Risk Factors and Distribution of MDROs Among Patients with Healthcare Associated Burn Wound Infection
Abstract
Introduction
Methods
Results
Discussion
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Mock, C.; Peck, M.; Peden, M.; et al. A WHO Plan for Burn Prevention and Care; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Bhat, V.G.; Vasaikar, S.D. Bacteriological profile and antibiogram of aerobic burn wound isolates in Mthatha, Eastern Cape, South Africa. South Afr J Epidemiol Infect 2010, 25, 16–19. [Google Scholar] [CrossRef]
- Fouzia, B.; Damle, A.S.; Maher, G. Changing patterns of burn infections IOSR. J Dent Med Sci 2013, 5, 11–14. [Google Scholar]
- Kumarasamy, K.K.; Toleman, M.A.; Walsh, T.R.; et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: A molecular, biological, and epidemiological study. Lancet Infect Dis 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lushniak, B.D. Antibiotic resistance: A public health crisis. Public Health Rep 2014, 129, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Vartak, A.M.; Patil, A.; Saldanha, J. Bacteriology of the burn wound at the Bai Jerbai Wadia Hospital for children, Mumbai, India—A 13 year study, Part I-Bacteriological profile. Indian J Plast Surg 2009, 42, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections. Available online: https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNos InfDef_current.pdf (accessed on 12 August 2018).
- Immunochemical methods used for organism detection. In AS Bailey & Scott’s Diagnostic Microbiology, 12th ed.; Forbes, B.A., Sham, D.F., Weissfeld, A.S., Eds.; Mosby: St. Louis, MO, USA, 2007; pp. 147–158. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard—Twelfth Edition. CLSI Document M02-A12. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement. CLSI Document M100-S25. