Kidney Function, Age, and Education as Contributors to Depression and Anxiety in Juvenile Systemic Lupus Erythematosus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Psychosocial Evaluation
2.3. Clinical and Laboratory Evaluation
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Clinical Characteristics of the JSLE Patient Cohort
3.3. Psychological Indicators between Groups
3.4. Clinical Comparisons between Groups
3.5. Factors Correlating with Depression and Anxiety in JSLE Patients
3.5.1. Survey Results
3.5.2. Correlations between Laboratory Values and Depression and Anxiety
3.5.3. Correlations between Treatments and Depression and Anxiety
3.5.4. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, E.M.D.; Lythgoe, H.; Midgley, A.; Beresford, M.W.; Hedrich, C.M. Juvenile-onset systemic lupus erythematosus: Update on clinical presentation, pathophysiology and treatment options. Clin. Immunol. 2019, 209, 108274. [Google Scholar] [CrossRef]
- Zulian, F.; Pluchinotta, F.; Martini, G.; Da Dalt, L.; Zacchello, G. Severe clinical course of systemic lupus erythematosus in the first year of life. Lupus 2008, 17, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, C.M.; Smith, E.; Beresford, M.W. Juvenile-onset systemic lupus erythematosus (jSLE)—Pathophysiological concepts and treatment options. Best Pract. Res. Clin. Rheumatol. 2017, 31, 488–504. [Google Scholar] [CrossRef]
- Ardoin, S.P.; Schanberg, L.E. Paediatric rheumatic disease: Lessons from SLE: Children are not little adults. Nat. Rev. Rheumatol. 2012, 8, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.M.; Trupin, L.; Katz, P.; Yelin, E.; Lawson, E.F. Depression Risk in Young Adults With Juvenile- and Adult-Onset Lupus: Twelve Years of Followup. Arthritis Care Res. 2018, 70, 475–480. [Google Scholar] [CrossRef]
- Demirkaya, E.; Bilginer, Y.; Aktay-Ayaz, N.; Yalnizoğlu, D.; Karli-Oğuz, K.; Işikhan, V.; Türker, T.; Topaloğlu, R.; Beşbaş, N.; Bakkaloğlu, A.; et al. Neuropsychiatric involvement in juvenile systemic lupus erythematosus. Turk. J. Pediatr. 2008, 50, 126–131. [Google Scholar] [PubMed]
- Benseler, S.M.; Silverman, E.D. Systemic Lupus Erythematosus. Pediatric Clin. N. Am. 2005, 52, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Sibbitt, W.L.; Brandt, J.R.; Johnson, C.R.; Maldonado, M.E.; Patel, S.R.; Ford, C.C.; Bankhurst, A.D.; Brooks, W.M. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J. Rheumatol. 2002, 29, 1536. [Google Scholar]
