Technological Perception with Rural and Urban Differentiation and Its Influence on the Quality of Life of Older People with Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Sampling
2.3. Method Description
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- The World Bank Data. Population Ages 65 and Above (% of Total Population). Available online: https://data.worldbank.org/indicator/SP.POP.65UP.TO.ZS (accessed on 23 November 2023).
- Varadaraj, V.; Ehrlich, J.R.; Swenor, B.K. Vision Impairment Has Implications for Aging and Health Outcomes, Beyond Ophthalmology. JAMA Netw. Open 2022, 5, e2214610. [Google Scholar] [CrossRef] [PubMed]
- Brunes, A.; Heir, T. Visual impairment and depression: Age-specific prevalence, associations with vision loss, and relation to life satisfaction. World J. Psychiatry 2020, 10, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Petri, D.; Maurutto, E.; Lucenteforte, E.; Menchini, F.; Lanzetta, P.; Varano, M.; van Nispen, R.M.A.; Virgili, G. Association Between Visual Impairment and Depression in Patients Attending Eye Clinics. JAMA Ophthalmol. 2021, 139, 753. [Google Scholar] [CrossRef] [PubMed]
- Demmin, D.L.; Silverstein, S.M. Visual Impairment and Mental Health: Unmet Needs and Treatment Options. Clin. Ophthalmol. 2020, 14, 4229–4251. [Google Scholar] [CrossRef] [PubMed]
- Heinze, N.; Davies, F.; Jones, L.; Castle, C.L.; Gomes, R.S.M. Conceptualizations of well-being in adults with visual impairment: A scoping review. Front. Psychol. 2022, 13, 964537. [Google Scholar] [CrossRef] [PubMed]
- Partow, S.; Cook, R.; McDonald, R. Coping with stigmatization and discrimination related to blindness and low vision. Rehabil. Psychol. 2021, 66, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, Z.; Xu, N.; Shao, S.; Ma, H.; He, Q.; Zhang, D.; Xu, H.; Qu, H. The relation between self-stigma and loneliness in visually impaired college students: Self-acceptance as mediator. Disabil. Health J. 2021, 14, 101054. [Google Scholar] [CrossRef]
- Ong, S.R.; Crowston, J.G.; Loprinzi, P.D.; Ramulu, P.Y. Physical activity, visual impairment, and eye disease. Eye 2018, 32, 1296–1303. [Google Scholar] [CrossRef]
- Hamedani, A.G.; VanderBeek, B.L.; Willis, A.W. Blindness and Visual Impairment in the Medicare Population: Disparities and Association with Hip Fracture and Neuropsychiatric Outcomes. Ophthalmic. Epidemiol. 2019, 26, 279–285. [Google Scholar] [CrossRef]
- Kotwal, A.A.; Holt-Lunstad, J.; Newmark, R.L.; Cenzer, I.; Smith, A.K.; Covinsky, K.E.; Escueta, D.P.; Lee, J.M.; Perissinotto, C.M. Social Isolation and Loneliness among San Francisco Bay Area Older Adults during the COVID-19 Shelter-in-Place Orders. J. Am. Geriatr. Soc. 2021, 69, 20–29. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, H.; Leng, M.; Wang, Z. Intrinsic Capacity to Predict Future Adverse Health Outcomes in Older Adults: A Scoping Review. Healthcare 2023, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Chuvarayan, Y.; Finger, R.P.; Köberlein-Neu, J. Economic burden of blindness and visual impairment in Germany from a societal perspective: A cost-of-illness study. Eur. J. Health Econ. 2020, 21, 115–127. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness; Vision Impairment Collaborators; the Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity1This paper has been edited by Neville N. Osborne, PhD, DSc, Nuffield Laboratory of Ophthalmology, University of Oxford, Walton Street, Oxford, UK; and Gerald J. Chader, The Foundation Fighting Blindness, Hunt Valley, MS.1. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Wang, J.J. Prevalence of Age-related Maculopathy in Australia. Ophthalmology 1995, 102, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The Prevalence of Age-Related Macular Degeneration in Asians. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The Natural History and Prognosis of Neovascular Age-Related Macular Degeneration. Ophthalmology 2008, 115, 116–126.e1. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-Related Macular Degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar]
- Instituto Nacional de Estadística 2021; Encuesta de Discapacidad, Autonomía Personal y Situaciones de Dependencia 2020. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176782&menu=resultados&idp=1254735573175#_tabs-1254736195764 (accessed on 2 February 2023).