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006, 42 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Strassle, P.D.; DiBiase, L.M.; et al. Timeline of healthcare-associated infections and pathogens after burn injuries. Am J Infect Control 2016, 44, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; McManus, A.; Nursing Committee of the International Society for Burn Injuries. Infection control in burn patients. Burns 2004, 30, A16–A24. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.; Kendrick, D. Epidemiology of burn injuries in the East Mediterranean Region: A systematic review. BMC Public Health 2010, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Maske, A.N.; Deshmukh, S.N. Clinico-epidemiological study of burns: Our experience with 500 patients. Int Surg J 2016, 3, 1234–1239. [Google Scholar] [CrossRef]
- Tripathee, S.; Basnet, S.J. Epidemiology of burn injuries in Nepal: A systemic review. Burns Trauma 2017, 5, 10. [Google Scholar] [PubMed]
- Güldoğan, C.E.; Kendirci, M.; Tikici, D.; Gündoğdu, E.; Yasti. Clinical infection in burn patients and its consequences. Ulus Travma Acil Cerrahi Derg 2017, 23, 466–471. [Google Scholar] [PubMed]
- El-Maghawry, H.A.M.M.; El Nem, W.; Sherif, N.; Hagag, S.A. An interventional study to decrease healthcare associated burn wound infections in the burn unit of Al Ahrar Hospital in Zagazig city, Sharkia Governorate. Int J Curr Microbiol Appp Sci 2016, 5, 566–578. [Google Scholar] [CrossRef][Green Version]
- Emami, S.A.; Karimi, H.; Alijanpour, A. Epidemiology of burn wound infection and its bacterial resistance, burn registry program. Merit Res J Med Med Sci 2015, 3, 135–139. [Google Scholar][Green Version]