- Levy, D.M.; Ardoin, S.P.; Schanberg, L.E. Neurocognitive impairment in children and adolescents with systemic lupus erythematosus. Nat. Clin. Pr. Rheumatol. 2009, 5, 106–114. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Figueiredo-Braga, M.; Cornaby, C.; Bernardes, M.; Figueiredo, M.; Mesquita, C.D.S.; Costa, L.; Poole, B.D. Correlation between physical markers and psychiatric health in a Portuguese systemic lupus erythematosus cohort: The role of suffering in chronic autoimmune disease. PLoS ONE 2018, 13, e0195579. [Google Scholar] [CrossRef]
- Figueiredo-Braga, M.; Cornaby, C.; Cortez, A.; Bernardes, M.; Terroso, G.; Figueiredo, M.; Mesquita, C.D.S.; Costa, L.; Poole, B.D. Influence of Biological Therapeutics, Cytokines, and Disease Activity on Depression in Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 5954897. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Braga, M.; Cornaby, C.; Cortez, A.; Bernardes, M.; Terroso, G.; Figueiredo, M.; Mesquita, C.D.S.; Costa, L.; Poole, B.D. Depression and anxiety in systemic lupus erythematosus: The crosstalk between immunological, clinical, and psychosocial factors. Medicine 2018, 97, e11376. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Pereira, M.G.; Duarte, S. Fadiga intensa em doentes com lúpus eritematoso sistémico: Estudo das características psicométricas da escala da intensidade da fadiga. Psicol. Saúde Doenças 2010, 11, 121–136. [Google Scholar] [CrossRef]
- Schmeding, A.; Schneider, M. Fatigue, health-related quality of life and other patient-reported outcomes in systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2013, 27, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: A systematic review. Arthritis Rheum. 2007, 57, 1348–1357. [CrossRef] [PubMed]
- Brennan, C.; Worrall-Davies, A.; McMillan, D.; Gilbody, S.; House, A. The Hospital Anxiety and Depression Scale: A diagnostic meta-analysis of case-finding ability. J. Psychosom. Res. 2010, 69, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, T.; Meneses, R.F.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.L. Development of the Portuguese version of MOS SF-36. Part I. Cultural and linguistic adaptation. Acta Med. Port. 2000, 13, 55–66. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, F.; Wang, J.; You, Y. Clinical aspects and risk factors of lupus nephritis: A retrospective study of 156 adult patients. J. Int. Med. Res. 2019, 47, 5070–5081. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.; Levinson, B.A.; Bornkamp, N.; Goldberg, J.D.; Buyon, J.; Belmont, H.M. Serum albumin at 1 year predicts long-term renal outcome in lupus nephritis. Lupus Sci. Med. 2018, 5, e000271. [Google Scholar] [CrossRef]