- Lin, X.; Lou, L.; Miao, Q.; Wang, Y.; Jin, K.; Shan, P.; Xu, Y. The pattern and gender disparity in global burden of age-related macular degeneration. Eur. J. Ophthalmol. 2021, 31, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kansal, K.; Khan, H. Environmental factors and eye health: Protecting your vision in a changing world. Int. J. Ophthalmol. Optometry 2023, 5, 33–35. [Google Scholar] [CrossRef]
- Swięch, A.; Dolar-Szczasny, J.; Wrobel-Dudzinska, D.; Kosior-Jarecka, E.; Mackiewicz, J. Quality of life among patients from urban and rural areas with advanced age-related macular degeneration assessed using the NEI-VFQ-25. Ann. Agric. Environ. Med. 2021, 28, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, M.M.; Zainullin, R.M.; Gilmanshin, T.R.; Kazakbaeva, G.M.; Rakhimova, E.M.; Rusakova, I.A.; Bolshakova, N.I.; Safiullina, K.R.; Yakupova, D.F.; Uzianbaeva, Y.V.; et al. Prevalence and Associated Factors of Age-Related Macular Degeneration in a Russian Population: The Ural Eye and Medical Study. Am. J. Ophthalmol. 2020, 210, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Abdiyeva, Y.J. Prevalence of age-related macular degeneration of retina among rural and urban population of the western region of Azerbaijan. Vestn. Oftalmol. 2021, 137, 160. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, S.; Tabuchi, H.; Ohsugi, H.; Enno, H.; Ishitobi, N.; Masumoto, H.; Kiuchi, Y. Accuracy of ultra-wide-field fundus ophthalmoscopy-assisted deep learning, a machine-learning technology, for detecting age-related macular degeneration. Int. Ophthalmol. 2019, 39, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, A.; Boneva, S.; Gruber, M.; Zhang, P.; Horres, R.; Bucher, F.; Auw-Haedrich, C.; Hansen, L.; Stahl, A.; Hilgendorf, I.; et al. Transcriptomic Characterization of Human Choroidal Neovascular Membranes Identifies Calprotectin as a Novel Biomarker for Patients with Age-Related Macular Degeneration. Am. J. Pathol. 2020, 190, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Hanrahan, B.W. The Effects of Technological Advances on Outcomes for Elderly Persons With Exudative Age-related Macular Degeneration. JAMA Ophthalmol. 2014, 132, 456. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Clark, W.L.; Nielsen, J.S.; Brill, J.V.; Greene, L.S.; Heggen, C.L. Optimizing Anti-VEGF Treatment Outcomes for Patients with Neovascular Age-Related Macular Degeneration. J. Manag. Care Spec. Pharm. 2018, 24, S3–S15. [Google Scholar] [CrossRef]
