The Linguistic–Cognitive Profile in an Adult Population with Parkinson’s Disease and Deep Brain Stimulation: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
2.4. Design
3. Results
3.1. Differences in the Linguistic Profile among the Groups
3.2. Differences in the Cognitive Profile among the Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayles, K.; Tomoeda, C. Cognitive-Communication Disorders of Dementia; Plural Publishing: San Diego, CA, USA, 2007. [Google Scholar]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Borghammer, P.; Van Den Berge, N. Brain-First versus Gut-First Parkinson’s Disease: A Hypothesis. J. Park. Dis. 2019, 9, S281–S295. [Google Scholar] [CrossRef]
- Onder, H.; Dilek, S.S.; Bahtiyarca, Z.T.; Comoglu, S. Analyses of the clinical factors and freezing of gait in association with the quality-of-life indexes in Parkinson’s disease subjects with and without STN-DBS therapy. Neurol. Res. 2024, 46, 207–212. [Google Scholar] [CrossRef]
- LaHue, S.C.; Ostrem, J.L.; Galifianakis, N.B.; San Luciano, M.; Ziman, N.; Wang, S.; Racine, C.A.; Starr, P.A.; Larson, P.S.; Katz, M. Parkinson’s disease patient preference and experience with various methods of DBS lead placement. Park. Relat. Disord. 2017, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non motor subtypes and Parkinson’s disease. Park. Relat. Disord. 2016, 22 (Suppl. S1), S41–S46. [Google Scholar] [CrossRef]
- Benabid, A.L. Deep brain stimulation for Parkinson’s disease. Curr. Opin. Neurobiol. 2003, 13, 696–706. [Google Scholar] [CrossRef]
- Mallet, L.; Mesnage, V.; Houeto, J.L.; Pelissolo, A.; Yelnik, J.; Behar, C.; Gargiulo, M.; Welter, M.L.; Bonnet, A.M.; Pillon, B.; et al. Compulsions, Parkinson’s disease, and stimulation. Lancet 2002, 360, 1302–1304. [Google Scholar] [CrossRef]
- Benabid, A.L.; Chabardes, S.; Mitrofanis, J.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009, 8, 67–81. [Google Scholar] [CrossRef]
- Parsons, T.D.; Rogers, S.A.; Braaten, A.J.; Woods, S.P.; Tröster, A.I. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: A meta-analysis. Lancet Neurol. 2006, 5, 578–588. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease and movement disorders: Moving forward. Lancet Neurol. 2008, 7, 9–11. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Hauser, R.A.; Zesiewicz, T.A. Advances in Parkinson’s disease. Park. Relat. Disord. 2011, 17, S1–S2. [Google Scholar]