- Bhangle, S.D.; Kramer, N.; Rosenstein, E.D. Corticosteroid-induced neuropsychiatric disorders: Review and contrast with neuropsychiatric lupus. Rheumatol. Int. 2013, 33, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Tyrovolas, S.; Koyanagi, A.; Chatterji, S.; Leonardi, M.; Ayuso-Mateos, J.L.; Tobiasz-Adamczyk, B.; Koskinen, S.; Rummel-Kluge, C.; Haro, J.M. The role of socio-economic status in depression: Results from the COURAGE (aging survey in Europe). BMC Public Health 2016, 16, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erol, R.Y.; Orth, U. Self-esteem development from age 14 to 30 years: A longitudinal study. J. Pers. Soc. Psychol. 2011, 101, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M. Adolescent depression: Diagnosis, treatment, and educational attainment. Health Econ. 2008, 17, 1215–1235. [Google Scholar] [CrossRef]
- Bauldry, S. Variation in the Protective Effect of Higher Education Against Depression. Soc. Ment. Health 2015, 5, 145–161. [Google Scholar] [CrossRef] [Green Version]


| Characteristics | Juvenile SLE Patients | Healthy Subjects | Adult SLE Patients | Depressed Patients | |||
|---|---|---|---|---|---|---|---|
| (N = 29) | (N = 22) | p-Value | (N = 73) | p-Value | (N = 32) | p-Value | |
| Gender, no. (%) | 0.625 | 0.021 | 1.000 | ||||
| Female | 26 (90%) | 21 (95%) | 73 (100%) | 29 (90%) | |||
| Age, mean ± SD | 23 ± 5.4 | 43 ± 11.5 | <0.001 | 45 ± 10.4 | <0.001 | 49 ± 14.0 | <0.001 |
| Education Level, no. (%) | 0.593 | <0.001 | <0.001 | ||||
| Primary | 0 (0%) | 0 (0%) | 17 (23%) | 8 (25%) | |||
| Middle School | 1 (3%) | 1 (5%) | 11 (15%) | 7 (21%) | |||
| High School | 2 (7%) | 2 (9%) | 11 (15%) | 7 (21%) | |||
| College | 16 (55%) | 8 (36%) | 24 (33%) | 5 (15%) | |||
| Graduate | 10 (34%) | 11 (50%) | 10 (14%) | 5 (15%) |
| Characteristics | HADS Depression | HADS Anxiety | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 29) | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | |
| Clinical Exam Results | |||||||||||
| Age at Diagnosis, median (range) | 16 (12–20) | <0.001 | 167.26 | −0.016 | 0.98 (0.90–1.07) | 0.701 | 0.003 | 181.31 | −0.019 | 0.98 (0.93–1.04) | 0.500 |
| Disease Duration (y), median (range) | 5 (1–23) | 0.011 | 165.57 | 0.023 | 1.02 (0.99–1.06) | 0.167 | 0.013 | 179.41 | 0.017 | 1.02 (1.00–1.04) | 0.119 |
| Body Mass Index | 23.43 ± 3.75 | 0.005 | 166.62 | 0.021 | 1.02 (0.97–1.07) | 0.364 | 0.015 | 179.15 | 0.026 | 1.03 (1.00–1.06) | 0.097 |
| SLEDAI, median (range) | 2 (0–12) | <0.001 | 167.41 | 0.001 | 1.00 (0.95–1.05) | 0.978 | 0.003 | 181.16 | 0.014 | 1.01 (0.98–1.05) | 0.432 |
| SLICC, median (range) | 0 (0–2) | 0.043 | 160.39 | 0.435 | 1.54 (1.13–2.06) | 0.004 | 0.019 | 178.46 | 0.215 | 1.24 (0.98–1.54) | 0.059 |
| Articular Manifestations, no. (%) | 15 (52%) | 0.017 | 164.59 | 0.315 | 1.37 (0.95–2.00) | 0.096 | 0.006 | 180.66 | −0.130 | 0.88 (0.69–1.12) | 0.293 |
| Mucocutaneous Manifestations, no. (%) | 24 (83%) | 0.015 | 165.03 | 0.417 | 1.52 (0.90–2.77) | 0.143 | 0.024 | 177.47 | 0.369 | 1.45 (1.02–2.12) | 0.048 |