- Prenner, J.L.; Halperin, L.S.; Rycroft, C.; Hogue, S.; Liu, Z.W.; Seibert, R. Disease Burden in the Treatment of Age-Related Macular Degeneration: Findings From a Time-and-Motion Study. Am. J. Ophthalmol. 2015, 160, 725–731.e1. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Muñoz, V.; Agulló-Tomás, M.S.; Forjaz, M.J.; Fernandez, E.; Rodriguez-Blazquez, C.; Ayala, A.; Fernandez-Mayoralas, G. Technology, Gender and COVID-19. Analysis of Perceived Health in Adults and Older People. In Human Aspects of IT for the Aged Population. Supporting Everyday Life Activities; Springer: Cham, Switzerland, 2021; pp. 363–379. [Google Scholar] [CrossRef]
- O’connor, S.R.; Treanor, C.; Ward, E.; Wickens, R.A.; O’connell, A.; Culliford, L.A.; Rogers, C.A.; Gidman, E.A.; Peto, T.; Knox, P.C.; et al. Patient Acceptability of Home Monitoring for Neovascular Age-Related Macular Degeneration Reactivation: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 13714. [Google Scholar] [CrossRef] [PubMed]
- Deffler, R.A.; Xu, J.; Bittner, A.K.; Bowers, A.R.; Hassan, S.E.; Ross, N.; Cooley, S.-S.L.; Doubt, A.; Davidorf, F.H.; Dougherty, B.E. Use and Perceptions of Advanced Driver Assistance Systems by Older Drivers with and without Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2022, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.A. The Psychosocial Impact of Macular Degeneration. Arch. Ophthalmol. 1998, 116, 514. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Bradley, C. Quality of life in age-related macular degeneration: A review of the literature. Health Qual. Life Outcomes 2006, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, M.R.; Calles-Monar, P.S.; Alonso-Tarancón, A.M.; Coco-Martín, R.M.; Mayo-Iscar, A. Impact of COVID-19 Confinement on Quality of Life of Patients with Age-Related Macular Degeneration: A Two-Wave Panel Study. J. Clin. Med. 2023, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, K.; Mitchell, P.; Detaram, H.D.; Burlutsky, G.; Liew, G.; Gopinath, B. Risk factors for poorer quality of life in patients with neovascular age-related macular degeneration: A longitudinal clinic-based study. Eye 2023, 37, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.B.; da Silva Borges, G.F.; Abe, R.Y.; de Souza, O.F.; Machado, M.C.; Ferreira, T.; José, N.K.; de Vasconcellos, J.P.C. The effects of age-related macular degeneration on quality of life in a Brazilian population. Int. J. Retina Vitreous 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Man, R.E.K.; Gan, A.T.L.; Fenwick, E.K.; Teo, K.Y.C.; Tan, A.C.; Cheung, G.C.M.; Teo, Z.L.; Kumari, N.; Wong, T.Y.; Lamoureux, E.L.; et al. Impact of incident age-related macular degeneration and associated vision loss on vision-related quality of life. Br. J. Ophthalmol. 2022, 106, 1063–1068. [Google Scholar] [CrossRef]
