Microstructural Evolution of a High-Strength Zr-Ti-Modified 2139 Aluminum Alloy for Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
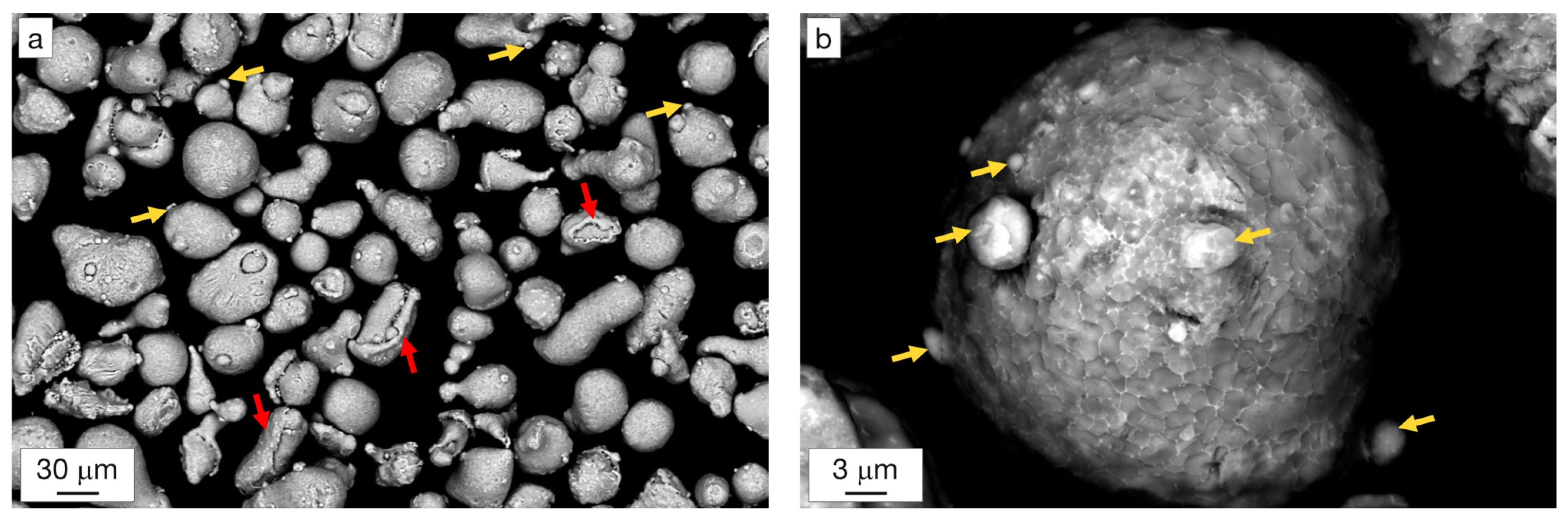
3.1. Powder Characterization
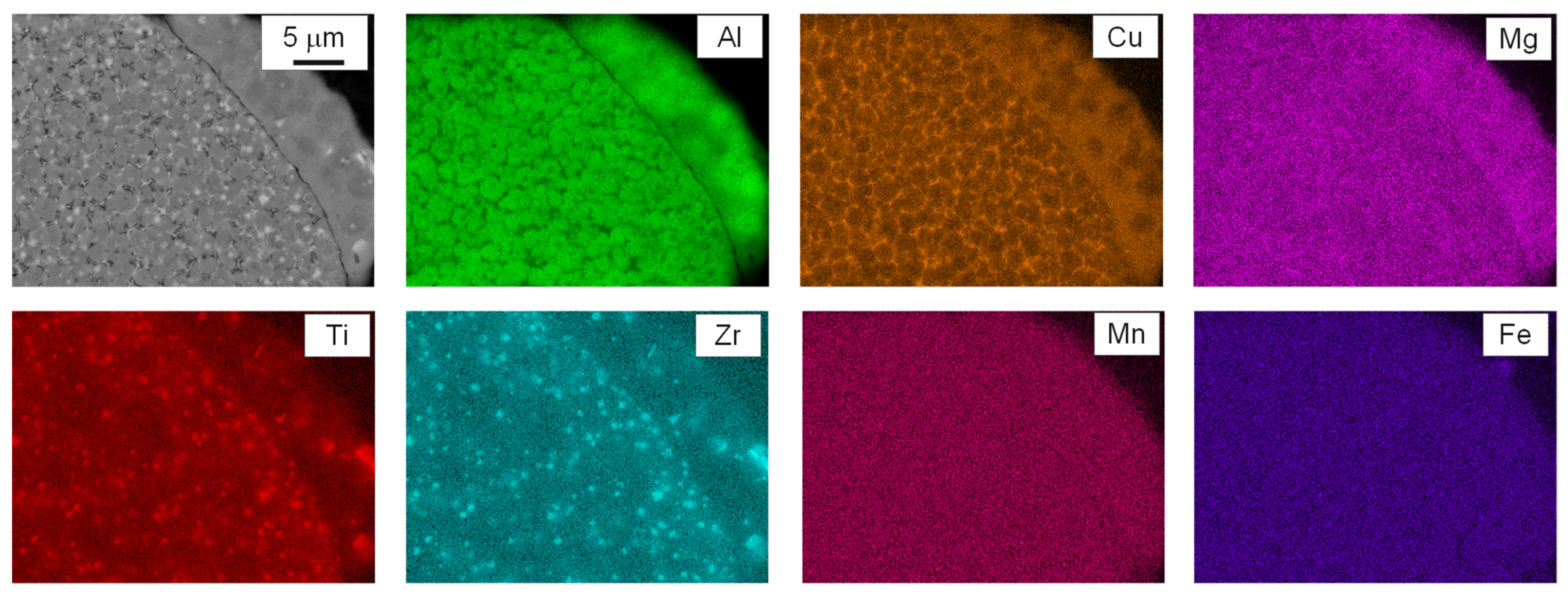
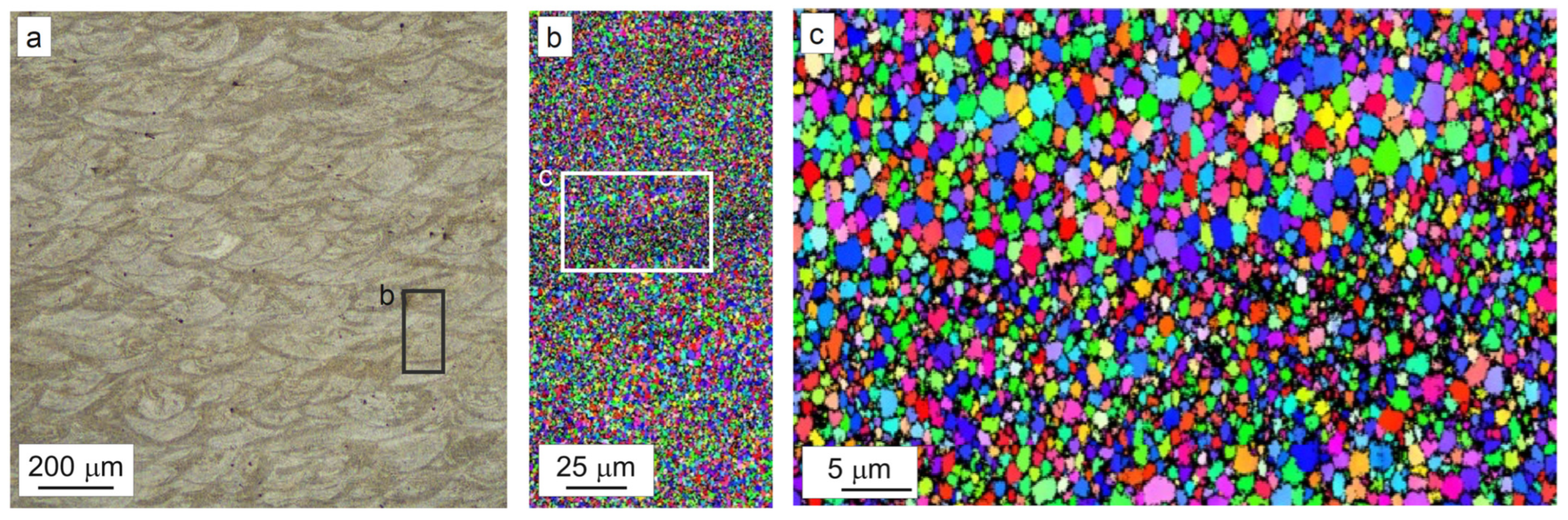
3.2. As-Built Microstructure and Microhardness
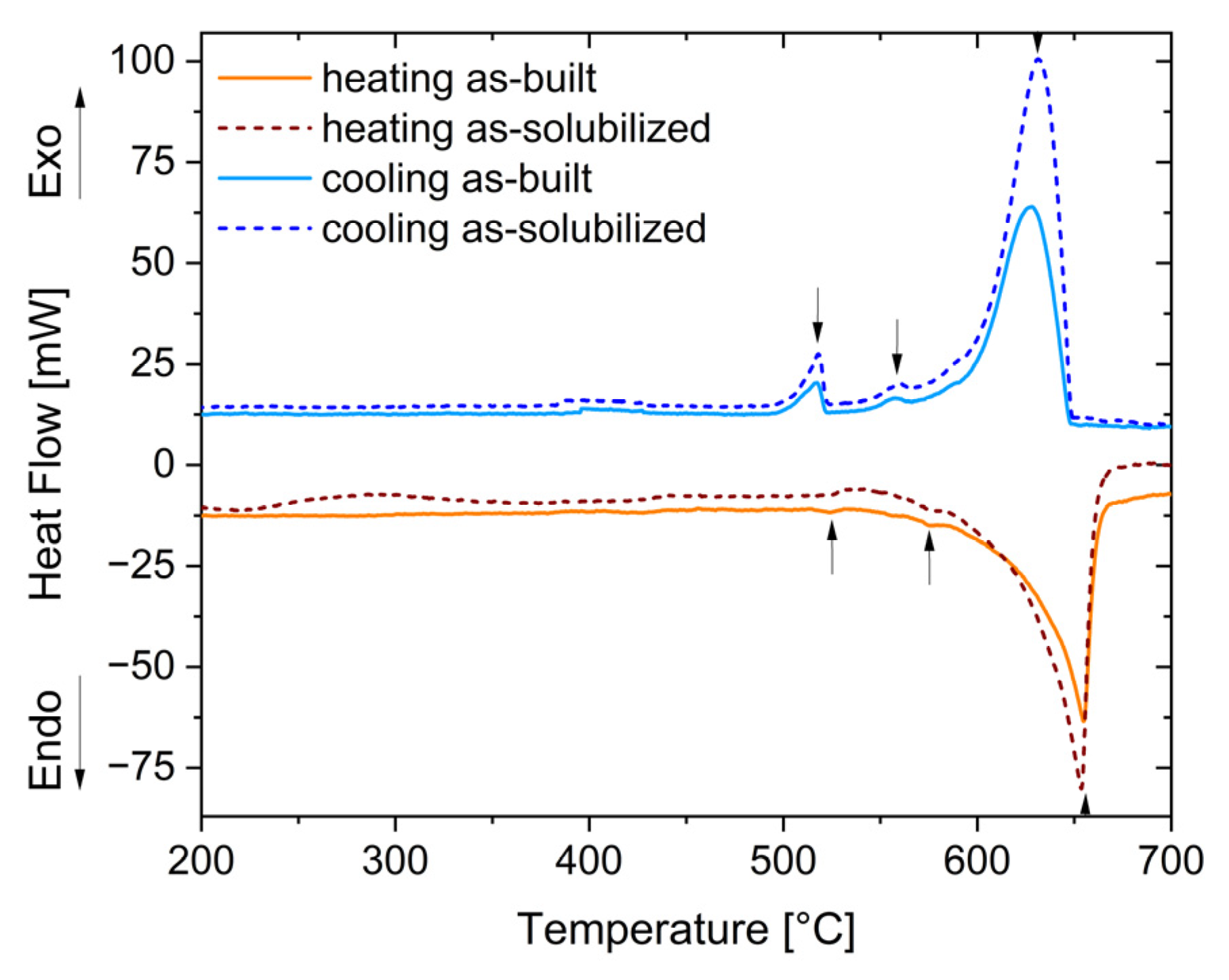
3.3. Phase Stability and Heat Treatment Optimization