| Hematological Manifestations, no. (%) | 13 (45%) | 0.008 | 166.14 | −0.212 | 0.81 (0.55–1.17) | 0.264 | 0.002 | 181.46 | −0.069 | 0.93 (0.73–1.19) | 0.581 |
| Renal Manifestations, no. (%) | 12 (41%) | 0.010 | 165.83 | 0.056 | 1.06 (0.97–1.15) | 0.206 | 0.026 | 177.24 | 0.063 | 1.07 (1.00–1.13) | 0.032 |
| Disease Management | |||||||||||
| Corticotherapy | 22 (76%) | 0.032 | 162.22 | 0.550 | 1.73 (1.08–2.96) | 0.032 | 0.033 | 175.89 | 0.375 | 1.46 (1.07–2.02) | 0.020 |
| Dosage (mg), mean ± SD | 7.78 ± 8.97 | 0.072 | 155.75 | 0.021 | 1.02 (1.00–1.04) | 0.021 | 0.102 | 163.70 | 0.015 | 1.01 (1.00–1.03) | 0.019 |
| Mycophenolic Acid | 8 (28%) | 0.002 | 167.03 | 0.126 | 1.13 (0.75–1.67) | 0.533 | 0.020 | 178.29 | 0.249 | 1.28 (0.99–1.65) | 0.059 |
| Lisinopril | 6 (21%) | 0.035 | 161.63 | 0.505 | 1.67 (1.10–2.44) | 0.013 | 0.043 | 174.14 | 0.392 | 1.48 (1.12–1.93) | 0.004 |
| Hydroxychloroquine | 26 (90%) | 0.066 | 156.70 | −0.816 | 0.44 (0.29–0.71) | <0.001 | 0.010 | 179.94 | −0.257 | 0.77 (0.55–1.13) | 0.163 |
| Pentoxifylline | 3 (10%) | 0.039 | 161.10 | 0.650 | 1.92 (1.16–3.01) | 0.007 | 0.003 | 181.16 | 0.151 | 1.16 (0.78–1.66) | 0.430 |
| Azathioprine | 5 (17%) | 0.017 | 164.68 | 0.376 | 1.46 (0.93–2.21) | 0.087 | 0.005 | 180.89 | 0.149 | 1.16 (0.85–1.56) | 0.341 |
| Characteristics | HADS Depression | HADS Anxiety | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 29) | Reference (Score Range) | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | |
| Fatigue Severity Scale, mean ± SD | 4.28 ± 1.38 | 0 (0–7) | 0.070 | 156.05 | 0.232 | 1.26 (1.10–1.44) | <0.001 | 0.019 | 178.40 | 0.083 | 1.09 (0.99–1.19) | 0.067 |
| Subject Happiness Scale, mean ± SD | 5.20 ± 1.04 | >20 (4–28) | 0.173 | 139.22 | −0.445 | 0.64 (0.55–0.75) | <0.001 | 0.0512 | 172.67 | −0.176 | 0.84 (0.75–0.94) | 0.002 |
| General Health | 48.62 ± 19.73 | (0–100) | 0.043 | 160.39 | −0.013 | 0.99 (0.98–1.00) | 0.009 | 0.048 | 173.22 | −0.009 | 0.99 (0.98–1.00) | 0.004 |
| Reported Health Transition | 58.62 ± 28.56 | (0–100) | 0.012 | 165.45 | −0.005 | 1.00 (0.99–1.00) | 0.160 | 0.005 | 180.96 | −0.002 | 1.00 (0.99–1.00) | 0.368 |
| Physical Function | 86.38 ± 15.46 | (0–100) | 0.017 | 164.60 | −0.009 | 0.99 (0.98–1.00) | 0.082 | 0.008 | 180.32 | −0.005 | 1.00 (0.99–1.00) | 0.221 |
| Physical Role Limitation | 81.48 ± 21.07 | (0–100) | 0.030 | 162.54 | −0.009 | 0.99 (0.98–1.00) | 0.024 | 0.003 | 181.32 | −0.002 | 1.00 (0.99–1.00) | 0.503 |
| Emotional Role Limitation | 77.87 ± 24.63 | (0–100) | 0.046 | 159.88 | −0.010 | 0.99 (0.98–1.00) | 0.005 | 0.069 | 169.62 | −0.009 | 0.99 (0.99–1.00) | <0.001 |