- Van Nispen, R.M.; de Boer, M.R.; Hoeijmakers, J.G.; Ringens, P.J.; van Rens, G.H. Co-morbidity and visual acuity are risk factors for health-related quality of life decline: Five-month follow-up EQ-5D data of visually impaired older patients. Health Qual. Life Outcomes 2009, 7, 18. [Google Scholar] [CrossRef]
- Marín, B.P.; Paniagua, N.M.G.; Gómez-Baldó, L.; Gallego-Pinazo, R. Burden of disease assessment in patients with neovascular age-related macular degeneration in Spain: Results of the AMD-MANAGE study. Eur. J. Ophthalmol. 2022, 32, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Cimas, M.; Ayala, A.; Sanz, B.; Agulló-Tomás, M.S.; Escobar, A.; Forjaz, M.J. Chronic musculoskeletal pain in European older adults: Cross-national and gender differences. Eur. J. Pain 2018, 22, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Metz, D.H. Mobility of older people and their quality of life. Transp. Policy (Oxf.) 2000, 7, 149–152. [Google Scholar] [CrossRef]
- Zorrilla-Muñoz, V.; García-Sedano, T.; Agulló-Tomás, M.S. Análisis socio-ergonómico en la agricultura. Evaluación del sector oleico desde una perspectiva de género y envejecimiento. Inf. Tec. Econ. Agrar. 2019, 115, 83–104. [Google Scholar] [CrossRef]
- Stevens, G.A.; White, R.A.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; Resnikoff, S.; et al. Global Prevalence of Vision Impairment and Blindness. Ophthalmology 2013, 120, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Fincher, C.L.; Thornhill, R. Why men have shorter lives than women: Effects of resource availability, infectious disease, and senescence. Am. J. Hum. Biol. 2009, 21, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, M. Age-related eye disease and gender. Maturitas 2016, 83, 19–26. [Google Scholar] [CrossRef]
- Zorrilla-Muñoz, V.; Veira-Ramos, A.; Agulló-Tomás, M.S.; Garcia-Aracil, N.; Fernandez, E. Effectiveness of Support Programmes for (in)Formal Caregivers of Older Dependent People to Design Technologies. In Human Aspects of IT for the Aged Population; Gao, Q., Zhou, J., Eds.; HCII 2023; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2023; Volume 14043. [Google Scholar] [CrossRef]