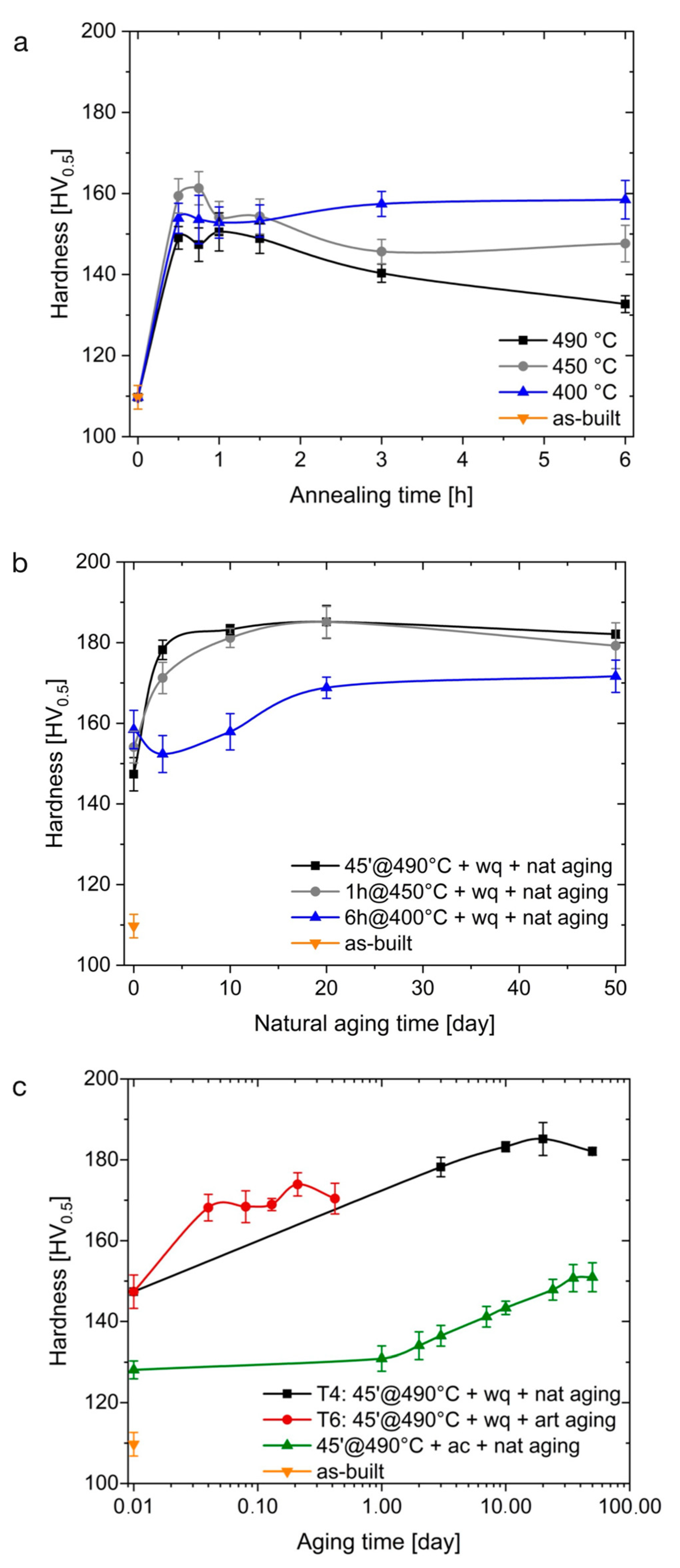
3.4. T4 State and Overaging Effects
4. Conclusions
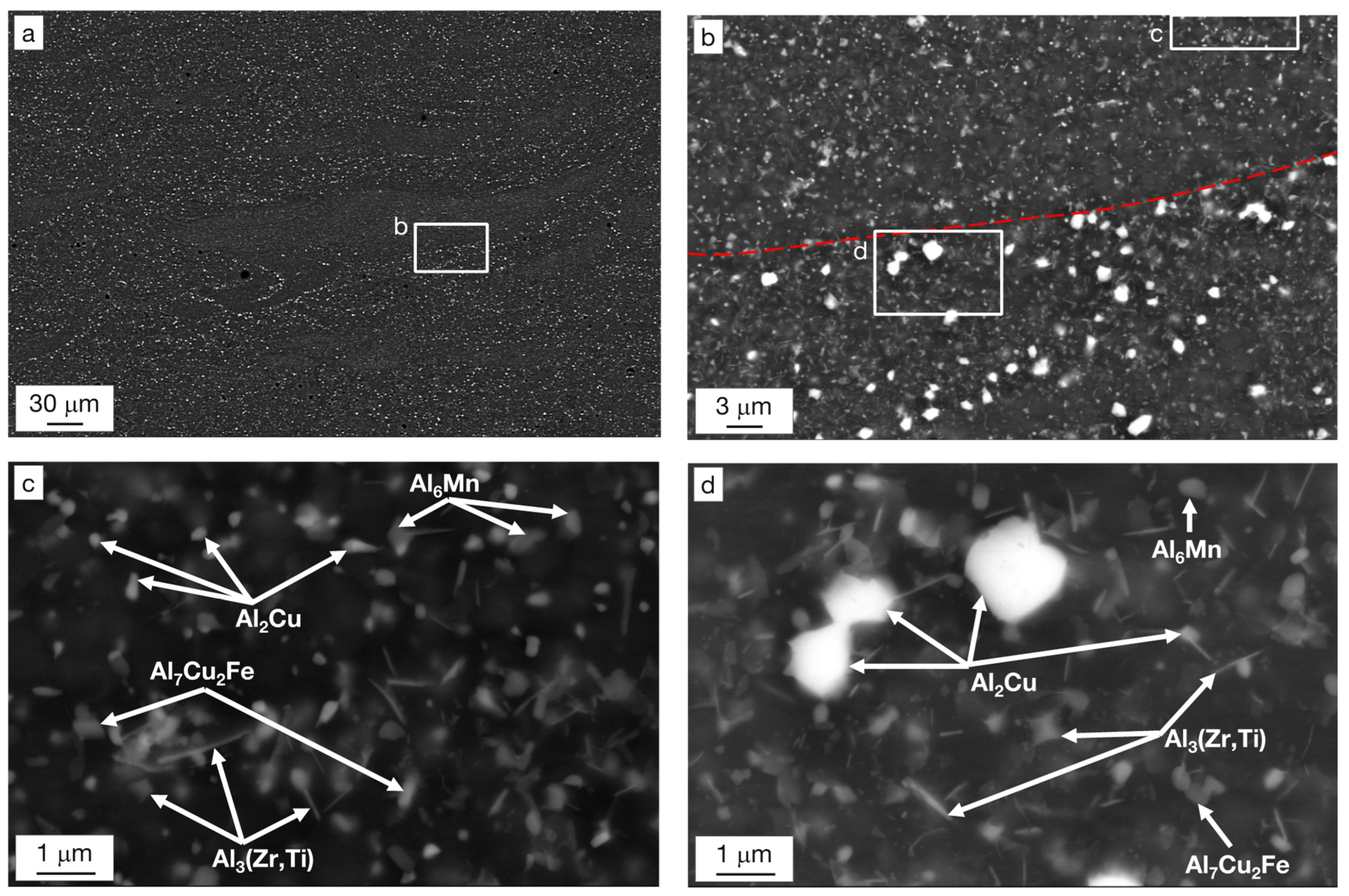
- The as-built state featured an extremely fine and equiaxed bimodal microstructure with grains 300–600 nm in size at the boundaries of the melt pools and grains 0.8–2.0 μm in size at their center. A complete columnar-to-equiaxed transition was therefore achieved by virtue of the combined action of Zr and Ti. Cubic nucleants corresponding to the Al3(Zr,Ti) phase were observed at the center of grains, while segregation of Cu and Mg was measured at the grain boundaries. No evidence of hot-cracks was detected in the investigated samples.
- A T4 treatment reached by holding the samples at 490 °C for 45 min, water quenching, and natural aging for at least 5 days allowed for achieving the highest microhardness value of 186.1 HV0.5. The T6 temper, consisting of the same solution-annealing treatment followed by artificial aging at 160 °C for 5 h, led to a microhardness of 173.9 HV0.5.
- Immediately after water quenching, the measured increase in microhardness from 109.7 to 147.4 HV0.5 was mainly related to the precipitation of additional Zr- and Ti-rich secondary particles during the annealing treatment, as predicted by Thermo-Calc ® simulations.
