Neuroendocrine | SYT1 | |
| 6 | Basal | KRT15 |
| Ionocytes | CFTR | |
| Cycling basal | GAPDH | |
| Suprabasal | KRT4, KRT15, KRT19 | |
| 7 | Secretory | CXCL17 |
| Basal | KRT15 | |
| Suprabasal | KRT4, KRT15 | |
| Serous | TCN1 | |
| Mucous | GOLM1 | |
| 8 | Secretory | CXCL17, TFF3 |
| Ionocytes | CFTR | |
| Suprabasal | KRT4, KRT15, TXN | |
| Serous | LTF, TCN1 | |
| Mucous | GOLM1, TFF3 | |
| 9 | Ciliated | CCDC113, FOXJ1, MLF1, TPPP3 |
| Secretory | MSMB | |
| Deuterosomal | CDC20B, PLK4, DEUP1, KIF9 | |
| Serous | LTF | |
| 10 | Ciliated | CCDC113, FOXJ1, MLF1, TPPP3, TUBB4B |
| Secretory | CYP2F1 | |
| Tuft/Brush | GNAT3 | |
| Cycling basal | PTTG1 | |
| Deuterosomal | CDC20B, PLK4, DEUP1, KIF9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quah, P.S.; Tran, B.M.; Corbin, V.D.A.; Chang, J.J.-Y.; Wong, C.Y.; Diaz-Méndez, A.; Hartley, C.A.; Zeng, W.; Hanssen, E.; Trifunovic, Z.; et al. Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease. Organoids 2023, 2, 82-101. https://doi.org/10.3390/organoids2020007
Quah PS, Tran BM, Corbin VDA, Chang JJ-Y, Wong CY, Diaz-Méndez A, Hartley CA, Zeng W, Hanssen E, Trifunovic Z, et al. Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease. Organoids. 2023; 2(2):82-101. https://doi.org/10.3390/organoids2020007
Chicago/Turabian StyleQuah, Pin Shie, Bang M. Tran, Vincent D.A. Corbin, Jessie J.-Y. Chang, Chinn Yi Wong, Andrés Diaz-Méndez, Carol A. Hartley, Weiguang Zeng, Eric Hanssen, Zlatan Trifunovic, and et al. 2023. "Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease" Organoids 2, no. 2: 82-101. https://doi.org/10.3390/organoids2020007
APA StyleQuah, P. S., Tran, B. M., Corbin, V. D. A., Chang, J. J.-Y., Wong, C. Y., Diaz-Méndez, A., Hartley, C. A., Zeng, W., Hanssen, E., Trifunovic, Z., Reading, P. C., Jackson, D. C., Vincan, E., Coin, L. J. M., & Deliyannis, G. (2023). Development of Matrix-Embedded Bovine Tracheal Organoids to Study the Innate Immune Response against Bovine Respiratory Disease. Organoids, 2(2), 82-101. https://doi.org/10.3390/organoids2020007