| Social Function | 75.86 ± 22.14 | (0–100) | 0.104 | 150.46 | −0.016 | 0.98 (0.98–0.99) | <0.001 | 0.049 | 172.99 | −0.008 | 0.99 (0.99–1.00) | 0.003 |
| Vitality | 53.68 ± 18.93 | (0–100) | 0.091 | 152.62 | −0.019 | 0.98 (0.97–0.99) | <0.001 | 0.073 | 168.87 | 0.003 | 0.99 (0.98–0.99) | 0.000 |
| Mental Health | 70.16 ± 17.08 | (0–100) | 0.178 | 138.44 | −0.031 | 0.97 (0.96–0.98) | <0.001 | 0.208 | 144.87 | -0.023 | 0.98 (0.97–0.98) | <0.001 |
| Bodily Pain | 53.10 ± 7.61 | (0–100) | 0.073 | 155.57 | 0.045 | 1.05 (1.02–1.07) | <0.001 | <0.001 | 181.61 | 0.003 | 1.00 (0.99–1.02) | 0.698 |
| Characteristics | HADS Depression | HADS Anxiety | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 29) | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | Pseudo R2 | AICc | Coefficient | Odds Ratio (95% CI) | p-Value | |
| Clinical Laboratory Results | |||||||||||
| Hemoglobin (g/dL) | 13.21 ± 1.40 | <0.001 | 167.35 | −0.016 | 0.98 (0.86–1.12) | 0.814 | 0.007 | 180.60 | 0.007 | 1.05 (0.96–1.14) | 0.279 |
| Leukocytes (109/L) | 6.12 ± 2.46 | 0.010 | 165.82 | 0.048 | 1.05 (0.97–1.13) | 0.203 | 0.013 | 179.44 | 0.038 | 1.04 (0.99–1.09) | 0.124 |
| Lymphocytes (109/L) | 1.85 ± 0.86 | 0.033 | 162.12 | −0.002 | 1.00 (0.80–1.24) | 0.984 | 0.024 | 177.53 | −0.032 | 0.97 (0.84–1.12) | 0.669 |
| Platelets (109/L) | 247.90 ± 69.39 | 0.058 | 158.02 | 0.004 | 1.00 (1.00–1.01) | 0.002 | 0.031563 | 176.17 | 0.032 | 1.00 (1.00–1.00) | 0.018 |
| Sedimentation Rate, ESR (mm/h) | 20.86 ± 19.76 | <0.001 | 167.31 | 0.002 | 1.00 (0.99–1.01) | 0.749 | <0.001 | 181.76 | 0.000 | 1.00 (0.99–1.01) | 0.980 |
| C-Reactive Protein, CRP (mg/L) | 2.59 ± 2.63 | <0.001 | 167.36 | −0.008 | 0.99 (0.92–1.06) | 0.818 | 0.004 | 181.11 | −0.020 | 0.98 (0.93–1.03) | 0.425 |
| Albumin (g/L) | 38.61 ± 9.33 | 0.035 | 161.78 | −0.020 | 0.98 (0.97–1.00) | 0.010 | 0.017 | 178.80 | −0.011 | 0.99 (0.98–1.00) | 0.071 |
| Anti-dsDNA (IU/mL) | 197.54 ± 272.99 | <0.001 | 167.25 | 0.000 | 1.00 (1.00–1.00) | 0.685 | <0.001 | 181.76 | 0.000 | 1.00 (1.00–1.00) | 0.937 |
| C3 (mg/dL) | 99.95 ± 22.33 | <0.001 | 167.39 | −0.001 | 1.00 (1.00–1.01) | 0.889 | 0.001 | 181.51 | 0.001 | 1.00 (1.00–1.01) | 0.612 |
| C4 (mg/dL) | 16.21 ± 7.72 | 0.012 | 165.50 | −0.018 | 0.98 (0.96–1.01) | 0.164 | <0.001 | 181.69 | −0.002 | 1.00 (0.98–1.01) | 0.787 |
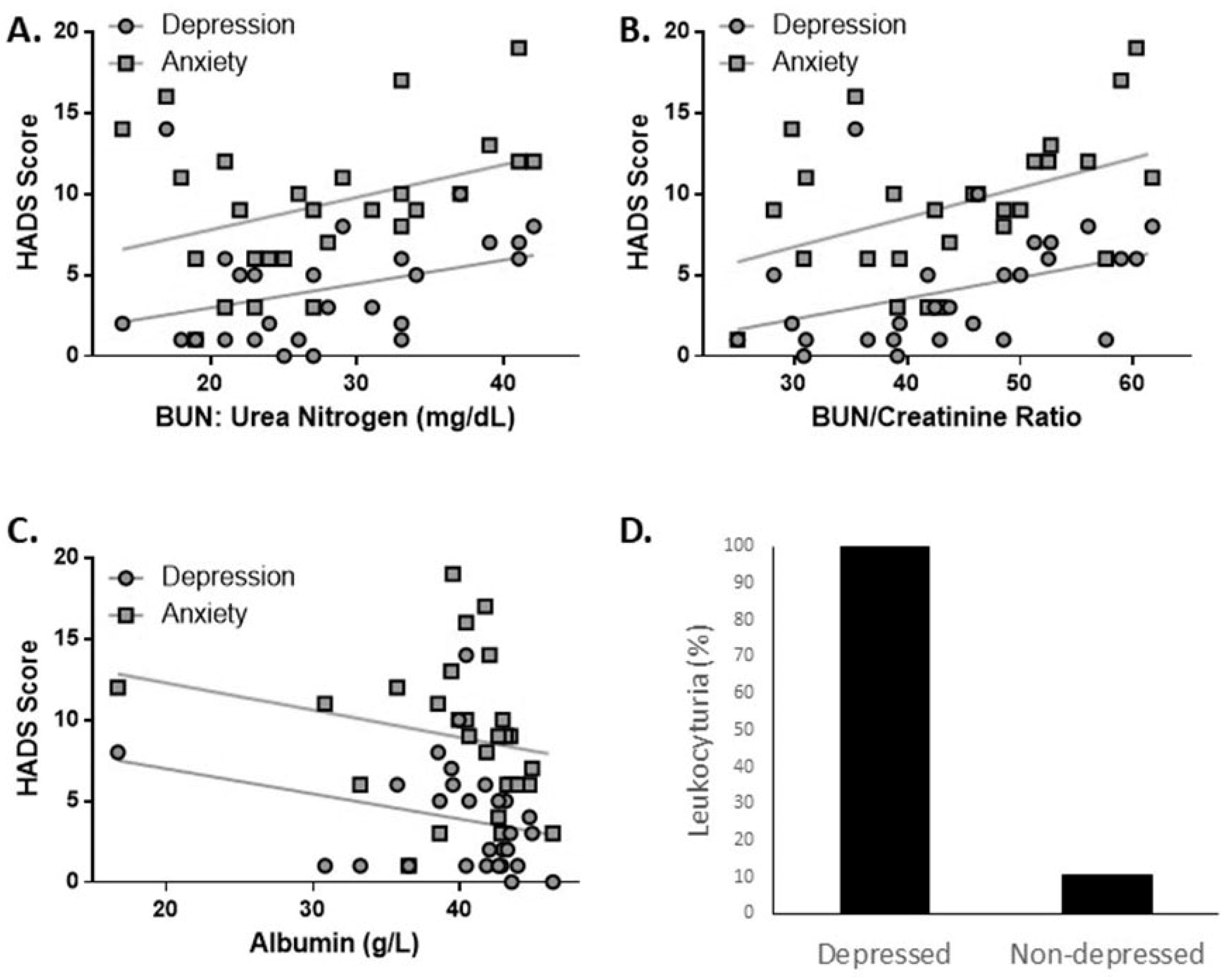
| BUN: Urea Nitrogen (mg/dL) | 25.76 ± 10.48 | 0.056 | 158.20 | 0.029 | 1.03 (1.01–1.05) | 0.003 | 0.066 | 170.13 | 0.021 | 1.02 (1.01–1.03) | <0.001 |
| Creatinine (mg/dL) | 0.63 ± 0.13 | <0.001 | 167.40 | −0.083 | 0.92 (0.22–4.02) | 0.911 | <0.001 | 181.64 | −0.175 | 0.84 (0.32–2.22) | 0.723 |