- Cruceanu, G.L.; Clemente-Belmonte, S.; Herrero-Sanz, R.; Ayala, A.; Zorrilla-Muñoz, V.; Agulló-Tomás, M.S.; Martínez-Miguelez, C.; Fernández-Mayoralas, G. Evaluation of Older People Digital Images: Representations from a Land, Gender and Anti-ageist Perspective. Land 2022, 12, 18. [Google Scholar] [CrossRef]
- Quesada, B.C.; Muñoz, V.Z.; Tomás, M.S.A. El uso de tecnologías de asistencia sanitaria digital por parte de la población mayor desde una perspectiva de género e intrageneracional. Teknokultura. Rev. Cult. Digit. Mov. Soc. 2021, 18, 103–113. [Google Scholar] [CrossRef]
- Mikołajczyk, B. Universal human rights instruments and digital literacy of older persons. Int. J. Hum. Rights 2023, 27, 403–424. [Google Scholar] [CrossRef]
- Orr, P.; Rentz, A.M.; Margolis, M.K.; Revicki, D.A.; Dolan, C.M.; Colman, S.; Fine, J.T.; Bressler, N.M. Validation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 3354. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.S.; Delbaere, K.; Lord, S.R.; Beaumont, P.; Vaegan; Madigan, M.C. Depressive symptoms and quality of life in people with age-related macular degeneration. Ophthalmic Physiol. Opt. 2011, 31, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Henning-Smith, C. The Unique Impact of COVID-19 on Older Adults in Rural Areas. J. Aging Soc. Policy 2020, 32, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Fuller, H.R.; Huseth-Zosel, A. Older Adults’ Loneliness in Early COVID-19 Social Distancing: Implications of Rurality. J. Gerontol. Ser. B 2022, 77, e100–e105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, T.; Dasgupta, R.K.; Xia, R. Narrowing the age-based digital divide: Developing digital capability through social activities. Inf. Syst. J. 2023, 33, 268–298. [Google Scholar] [CrossRef]
- Tirado-Morueta, R.; Rodríguez-Martín, A.; Álvarez-Arregui, E.; Ortíz-Sobrino, M.Á.; Aguaded-Gómez, J.I. The digital inclusion of older people in Spain: Technological support services for seniors as predictor. Ageing Soc. 2023, 43, 1409–1435. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, K.H.; Hwang, J.G.; Jun, S.; Yu, J.; Lee, C. Technological Opportunity Analysis: Assistive Technology for Blind and Visually Impaired People. Sustainability 2020, 12, 8689. [Google Scholar] [CrossRef]
- LaMonica, H.M.; Davenport, T.A.; Roberts, A.E.; Hickie, I.B. Understanding Technology Preferences and Requirements for Health Information Technologies Designed to Improve and Maintain the Mental Health and Well-Being of Older Adults: Participatory Design Study. JMIR Aging 2021, 4, e21461. [Google Scholar] [CrossRef]
- Armstrong, G.W.; Miller, J.B. Telemedicine for the Diagnosis and Management of Age-Related Macular Degeneration: A Review. J. Clin. Med. 2022, 11, 835. [Google Scholar] [CrossRef]