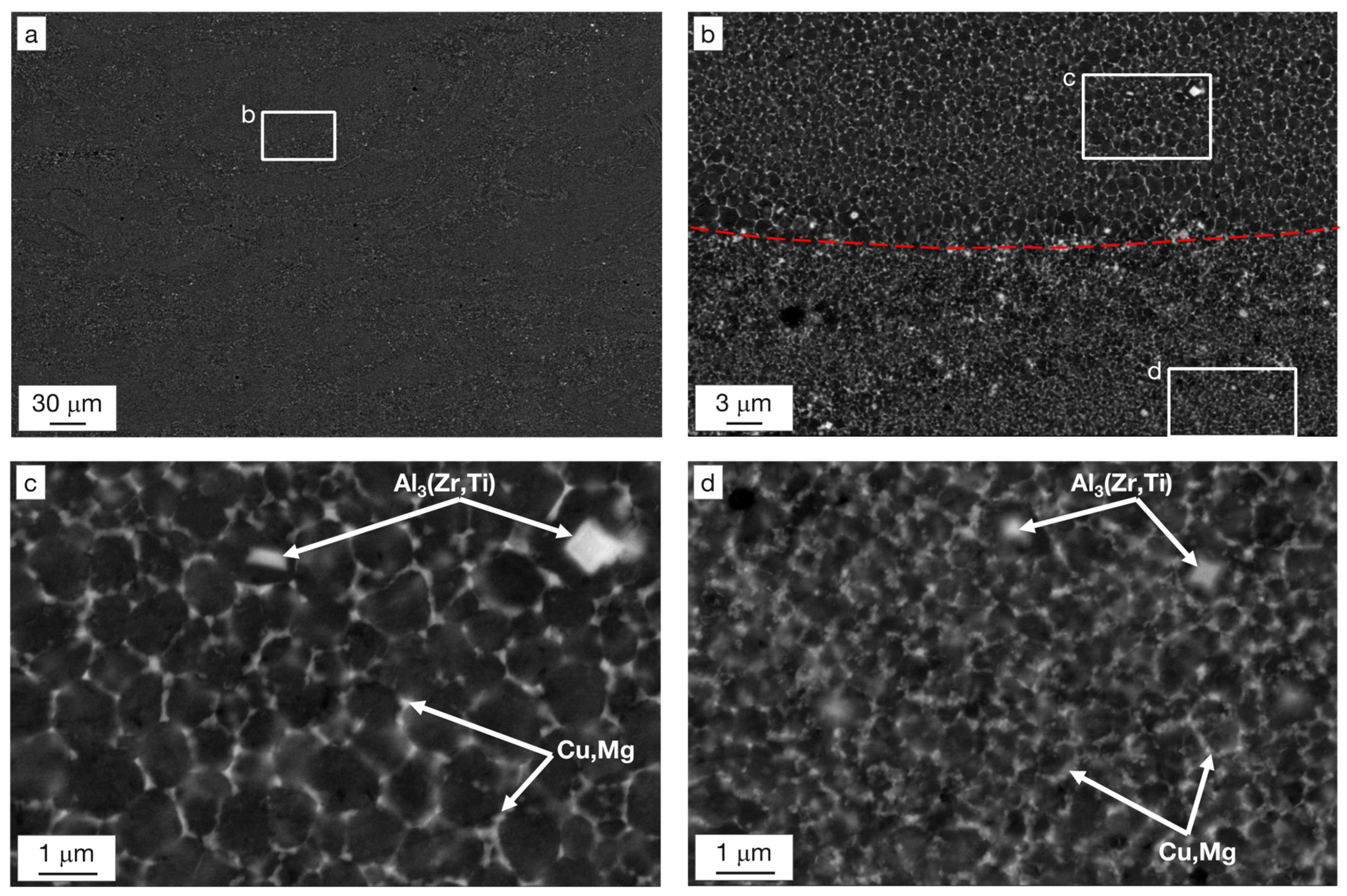
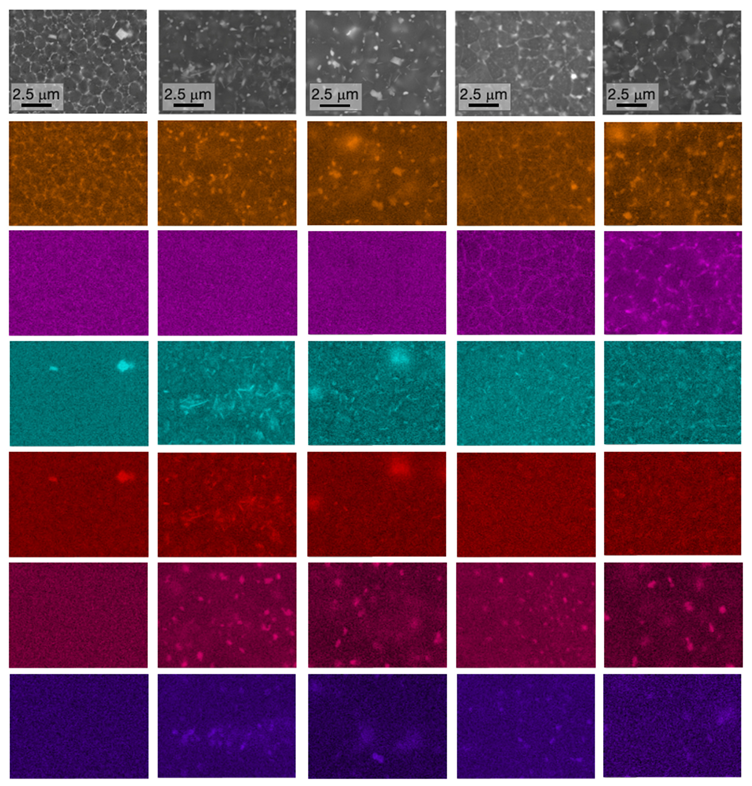
- The complex microstructure of the T4 temper was characterized by a large number of precipitates on the micrometer and sub-micrometer size scales. Coarse precipitates, 0.5–1 μm in size, corresponded to Al2Cu particles mainly disposed at the edge of the molten pools. Cubic precipitates of Al3(Zr,Ti) were observed at the center of the grains and were assumed to be the nucleants promoting their formation. Rod-shaped Al3(Zr,Ti) and quasi-spherical precipitates of about 100–300 nm rich in Cu, Mn, and Fe were arranged within the grains. Finally, particles smaller than 80 nm were assumed to be the expected Ω-phase (Al2Cu) strengthening precipitates.