- Fahn, S.; Jankovic, J.; Hallett, M. (Eds.) Principles and Practice of Movement Disorders, 2nd ed.; Elsevier: Madrid, Spain, 2015. [Google Scholar]
- Shulman, L.M.; Tanner, C.M. Parkinson’s Disease: Diagnosis and Clinical Management, 2nd ed.; Demos Medical Publishing: London, UK, 2017. [Google Scholar]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive impairment in Parkinson’s disease: The dual syndrome hypothesis. Neuro-Degener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Foltynie, T.; Brayne, C.E.; Robbins, T.W.; Barker, R.A. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain A J. Neurol. 2007, 130 Pt 7, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Muslimovic, D.; Post, B.; Speelman, J.D.; Schmand, B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005, 65, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A.; Kragh-Sorensen, P. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch. Neurol. 2003, 60, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.L. Language and Parkinson’s disease. Annu. Rev. Appl. Linguist. 2008, 28, 113–127. [Google Scholar] [CrossRef]
- Altmann, L.J.; Troche, M.S. High-level language production in Parkinson’s disease: A review. Park. Dis. 2011, 2011, 238956. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, S.; Mondini, S.; Signorini, M.; Marchetto, A.; Bambini, V.; Arcara, G. Pragmatic Language Disorder in Parkinson’s Disease and the Potential Effect of Cognitive Reserve. Front. Psychol. 2019, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Iribe, Y.; Kitaoka, N.; Tsuboi, T.; Hiraga, K.; Satake, Y.; Hattori, M.; Tanaka, Y.; Sato, M.; Hori, A.; et al. Analysis of spontaneous speech in Parkinson’s disease by natural language processing. Park. Relat. Disord. 2023, 113, 105411. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, J.; Nakano, H.; Lieberman, P.; Friedman, J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain Lang. 2006, 97, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Noble, E.; Jones, D.; Burn, D. Life with communication changes in Parkinson’s disease. Age Ageing 2006, 35, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Prieto, F.; Radanovic, M.; Schmitt, C.; Barbosa, E.R.; Mansur, L.L. Compreensão de sentenças na doença de Parkinson. Dement. Neuropsychol. 