- Perepelkina, T.; Fulton, A.B. Aplicaciones de inteligencia artificial (IA) para la degeneración macular relacionada con la edad (DMAE) y otras distrofias de retina. Semin. Ophthalmol. 2021, 36, 304–309. [Google Scholar] [CrossRef]
- Russell, C.D.; Lone, N.I.; Baillie, J.K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 2023, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nalla, L.V.; Sharma, M.; Sharma, N.; Singh, A.A.; Malim, F.M.; Ghatage, M.; Mukarram, M.; Pawar, A.; Parihar, N.; et al. Association of COVID-19 with Comorbidities: An Update. ACS Pharmacol. Transl. Sci. 2023, 6, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Wu, J.; Qian, L.; Lewin, B.; Sy, L.S.; Lin, I.-C.; Ku, J.H.; Tseng, H.F. Risk of herpes zoster following mRNA COVID-19 vaccine administration. Expert Rev. Vaccines 2023, 22, 643–649. [Google Scholar] [CrossRef]
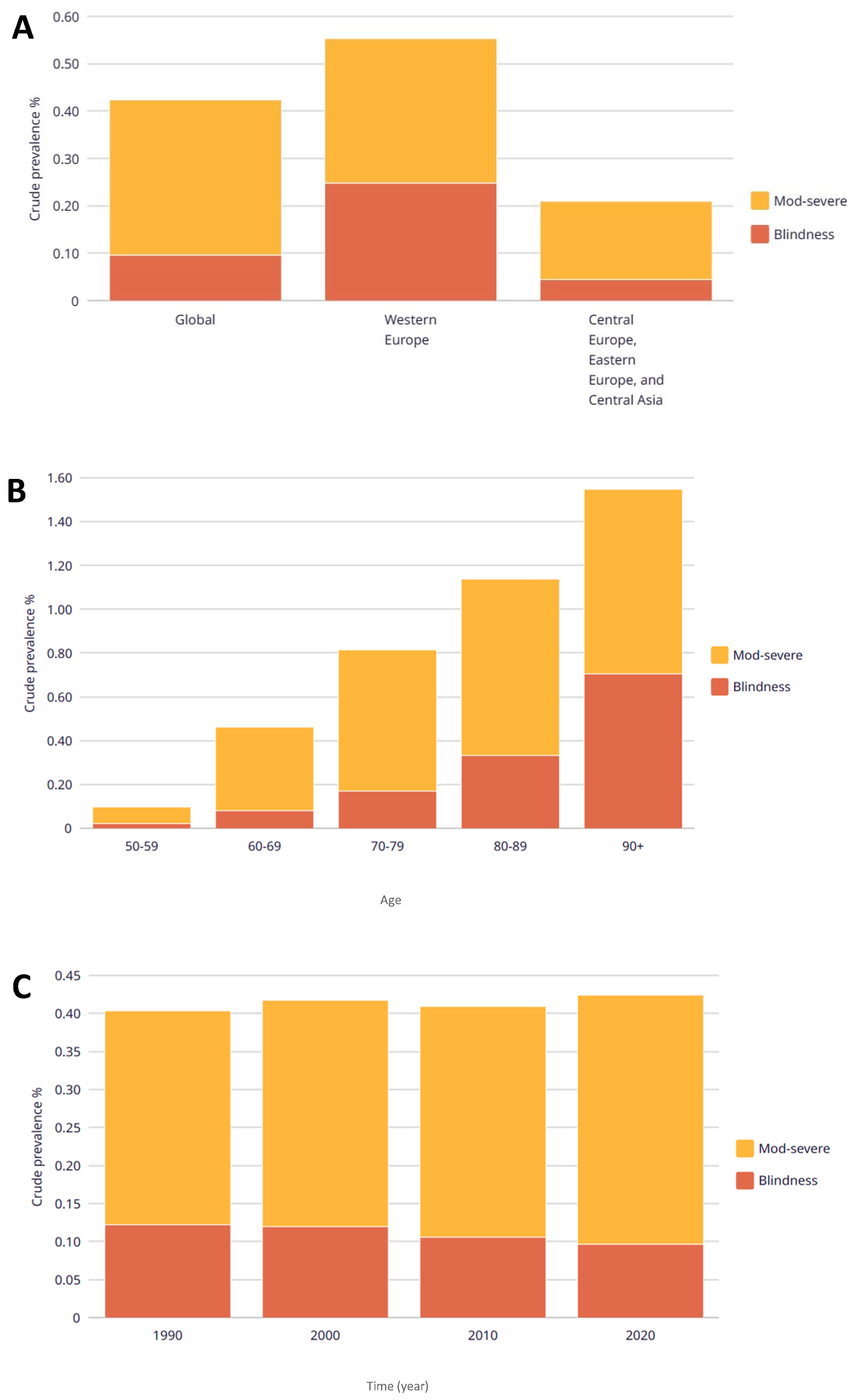
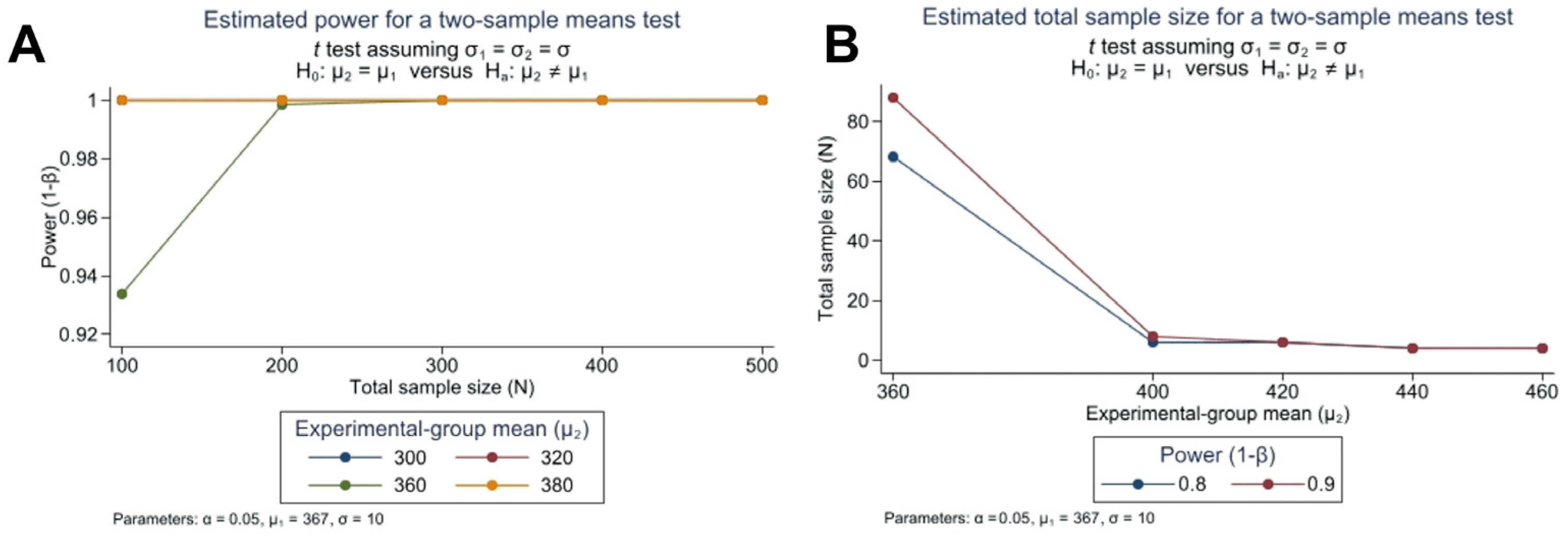

| Sociodemographics Variables | Totals (n = 2405) | Rural Areas (n = 855) ˂ 20,000 Habitants | Urban Areas (n = 1550) ≥ 20,000 Habitants | |||||
|---|---|---|---|---|---|---|---|---|
| Totals (n = 2405) | AMD (n = 367) | Non-AMD (n = 2038) | AMD Persons (n = 109) | Non-AMD Persons (n = 746) | AMD Persons (n = 258) | Non-AMD Persons (n = 1292) | ||
| [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | [Mean ± SD] | ||
| [N(%)] | [N(%)] | [N(%)] | [N(%)] | [N(%)] | [N(%)] | [N(%)] | ||
| AMD diagnosis | Persons with AMD diagnosis | 367 (15.26) | 367 (100) | -- | 109 (100) | 258 (100) | -- | |
| Persons with non-AMD diagnosis | 2038 (84.74) | -- | 2038 (100) | -- | 746 (100) | -- | 1292 (100) | |
| Sex | Men | 870 (36.55) | 110 (29.97) | 760 (37.29) | 33 (30.28) | 292 (39.14) | 77 (29.84) | 468 (36.22) |
| Women | 1535 (63.83) | 257 (70.03) | 1278 (62.71) | 76 (69.72) | 454 (60.86) | 181 (70.16) | 824 (63.78) | |
| Age (≥50 years) | Total (n = 2405) | 79.20 ± 0.27 | 79.29 ± 0.63 | 73.27 ± 0.29 | 77.38 ± 1.18 | 74.77 ± 0.47 | 80.24 ± 0.74 | 72.40 ± 0.36 |
| Women (n = 1535) | 72.81 ± 0.43 | 77.94 ± 1.14 | 72.08 ± 0.46 | 79.22 ± 1.33 | 75.32 ± 0.61 | 80.34 ± 0.92 | 73.23 ± 0.45 | |
| Men (n = 870) | 74.99 ± 0.34 | 80.01 ± 0.76 | 73.97 ± 0.37 | 73.12 ± 2.27 | 73.91 ± 0.73 | 80.01 ± 1.25 | 70.93 ± 0.59 | |
| Total (n = 2405) | Rural Areas (n = 855) | Urban Areas (n = 1550) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 2405) | AMD (n = 367) | Non-AMD (n = 2038) | AMD Persons (n = 109) | Non-AMD Persons (n = 746) | AMD Persons (n = 258) | Non-AMD Persons (n = 1292) | |||||||||