- Substantial microstructural changes occurred in T4 samples when held for 50 h at 250 °C, with evidence of Cu and Mg segregation at the grain boundaries and slight grain coarsening (reaching a size of 0.8–2.2 µm), which contributed to a decrease in microhardness (from 186.1 to 119.3 HV0.5). The exposure for 50 h at 300 °C led to a further decrease in hardness down to 110.3 HV0.5 and stimulated the spheroidization of Cu and Mg at the grain boundaries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, T.; Kennedy, J.V.; Potgieter, J. A Comparison of Traditional Manufacturing vs Additive Manufacturing, the Best Method for the Job. In Procedia Manufacturing; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 30, pp. 11–18. [Google Scholar]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A Review of Selective Laser Melting of Aluminum Alloys: Processing, Microstructure, Property and Developing Trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, X.J.; Saunders, M.; Suvorova, A.; Zhang, L.C.; Liu, Y.J.; Fang, M.H.; Huang, Z.H.; Sercombe, T.B. A Selective Laser Melting and Solution Heat Treatment Refined Al-12Si Alloy with a Controllable Ultrafine Eutectic Microstructure and 25% Tensile Ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Casati, R.; Hamidi Nasab, M.; Tirelli, V.; Vedani, M. Effect of Different Heat Treatment Routes on Microstructure and Mechanical Properties of AlSi7Mg, AlSi10Mg and Al-Mg-Zr-Sc Alloys Produced by Selective Laser Melting. In Proceedings of the EuroPM 2018, Bilbao, Spain, 14–18 October 2018; EPMA European Powder Metallurgy Association: Chantilly, France, 2018. Available online: https://www.epma.com/publications/euro-pm-proceedings/product/ep18-3992767 (accessed on 3 April 2023).
- Saravana Kumar, M.; Javidrad, H.R.; Shanmugam, R.; Ramoni, M.; Adediran, A.A.; Pruncu, C.I. Impact of Print Orientation on Morphological and Mechanical Properties of L-PBF Based AlSi7Mg Parts for Aerospace Applications. Silicon 2022, 14, 7083–7097. [Google Scholar] [CrossRef]
- Zhao, L.; Song, L.; Santos Macías, J.G.; Zhu, Y.; Huang, M.; Simar, A.; Li, Z. Review on the Correlation between Microstructure and Mechanical Performance for Laser Powder Bed Fusion AlSi10Mg. Addit. Manuf. 2022, 56, 102914. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D Printing of High-Strength Aluminium Alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Dixit, S.; Liu, S. Laser Additive Manufacturing of High-Strength Aluminum Alloys: Challenges and Strategies. J. Manuf. Mater. Process. 2022, 6, 156. [Google Scholar] [CrossRef]
- Elambasseril, J.; Benoit, M.J.; Zhu, S.; Easton, M.A.; Lui, E.; Brice, C.A.; Qian, M.; Brandt, M. Effect of Process Parameters and Grain Refinement on Hot Tearing Susceptibility of High Strength Aluminum Alloy 2139 in Laser Powder Bed Fusion. Prog. Addit. Manuf. 2022, 7, 887–901. [Google Scholar] [CrossRef]
- Del Guercio, G.; Simonelli, M. Increasing the Build Rate of High-Strength Aluminium Alloys Produced by Laser Powder Bed Fusion. Opt. Laser Technol. 2023, 161, 109133. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, Z.; Zhang, H.; Nie, X.; Zhang, C.; Zhu, H. High Strength Al–Li Alloy Development for Laser Powder Bed Fusion. Addit. Manuf. 2021, 47, 102249. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Guo, C.; Zhou, Y.; Tan, Q.; Qu, W.; Li, X.; Hu, X.; Zhang, M.X.; Zhu, Q. Investigation into the Effect of Energy Density on Densification, Surface Roughness and Loss of Alloying Elements of 7075 Aluminium Alloy Processed by Laser Powder Bed Fusion. Opt. Laser Technol. 2022, 147, 107621. [Google Scholar] [CrossRef]
- Belelli, F.; Casati, R.; Vedani, M. Effect of Cu Content on Hot-Crack Resistance of Al-Cu-Mg Alloys Produced by Laser Powder Bed Fusion. Philos Mag. Lett. 2022, 102, 111–119. [Google Scholar] [CrossRef]
- Belelli, F.; Casati, R.; Vedani, M.; Volpp, J. Design and Characterization of Al–Mg–Si–Zr Alloys with Improved Laser Powder Bed Fusion Processability. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 331–343. [Google Scholar] [CrossRef]