2007, 1, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Hoz, M.; Garrido Del Águila, D.; García Retamero, R. Alteraciones lingüísticas en pacientes con deterioro cognitivo leve. Rev. Neurol. 2021, 72, 67–76. [Google Scholar]
- Tremblay, C.; Vachon-Joannette, J.; Chantal, S.; Langlois, M.; Monetta, M. Is there an Association between Pragmatic Language, Social Cognition and Executive Deficits in Parkinson’s Disease? Procedia-Soc. Behav. Sci. 2012, 61, 185–186. [Google Scholar] [CrossRef][Green Version]
- Cano Villagrasa, A.; Suárez Torres, M.; Valles-González, B. Diagnósticos Fonoaudiológicos y Síntomas no Motores en Pacientes con Enfermedad de Parkinson. Areté Rev. Fonoaudiol. 2020, 20, 63–71. [Google Scholar]
- Cummings, N.A. Emergence of the mental health complex: Adaptive and maladaptive responses. Prof. Psychol. Res. Pract. 1988, 19, 308–315. [Google Scholar] [CrossRef]
- Goldman, J.G.; Vernaleo, B.A.; Camicioli, R.; Dahodwala, N.; Dobkin, R.D.; Ellis, T.; Galvin, J.E.; Marras, C.; Edwards, J.; Fields, J.; et al. Cognitive impairment in Parkinson’s disease: A report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Park. Dis. 2018, 4, 19. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nature reviews. Neurology 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Aracil-Bolaños, I.; Sampedro, F.; Marín-Lahoz, J.; Horta-Barba, A.; Martínez-Horta, S.; Botí, M.; Izquierdo, C. A divergent breakdown of neurocognitive networks in Parkinson’s Disease mild cognitive impairment. Hum. Brain Mapp. 2019, 40, 3233–3242. [Google Scholar] [CrossRef]
- Herrera Gómez, E.; Cuetos Vega, F. Alteraciones Cognitivas y Lingüísticas en la Enfermedad de Parkinson. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 2013. [Google Scholar]
- Cilia, R.; Tunesi, S.; Marotta, G.; Cereda, E.; Siri, C.; Tesei, S.; Zecchinelli, A.L.; Canesi, M.; Mariani, C.B.; Meucci, N.; et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 2016, 80, 662–673. [Google Scholar] [CrossRef]