| BUN/Creatinine Ratio | 41.21 ± 15.15 | 0.065 | 156.83 | 0.023 | 1.03 (1.01–1.04) | 0.002 | 0.080 | 167.57 | 0.017 | 1.02 (1.01–1.03) | <0.001 |
| Urine (abnorml), no. (%) | 14 (48%) | 0.008 | 166.17 | 0.207 | 1.23 (0.85–1.78) | 0.266 | 0.005 | 180.90 | 0.115 | 1.12 (0.88–1.43) | 0.353 |
| Proteinuria, no. (%) | 13 (93%) | ||||||||||
| Proteinuria (g/dL), mean ± SD | 0.72 ± 0.94 | 0.471 | 91.48 | −0.059 | 0.94 (0.67–1.26) | 0.713 | 0.522 | 89.98 | 0.037 | 1.04 (0.85–1.24) | 0.700 |
| Leukocyturia, no. (%) | 7 (50%) | ||||||||||
| Leukocyturia (cells/uL), mean ± SD | 326.14 ± 463.21 | 0.743 | 48.91 | −0.002 | 1.00 (0.99–1.00) | 0.033 | 0.823 | 38.32 | −0.001 | 1.00 (1.00–1.00) | 0.033 |
| Erythrocyturia, no. (%) | 2 (14%) | ||||||||||
| Erythrocyturia (cells/uL), mean ± SD | 282.15 ± 310.77 | 0.962 | −1.84 | 0.005 | 1.01 (1.00–1.01) | 0.028 | 0.958 | <0.001 | 0.002 | 1.00 (1.00–1.01) | 0.121 |
| Depression | |||
|---|---|---|---|
| Variable | Coefficient | Odds Ratio (95% CI) | p-value |
| BUN/Creatinine Ratio | 0.021 | 1.02 (1.00–1.04) | 0.043 |
| Model Fit Summary | Pseudo R2 | AICc | |
| 0.513 | 90.21 | ||
| Anxiety | |||
| Variable | Coefficient | Odds Ratio (95% CI) | p-value |
| BUN/Creatinine Ratio | 0.018 | 1.02 (1.01–1.03) | <0.001 |
| Model Fit Summary | Pseudo R2 | AICc | |
| 0.137 | 162.71 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo-Braga, M.; Silva, B.; Ganhão, S.; Aguiar, F.; Cornaby, C.; Brito, I.; Poole, B.D. Kidney Function, Age, and Education as Contributors to Depression and Anxiety in Juvenile Systemic Lupus Erythematosus. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 1503-1515. https://doi.org/10.3390/ejihpe11040107
Figueiredo-Braga M, Silva B, Ganhão S, Aguiar F, Cornaby C, Brito I, Poole BD. Kidney Function, Age, and Education as Contributors to Depression and Anxiety in Juvenile Systemic Lupus Erythematosus. European Journal of Investigation in Health, Psychology and Education. 2021; 11(4):1503-1515. https://doi.org/10.3390/ejihpe11040107
Chicago/Turabian StyleFigueiredo-Braga, Margarida, Beatriz Silva, Sara Ganhão, Francisca Aguiar, Caleb Cornaby, Iva Brito, and Brian D. Poole. 2021. "Kidney Function, Age, and Education as Contributors to Depression and Anxiety in Juvenile Systemic Lupus Erythematosus" European Journal of Investigation in Health, Psychology and Education 11, no. 4: 1503-1515. https://doi.org/10.3390/ejihpe11040107
APA StyleFigueiredo-Braga, M., Silva, B., Ganhão, S., Aguiar, F., Cornaby, C., Brito, I., & Poole, B. D. (2021). Kidney Function, Age, and Education as Contributors to Depression and Anxiety in Juvenile Systemic Lupus Erythematosus. European Journal of Investigation in Health, Psychology and Education, 11(4), 1503-1515. https://doi.org/10.3390/ejihpe11040107