- Coetzee, E.; Rode, H.; Kahn, D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg 2013, 51, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Essayagh, M.; Essayagh, T.; Essayagh, S.; El Hamzaoui, S. Epidemiology of burn wound infection in Rabat, Morocco: Three-year review. Med Sante Trop 2014, 24, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Bayram, Y.; Parlak, M.; Aypak, C.; Bayram, I. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int J Med Sci 2013, 10, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rathod, V.S.; Kasturi, R.S. Emergence of multi-drug resistant strains among bacterial isolates in burn wound swabs in a tertiary care centre, Nanded, Maharashtra, India. Int J Res Med Sci 2017, 5, 973–977. [Google Scholar] [CrossRef]
- Mohamed, H. One year prevalence of critically ill burn wound bacterial infections in surgical ICU in Egypt: Retrospective study. Egypt J Anesth 2016, 32, 431–434. [Google Scholar] [CrossRef]
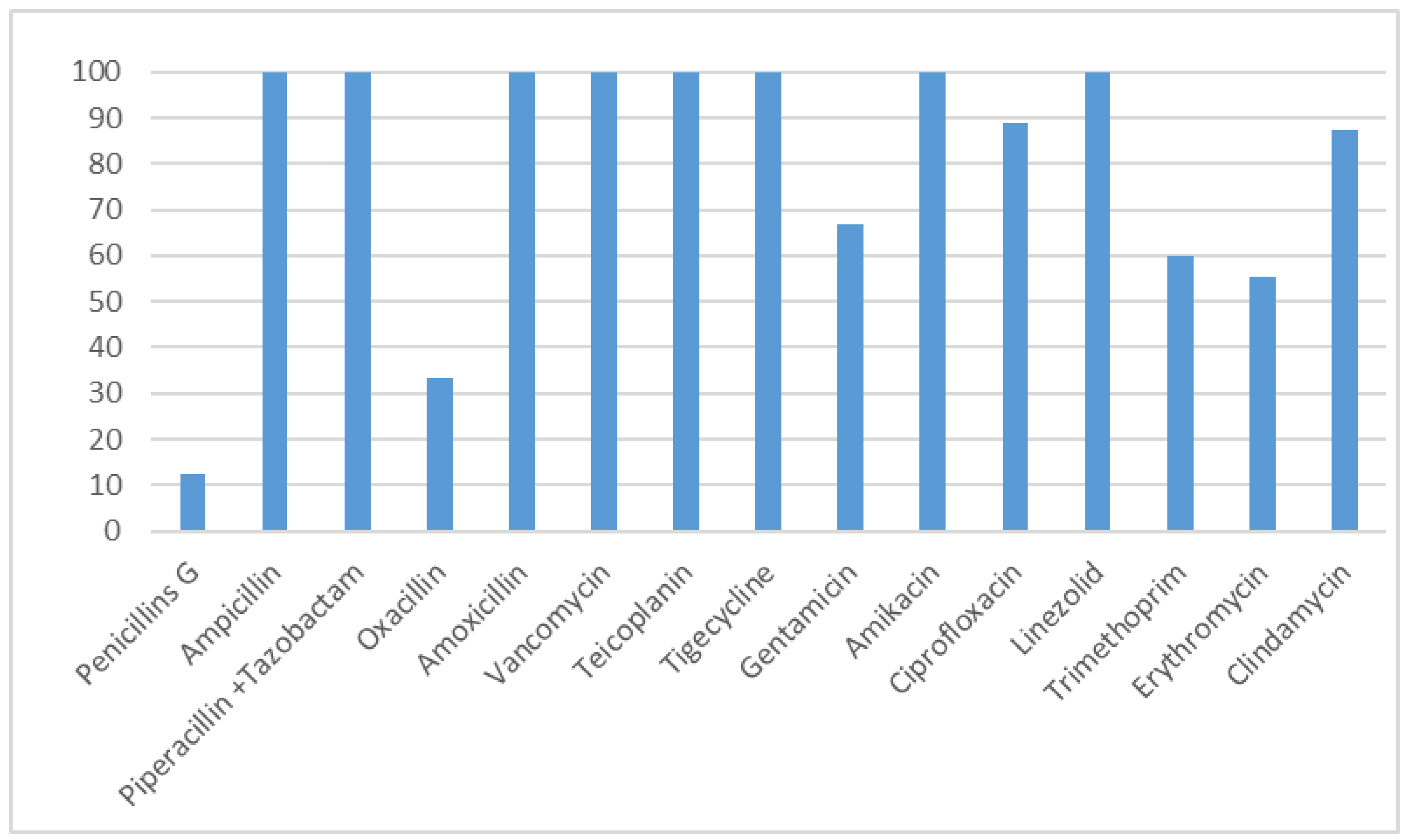
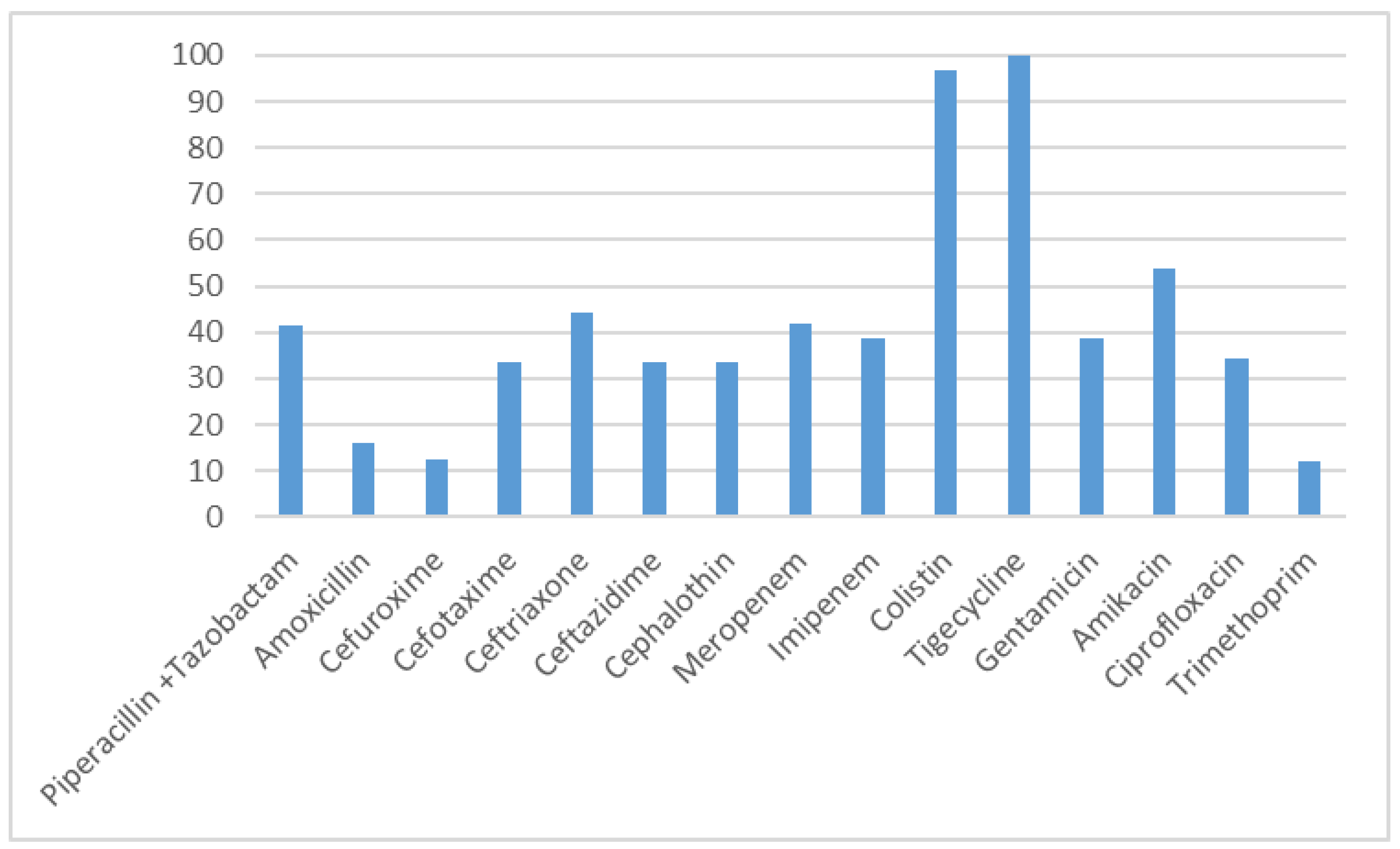
| Risk Factor Category | N | % |
|---|---|---|
| Gender | ||
| Male | 123 | 61.19 |
| Female | 78 | 38.81 |
| Type of Burn | ||
| Flame | 126 | 62.69 |
| Scald | 56 | 27.86 |
| Contact | 10 | 4.98 |
| Electric | 8 | 3.98 |
| Chemical | 1 | 0.50 |
| TBSA | ||
| >35% | 25 | 12.44 |
| ≤35% | 176 | 87.56 |
| Length of hospital stay | ||
| >14 days | 58 | 28.86 |
| ≤14 days | 143 | 71.14 |
| Risk Factor | Patient with Healthcare Associated Burn Infection | Patient Without Healthcare Associated Burn Infection | Relative Risk | Confidence Interval |
|---|---|---|---|---|
| Male | 11 | 112 | 1.163 | 0.448—3.017 |
| Female | 6 | 72 | ||
| Flame burn | 17 | 109 | 20.945 | 1.278—343.3 |
| Other burn injuries | 0 | 75 | ||
| Patient with >35 TBSA burn | 10 | 15 | 10.057 | 4.212—24.013 |
| Patient with ≤35 TBSA burn | 7 | 169 | ||
| LOS >14 days | 12 | 46 | 5.917 | 2.182–16.047 |
| LOS ≤14 | 5 | 138 |