| N | % total | N | % AMD | N | % Non-AMD | N | % AMD | N | % Non-AMD | N | % AMD | N | % Non-AMD | ||
| Diagnosis related to vision | Retinitis pigmentosa | 74 | 3.08 | 15 | 4.09 | 59 | 2.89 | 6 | 5.50 | 25 | 3.35 | 9 | 3.49 | 34 | 2.63 |
| Magma myopia | 257 | 10.69 | 56 | 15.26 | 201 | 9.86 | 17 | 15.60 | 74 | 9.92 | 39 | 15.12 | 127 | 9.83 | |
| Diabetic retinopathy | 150 | 6.24 | 21 | 5.72 | 129 | 6.33 | 5 | 4.59 | 45 | 6.03 | 16 | 6.20 | 84 | 6.50 | |
| Glaucoma | 345 | 14.35 | 73 | 19.89 | 272 | 13.35 | 26 | 23.85 | 94 | 12.60 | 47 | 18.22 | 178 | 13.78 | |
| Cataract | 1207 | 50.19 | 195 | 53.13 | 1012 | 49.66 | 56 | 51.38 | 381 | 51.07 | 139 | 53.88 | 631 | 48.84 | |
| Diagnosis non-related to vision | Cancer/malignant tumor | 268 | 11.14 | 50 | 13.62 | 218 | 10.70 | 16 | 14.68 | 76 | 10.19 | 34 | 13.18 | 142 | 10.99 |
| Diabetes | 678 | 28.19 | 83 | 22.62 | 595 | 29.20 | 31 | 28.44 | 221 | 29.62 | 42 | 16.28 | 374 | 28.95 | |
| Chronic depression | 464 | 19.29 | 72 | 19.62 | 392 | 19.23 | 25 | 22.94 | 118 | 15.82 | 47 | 18.22 | 274 | 21.21 | |
| Chronic anxiety | 423 | 17.59 | 53 | 14.44 | 370 | 18.16 | 21 | 19.27 | 110 | 14.75 | 32 | 12.40 | 260 | 20.12 | |
| Parkinson | 80 | 3.33 | 13 | 3.54 | 67 | 3.29 | 5 | 4.59 | 16 | 2.14 | 8 | 3.10 | 51 | 3.95 | |
| Alzheimer | 260 | 10.81 | 27 | 7.36 | 233 | 11.43 | 10 | 9.17 | 46 | 6.17 | 17 | 6.59 | 87 | 6.73 | |
| Muscular dystrophy | 149 | 6.20 | 25 | 6.81 | 124 | 6.08 | 10 | 9.17 | 37 | 4.96 | 15 | 5.81 | 87 | 6.73 | |
| Stroke | 250 | 10.40 | 36 | 9.81 | 214 | 10.50 | 13 | 11.93 | 86 | 11.53 | 23 | 8.91 | 128 | 9.91 | |
| Myocardial infarction | 222 | 9.23 | 38 | 10.35 | 184 | 9.03 | 12 | 11.01 | 72 | 9.65 | 26 | 10.08 | 112 | 8.67 | |
| Arthritis | 720 | 29.94 | 124 | 33.79 | 596 | 29.24 | 35 | 32.11 | 228 | 30.56 | 89 | 34.50 | 368 | 28.48 | |
| Osteoarthritis | 1242 | 51.64 | 221 | 60.22 | 1021 | 50.10 | 61 | 55.96 | 382 | 51.21 | 160 | 62.02 | 369 | 28.56 | |
| Total (n = 2405) | Rural Areas (n = 855) ˂ 20,000 Habitants | Urban Areas (n = 1550) ≥ 20,000 Habitants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMD (n = 367) | Non-AMD (n = 2038) | AMD Persons (n = 109) | Non-AMD Persons (n = 746) | AMD Persons (n = 258) | Non-AMD Persons (n = 1292) | |||||||||||||
| ITEM | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient | Average Interitem Covariance | Number of Items in the Scale | Scale Reliability Coefficient |