- Dashtipour, K.; Johnson, E.; Kani, C.; Kani, K.; Hadi, E.; Ghamsary, M.; Chen, J.J. European Parkinson´s Disease Association. In Life with Parkinson´s: Non-Motor Symptoms; EPDA: Brussels, Belgium, 2010. [Google Scholar]
- Stegemöller, E.L.; Hibbing, P.; Radig, H.; Wingate, J. Therapeutic singing as an early intervention for swallowing in persons with Parkinson’s disease. Complement. Ther. Med. 2017, 31, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wengel, S.P.; Bohac, D.; Burke, W.J. Depression in Parkinson’s Disease. In Parkinson’s Disease; CRC Press: Boca Raton, FL, USA, 2004; pp. 329–338. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Pennell, K.D.; Miller, G.W. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 2008, 29, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.T.; Luchesi, K.F. The difficulties in the care of the patient with neurodegenerative diseases: The speech-language therapist and the multi-professional team. Audiol. Commun. Res. 2019, 24, e2063. [Google Scholar] [CrossRef]
- Pedraza, O.L.; Salazar, A.M.; Sierra, F.A.; Soler, D.; Castro, J.; Castillo, P.; Hernández, A.; Piñeros, C. Confiabilidad, validez de criterio y discriminante del Montreal Cognitive Assessment (MoCA) test, en un grupo de adultos de Bogotá. Acta Médica Colomb. 2016, 41, 221–228. [Google Scholar] [CrossRef]
- Matias-Guiu, J.A.; de Bobadilla, R.F.; Escudero, G.; Pérez-Pérez, J.; Cortés, A.; Morenas-Rodríguez, E.; Matías-Guiu, J. Validación de la versión española del test Addenbrooke’s Cognitive Examination III para el diagnóstico de demencia. Neurologia 2015, 30, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Rosell Clari, V.Y.; Hernández Sacristán, C. MetAphAs. Protocolo de Exploración de Habilidades Metalingüísticas Naturales en la Afasia; Nau Llibres: Valencia, Spain, 2014. [Google Scholar]
- Bocanegra, Y.; García, A.M.; Lopera, F.; Pineda, D.; Baena, A.; Ospina, P.; Gómez, F. Differential linguistic impairment in patients with Parkinson’s disease and genetic Parkinsonisms. J. Neurolinguistics 2021, 60, 101003. [Google Scholar]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’.s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Larsen, J.P.; Tysnes, O.B.; Alves, G. Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study. Neurology 2017, 88, 767–774. [Google Scholar] [CrossRef] [PubMed]