| Risk Factor | Blood Culture with MDRO in Patients with Healthcare Associated Burn Infection | Blood Culture Without MDRO in Patients with Healthcare Associated Burn Infection | Relative Risk | Confidence Interval |
|---|---|---|---|---|
| Male | 15 | 12 | 0.648 | 0.435–0.966 |
| Female | 12 | 2 | ||
| Patient with >35 TBSA burn Patient with ≤35 | 20 7 | 9 5 | 1.182 | 0.691–2.025 |
| TBSA burn LOS >14 days | 18 | 11 | 0.827 | 0.536–1.276 |
| LOS ≤14 | 9 | 3 |
| Microorganism | No. of Isolates | Percentage | Number of MDR Isolates | Percentage of MDR Isolates * |
|---|---|---|---|---|
| Acinetobacter baumannii | 17 | 41.46 | 17 | 100 |
| Klebsiella pneumoniae | 8 | 19.51 | 4 | 50 |
| Staphylococcus aureus | 6 | 14.63 | 4 | 66.67 |
| Pseudomonas aeruginosa | 6 | 14.63 | 1 | 16.66 |
| Enterococcus faecalis | 2 | 4.88 | 0 | 0 |
| Serratia marcescens | 1 | 2.44 | 1 | 100 |
| Enterococcus gallinarum | 1 | 2.44 | 0 | 0 |
| TOTAL | 41 | 100 | 27 | 65.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfadli, M.; El-Sehsah, E.M.; Ramadan, M.A.-M. Risk Factors and Distribution of MDROs Among Patients with Healthcare Associated Burn Wound Infection. Germs 2018, 8, 199-206. https://doi.org/10.18683/germs.2018.1147
Alfadli M, El-Sehsah EM, Ramadan MA-M. Risk Factors and Distribution of MDROs Among Patients with Healthcare Associated Burn Wound Infection. Germs. 2018; 8(4):199-206. https://doi.org/10.18683/germs.2018.1147
Chicago/Turabian StyleAlfadli, Mariam, Eman M. El-Sehsah, and Moustapha Ahmed-Maher Ramadan. 2018. "Risk Factors and Distribution of MDROs Among Patients with Healthcare Associated Burn Wound Infection" Germs 8, no. 4: 199-206. https://doi.org/10.18683/germs.2018.1147
APA StyleAlfadli, M., El-Sehsah, E. M., & Ramadan, M. A.-M. (2018). Risk Factors and Distribution of MDROs Among Patients with Healthcare Associated Burn Wound Infection. Germs, 8(4), 199-206. https://doi.org/10.18683/germs.2018.1147