- Belelli, F.; Casati, R.; Riccio, M.; Rizzi, A.; Kayacan, M.Y.; Vedani, M. Development of a Novel High-Temperature al Alloy for Laser Powder Bed Fusion. Metals 2021, 11, 35. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for Developing Castable, Creep-Resistant Aluminum-Based Alloys-A Review. Z. Met. 2006, 97, 246–265. [Google Scholar] [CrossRef]
- Schmidtke, K.; Palm, F.; Hawkins, A.; Emmelmann, C. Process and Mechanical Properties: Applicability of a Scandium Modified Al-Alloy for Laser Additive Manufacturing. In Physics Procedia; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 12, pp. 369–374. [Google Scholar]
- Schimbäck, D.; Mair, P.; Bärtl, M.; Palm, F.; Leichtfried, G.; Mayer, S.; Uggowitzer, P.J.; Pogatscher, S. Alloy Design Strategy for Microstructural-Tailored Scandium-Modified Aluminium Alloys for Additive Manufacturing. Scr. Mater. 2022, 207, 114277. [Google Scholar] [CrossRef]
- Deillon, L.; Jensch, F.; Palm, F.; Bambach, M. A New High Strength Al–Mg–Sc Alloy for Laser Powder Bed Fusion with Calcium Addition to Effectively Prevent Magnesium Evaporation. J. Mater. Process. Technol. 2022, 300, 117416. [Google Scholar] [CrossRef]
- Geng, Y.; Tang, H.; Xu, J.; Zhang, Z.; Xiao, Y.; Wu, Y. Strengthening Mechanisms of High-Performance Al-Mn-Mg-Sc-Zr Alloy Fabricated by Selective Laser Melting. Sci. China Mater. 2021, 64, 3131–3137. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Park, S.; Huynh, T.; Mehta, A.; Thapliyal, S.; Mishra, R.S.; Sohn, Y. Elimination of Extraordinarily High Cracking Susceptibility of Aluminum Alloy Fabricated by Laser Powder Bed Fusion. J. Mater. Sci. Technol. 2022, 103, 50–58. [Google Scholar] [CrossRef]
- M4p StrengthAl Material Data Sheet. Available online: https://www.metals4printing.com/wp-content/uploads/datasheets/de/Al-Basis/m4p_Datenblatt_StrengthAl_DE.pdf (accessed on 3 April 2023).
- Belelli, F.; Casati, R.; Andrianopoli, C.; Cuccaro, F.; Vedani, M. Investigation and Characterization of an Al-Mg-Zr-Sc Alloy with Reduced Sc Content for Laser Powder Bed Fusion. J. Alloys Compd. 2022, 924, 166519. [Google Scholar] [CrossRef]
- Addalloy NanoAl Material Data Sheet. Available online: https://www.nanoal.com/addalloy-powder-additive-manufacturing (accessed on 3 April 2023).
- Griffiths, S.; Rossell, M.D.; Croteau, J.; Vo, N.Q.; Dunand, D.C.; Leinenbach, C. Effect of Laser Rescanning on the Grain Microstructure of a Selective Laser Melted Al-Mg-Zr Alloy. Mater. Charact. 2018, 143, 34–42. [Google Scholar] [CrossRef]
- Belelli, F.; Casati, R.; Larini, F.; Riccio, M.; Vedani, M. Investigation on Two Ti–B Reinforced Al Alloys for Laser Powder Bed Fusion. Mater. Sci. Eng. A 2021, 808, 140944. [Google Scholar] [CrossRef]
- Li, G.; Brodu, E.; Soete, J.; Wei, H.; Liu, T.; Yang, T.; Liao, W.; Vanmeensel, K. Exploiting the Rapid Solidification Potential of Laser Powder Bed Fusion in High Strength and Crack-Free Al-Cu-Mg-Mn-Zr Alloys. Addit. Manuf. 2021, 47, 102210. [Google Scholar] [CrossRef]
- A20X Eckart Material Data Sheet. Available online: https://www.eckart.net/us/en/am/a20x (accessed on 3 April 2023).
- Barode, J.; Vayyala, A.; Virgillito, E.; Aversa, A.; Mayer, J.; Fino, P.; Lombardi, M. Revisiting Heat Treatments for Additive Manufactured Parts: A Case Study of A20X Alloy. Mater. Des. 2023, 225, 111566. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Zhu, Y.; Wu, X.; Huang, A. Review of High-Strength Aluminium Alloys for Additive Manufacturing by Laser Powder Bed Fusion. Mater. Des. 2022, 219, 110779. [Google Scholar] [CrossRef]
- Leirmo, J.L. High Strength Aluminium Alloys in Laser-Based Powder Bed Fusion—A Review. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 104, pp. 1747–1752. [Google Scholar]
- Schuster, M.; De Luca, A.; Mathur, A.; Hosseini, E.; Leinenbach, C. Precipitation in a 2xxx Series Al-Cu-Mg-Zr Alloy Fabricated by Laser Powder Bed Fusion. Mater. Des. 2021, 211, 110131. [Google Scholar] [CrossRef]
- EOS Aluminium Al2139 AM Material Data Sheet. Available online: https://www.eos.info/en/3d-printing-materials/metals/aluminum-al (accessed on 3 April 2023).