| n | Percentage | ||
|---|---|---|---|
| Sex | Men | 31 | 51.6 |
| Women | 29 | 48.4 | |
| Diagnosis | Stage I | 20 | 33.3 |
| Stage II | 20 | 33.4 | |
| Stage III | 20 | 33.3 | |
| Years of treatment | 0–5 years | 13 | 21.7 |
| 5–10 years | 34 | 56.6 | |
| 10–15 years | 13 | 21.7 | |
| Grade of incapacity | Less than 33% | 0 | 0 |
| Between 33% and 66% | 47 | 78.3 | |
| More than 66% | 13 | 21.7 | |
| Medication | Levodopa | 52 | 86.7 |
| Dopamine agonist | 4 | 6.7 | |
| MAO-B enzyme inhibitors | 2 | 3.3 | |
| Catechol-O-methyltransferase inhibitors | 2 | 3.3 | |
| Years since diagnosis | 14–18 years | 21 | 35 |
| 9–13 years | 19 | 31.7 | |
| 4–8 years | 18 | 30 | |
| 1–3 years | 2 | 3.3 | |
| Carer | No | 0 | 0 |
| Yes | 60 | 100 | |
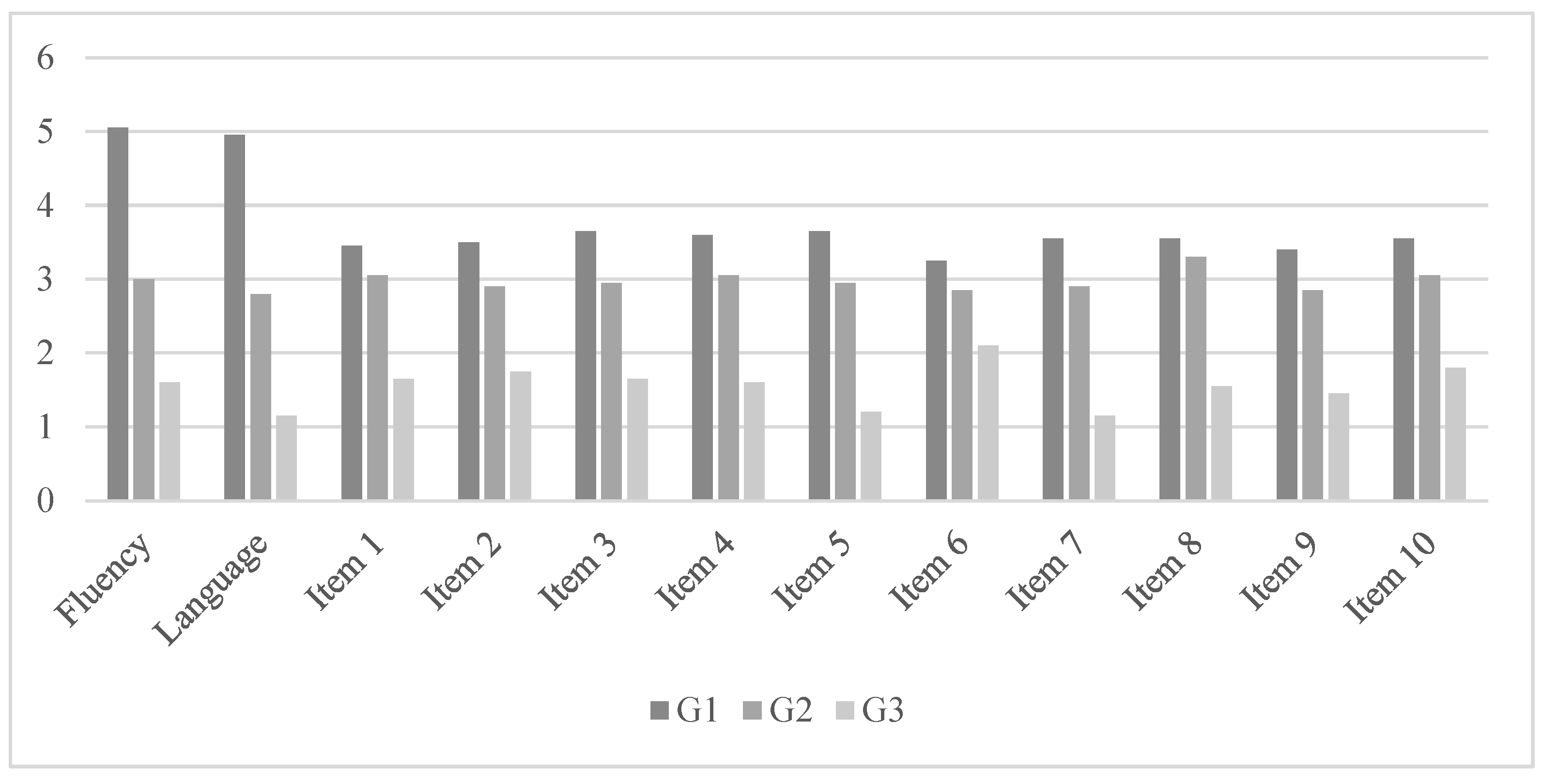
| Profile Linguistic | G1 (n = 20) | G2 (n = 20) | G3 (n = 20) | F (6,52) | η2P | Differences between Groups | |||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||||
| ACE—III | |||||||||
| Fluency | 5.05 | 0.75 | 3.00 | 0.79 | 1.60 | 0.94 | 86.34 * | 0.75 | G3 < G2 < G1 |
| Language | 4.95 | 0.68 | 2.80 | 0.69 | 1.15 | 1.13 | 96.93 * | 0.77 | G3 < G2 < G1 |
| Section 6 of the MetAphAs test | |||||||||
| Item 1 | 3.45 | 0.51 | 3.05 | 0.94 | 1.65 | 1.30 | 18.70 * | 0.39 | G3 < G2 < G1 |
| Item 2 | 3.50 | 0.51 | 2.90 | 0.85 | 1.75 | 1.20 | 19.36 * | 0.40 | G3 < G2 < G1 |
| Item 3 | 3.65 | 0.48 | 2.95 | 0.75 | 1.65 | 1.13 | 29.31 * | 0.50 | G3 < G2 < G1 |
| Item 4 | 3.60 | 0.50 | 3.05 | 0.88 | 1.60 | 1.09 | 28.60 * | 0.50 | G3 < G2 < G1 |
| Item 5 | 3.65 | 0.48 | 2.95 | 0.88 | 1.20 | 1.10 | 42.51 * | 0.59 | G3 < G2 < G1 |
| Item 6 | 3.25 | 0.44 | 2.85 | 0.87 | 2.10 | 1.02 | 10.19 * | 0.26 | G3 < G2 < G1 |
| Item 7 | 3.55 | 0.51 | 2.90 | 0.85 | 1.15 | 1.08 | 42.53 * | 0.59 | G3 < G2 < G1 |
| Item 8 | 3.55 | 0.78 | 3.30 | 0.80 | 1.55 | 1.14 | 32.15 * | 0.53 | G3 < G2 < G1 |
| Item 9 | 3.40 | 0.50 | 2.85 | 0.74 | 1.45 | 0.88 | 38.03 * | 0.57 | G3 < G2 < G1 |
| Item 10 | 3.55 | 0.51 | 3.05 | 0.82 | 1.80 | 1.10 | 22.53 * | 0.44 | G3 < G2 < G1 |
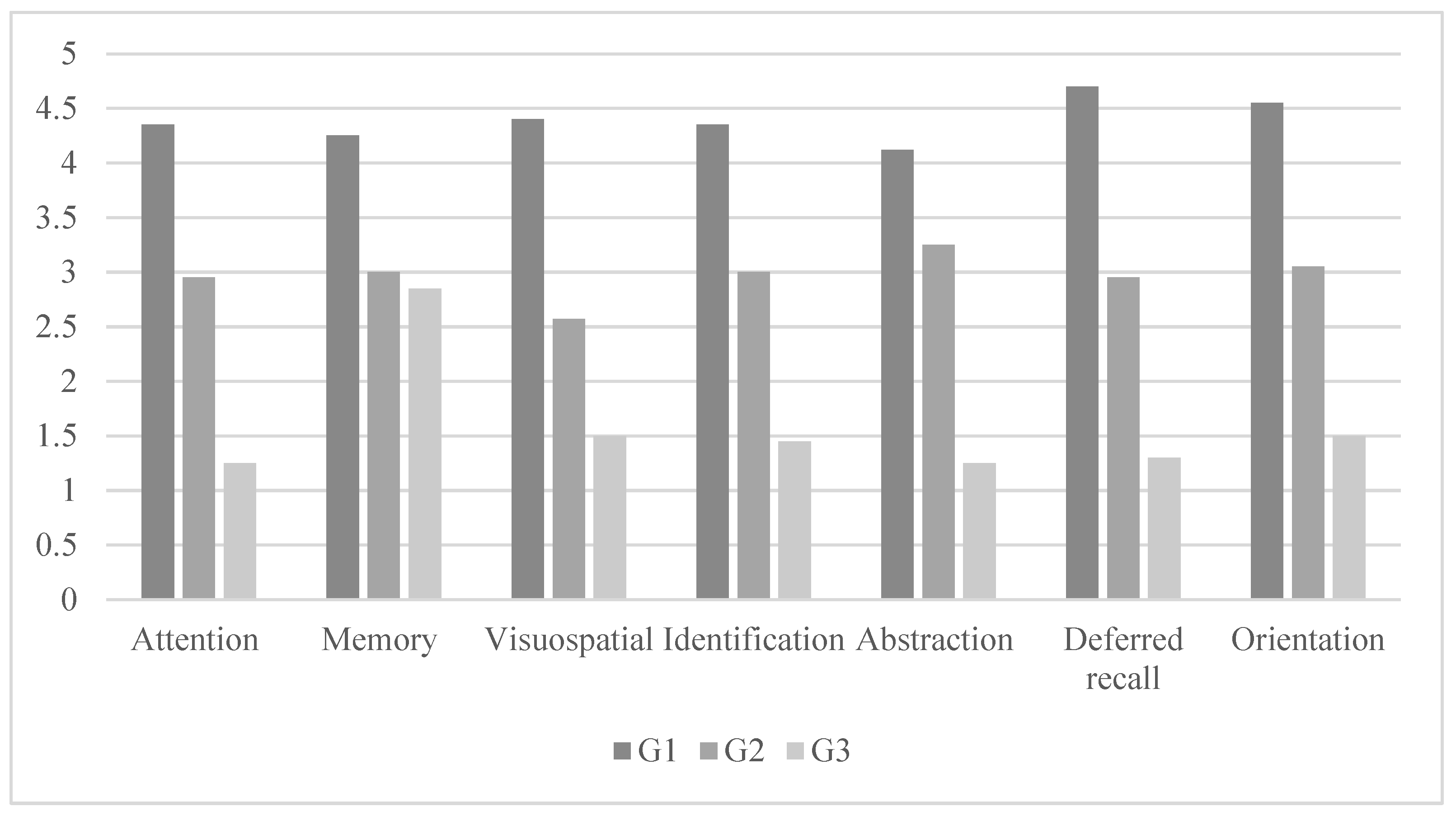