| Visibility | 0.1938 1 | 3 | 0.6314 2 | 0.2628 1 | 3 | 0.7353 2 | --- | 1 | --- | 0.0742 | 6 | 0.2628 | --- | 1 | --- | 0.2560 1 | 3 | 0.7181 2 |
| Communication | --- | 1 | --- | 0.3244 1 | 6 | 0.8741 3 | --- | 0 | --- | 0.3816 1 | 5 | 0.8886 3 | --- | 0 | --- | --- | 0 | --- |
| Learning | 0.3410 1 | 7 | 0.8642 3 | 0.2225 1 | 4 | 0.7573 2 | --- | 0 | --- | --- | 1 | --- | --- | 0 | --- | --- | 0 | --- |
| Mobility | 0.2799 1 | 8 | 0.8511 3 | 0.2781 1 | 14 | 0.9119 3 | --- | 1 | --- | 0.2494 1 | 10 | 0.8812 3 | 0.2753 1 | 4 | 0.7243 2 | 0.2881 1 | 9 | 0.8772 3 |
| Selfcare | --- | 1 | --- | 0.3573 1 | 9 | 0.8806 3 | --- | 0 | --- | 0.5014 | 6 | 0.8535 3 | --- | 1 | --- | 0.2651 1 | 3 | 0.7213 2 |
| Domestic life | 0.3815 1 | 3 | 0.7744 2 | 0.3983 1 | 6 | 0.8474 3 | 0.2966 1 | 2 | 0.7428 2 | 0.3848 1 | 5 | 0.8314 3 | --- | 1 | --- | 0.4026 1 | 6 | 0.8490 3 |
| Interpersonal relationships | --- | 0 | --- | 0.2399 1 | 4 | 0.7646 2 | --- | 0 | --- | 0.3043 1 | 3 | 0.7937 2 | --- | 0 | --- | 0.2882 1 | 2 | 0.8094 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Sanchez, A.; Zorrilla-Muñoz, V.; Martinez-Navarrete, G.; Fernandez, E. Technological Perception with Rural and Urban Differentiation and Its Influence on the Quality of Life of Older People with Age-Related Macular Degeneration. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 1470-1488. https://doi.org/10.3390/ejihpe14050097
Parra-Sanchez A, Zorrilla-Muñoz V, Martinez-Navarrete G, Fernandez E. Technological Perception with Rural and Urban Differentiation and Its Influence on the Quality of Life of Older People with Age-Related Macular Degeneration. European Journal of Investigation in Health, Psychology and Education. 2024; 14(5):1470-1488. https://doi.org/10.3390/ejihpe14050097
Chicago/Turabian StyleParra-Sanchez, Angel, Vanessa Zorrilla-Muñoz, Gema Martinez-Navarrete, and Eduardo Fernandez. 2024. "Technological Perception with Rural and Urban Differentiation and Its Influence on the Quality of Life of Older People with Age-Related Macular Degeneration" European Journal of Investigation in Health, Psychology and Education 14, no. 5: 1470-1488. https://doi.org/10.3390/ejihpe14050097
APA StyleParra-Sanchez, A., Zorrilla-Muñoz, V., Martinez-Navarrete, G., & Fernandez, E. (2024). Technological Perception with Rural and Urban Differentiation and Its Influence on the Quality of Life of Older People with Age-Related Macular Degeneration. European Journal of Investigation in Health, Psychology and Education, 14(5), 1470-1488. https://doi.org/10.3390/ejihpe14050097