- Croteau, J.R.; Griffiths, S.; Rossell, M.D.; Leinenbach, C.; Kenel, C.; Jansen, V.; Seidman, D.N.; Dunand, D.C.; Vo, N.Q. Microstructure and Mechanical Properties of Al-Mg-Zr Alloys Processed by Selective Laser Melting. Acta Mater. 2018, 153, 35–44. [Google Scholar] [CrossRef]
- Zhang, D.; Prasad, A.; Bermingham, M.J.; Todaro, C.J.; Benoit, M.J.; Patel, M.N.; Qiu, D.; Stjohn, D.H.; Qian, M.A.; Easton, M.A. Grain Refinement of Alloys in Fuson-Based Additive Manufacturing Processes. Metall. Mater. Trans. A 2020, 51A, 4341–4359. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Zhou, L.; Lu, S.Z. A new Concept for Growth Restriction during Solidification. Acta Mater. 2018, 152, 248–252. [Google Scholar] [CrossRef]
- Zhang, D.; Atkinson, H.V.; Dong, H.; Zhu, Q. Differential Scanning Calorimetry (DSC) and Thermodynamic Prediction of Liquid Fraction vs Temperature for Two High-Performance Alloys for Semi-Solid Processing (Al-Si-Cu-Mg (319s) and Al-Cu-Ag (201)). Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 4701–4712. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Bai, S.; Ying, P.; Lin, L. Effect of Ag Additions on the Lengthening Rate of Ω Plates and Formation of σ Phase in Al-Cu-Mg Alloys during Thermal Exposure. Mater. Charact. 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Xiao, D.H.; Wang, J.N.; Ding, D.Y.; Chen, S.P. Effect of Cu Content on the Mechanical Properties of an Al-Cu-Mg-Ag Alloy. J. Alloys Compd. 2002, 343, 77–81. [Google Scholar] [CrossRef]
- Chen, J.; Ling, K.; Deng, P.; Mo, W.; Tang, C.; Ouyang, Z.; Luo, B.; Bai, Z. Effect of Mg Content on Microstructure, Mechanical Properties and Intergranular Corrosion Properties of Al-Cu-Mg-Ag Alloys. Mater. Today Commun. 2023, 34, 105363. [Google Scholar] [CrossRef]
- Bai, S.; Zhou, X.; Liu, Z.; Xia, P.; Liu, M.; Zeng, S. Effects of Ag Variations on the Microstructures and Mechanical Properties of Al-Cu-Mg Alloys at Elevated Temperatures. Mater. Sci. Eng. A 2014, 611, 69–76. [Google Scholar] [CrossRef]
- Rakhmonov, J.U.; Vo, N.Q.; Croteau, J.R.; Dorn, J.; Dunand, D.C. Laser-Melted Al-3.6Mn-2.0Fe-1.8Si-0.9Zr (Wt%) Alloy with Outstanding Creep Resistance via Formation of α-Al(FeMn)Si Precipitates. Addit. Manuf. 2022, 60, 103285. [Google Scholar] [CrossRef]


| Al | Cr | Cu | Fe | Mg | Mn | Si | Zn | Ag | V | Ti | Zr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal | Bal. | <0.05 | 4.5–5.5 | <0.15 | >0.8 | 0.2–0.6 | <0.1 | <0.25 | 0.15–0.60 | <0.05 | Ti + Zr < 4.0 | |
| Measured | Bal. | 0.02 | 5.41 | 0.11 | 0.70 | 0.57 | 0.04 | - | 0.44 | - | 1.08 | 1.80 |
| As-Built | T4 | T4 + 50 h @ 150 °C | T4 + 50 h @ 250 °C | T4 + 50 h @ 300 °C | |
|---|---|---|---|---|---|
| HV0.5 | 109.7 | 186.1 | 167.9 | 119.3 | 110.3 |
 | |||||
| Cu | |||||
| Mg | |||||
| Zr | |||||
| Ti | |||||
| Mn | |||||
| Fe | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larini, F.; Casati, R.; Marola, S.; Vedani, M. Microstructural Evolution of a High-Strength Zr-Ti-Modified 2139 Aluminum Alloy for Laser Powder Bed Fusion. Metals 2023, 13, 924. https://doi.org/10.3390/met13050924
Larini F, Casati R, Marola S, Vedani M. Microstructural Evolution of a High-Strength Zr-Ti-Modified 2139 Aluminum Alloy for Laser Powder Bed Fusion. Metals. 2023; 13(5):924. https://doi.org/10.3390/met13050924
Chicago/Turabian StyleLarini, Federico, Riccardo Casati, Silvia Marola, and Maurizio Vedani. 2023. "Microstructural Evolution of a High-Strength Zr-Ti-Modified 2139 Aluminum Alloy for Laser Powder Bed Fusion" Metals 13, no. 5: 924. https://doi.org/10.3390/met13050924
APA StyleLarini, F., Casati, R., Marola, S., & Vedani, M. (2023). Microstructural Evolution of a High-Strength Zr-Ti-Modified 2139 Aluminum Alloy for Laser Powder Bed Fusion. Metals, 13(5), 924. https://doi.org/10.3390/met13050924