| Profile Cognitive | G1 (n = 20) | G2 (n = 20) | G3 (n = 20) | F (6,52) | η2P | Differences between Groups | |||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||||
| Attention | 4.35 | 0.48 | 2.95 | 0.82 | 1.25 | 1.07 | 69.99 * | 0.711 | G3 < G2 < G1 |
| Memory | 4.25 | 0.44 | 3.00 | 0.91 | 2.85 | 0.74 | 50.55 * | 0.639 | G3 < G2 < G1 |
| Visuospatial | 4.40 | 0.50 | 2.57 | 1.09 | 1.50 | 1.10 | 50.54 * | 0.639 | G3 < G2 < G1 |
| Identification | 4.35 | 0.48 | 3.00 | 0.85 | 1.45 | 1.23 | 61.14 * | 0.682 | G3 < G2 < G1 |
| Abstraction | 4.12 | 0.43 | 3.25 | 0.78 | 1.25 | 1.25 | 79.69 * | 0.737 | G3 < G2 < G1 |
| Deferred recall | 4.70 | 0.47 | 2.95 | 0.75 | 1.30 | 1.17 | 61.80 * | 0.684 | G3 < G2 < G1 |
| Orientation | 4.55 | 0.51 | 3.05 | 0.82 | 1.50 | 1.14 | 55.97 * | 0.663 | G3 < G2 < G1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Villagrasa, A.; López-Zamora, M.; Romero-Moreno, L.; Valles-González, B. The Linguistic–Cognitive Profile in an Adult Population with Parkinson’s Disease and Deep Brain Stimulation: A Comparative Study. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 385-398. https://doi.org/10.3390/ejihpe14020026
Cano-Villagrasa A, López-Zamora M, Romero-Moreno L, Valles-González B. The Linguistic–Cognitive Profile in an Adult Population with Parkinson’s Disease and Deep Brain Stimulation: A Comparative Study. European Journal of Investigation in Health, Psychology and Education. 2024; 14(2):385-398. https://doi.org/10.3390/ejihpe14020026
Chicago/Turabian StyleCano-Villagrasa, Alejandro, Miguel López-Zamora, Lorena Romero-Moreno, and Beatriz Valles-González. 2024. "The Linguistic–Cognitive Profile in an Adult Population with Parkinson’s Disease and Deep Brain Stimulation: A Comparative Study" European Journal of Investigation in Health, Psychology and Education 14, no. 2: 385-398. https://doi.org/10.3390/ejihpe14020026
APA StyleCano-Villagrasa, A., López-Zamora, M., Romero-Moreno, L., & Valles-González, B. (2024). The Linguistic–Cognitive Profile in an Adult Population with Parkinson’s Disease and Deep Brain Stimulation: A Comparative Study. European Journal of Investigation in Health, Psychology and Education, 14(2), 385-398. https://doi.org/10.3390/ejihpe14020026







