IL-17 and IL-22 Genetic Polymorphisms in HBV Vaccine Non- and Low-Responders Among Healthcare Workers
Abstract
Background
Methods
Results
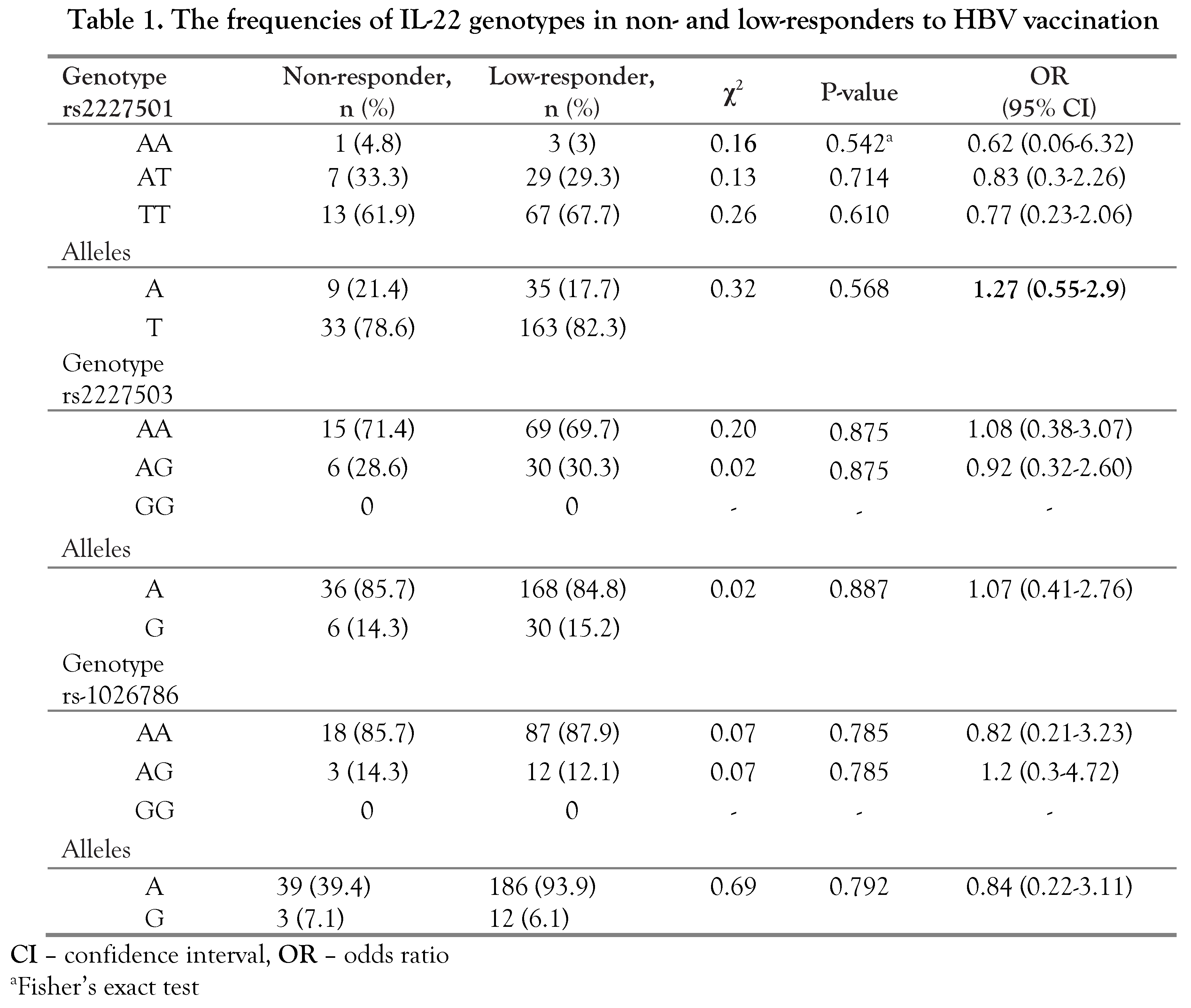
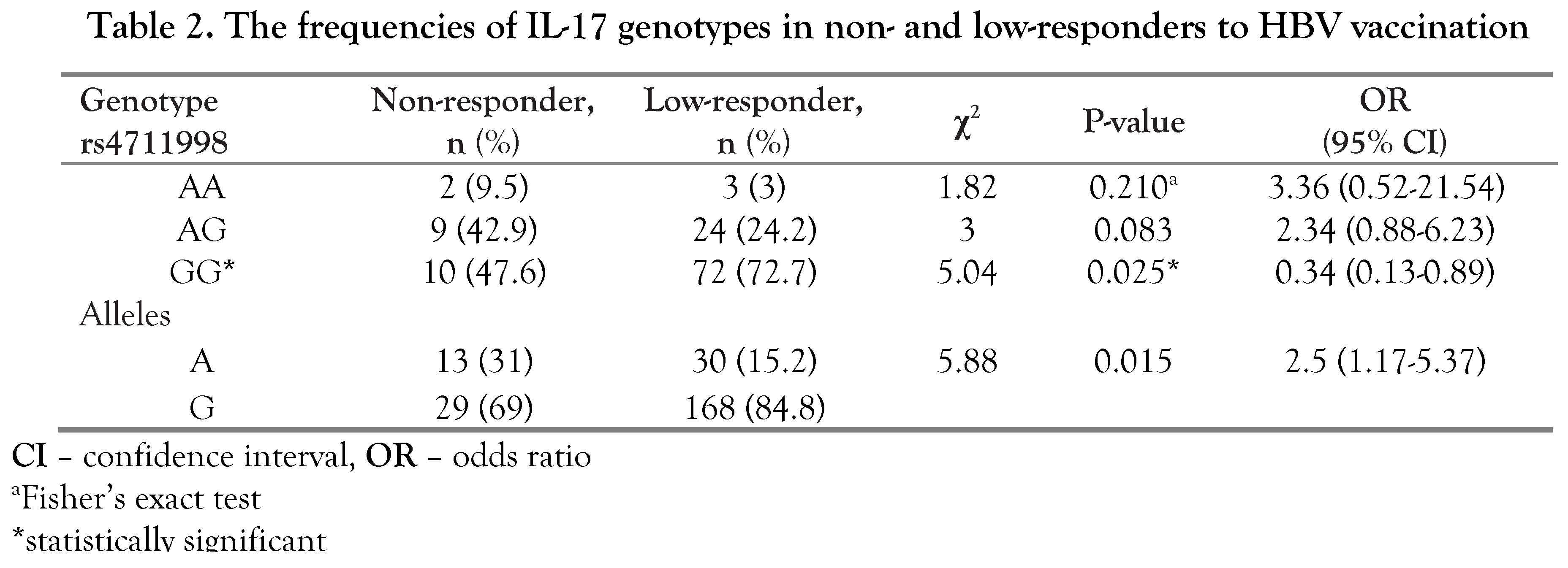
Discussion
Conclusions
Author Contributions
Conflicts of Interest
References
- Adibi, P.; Rezailashkajani, M.; Roshandel, D.; et al. An economic analysis of premarriage prevention of hepatitis B transmission in Iran. BMC Infect Dis 2004, 4, 31. [Google Scholar] [CrossRef]
- Malka, E.; Streinu-Cercel, A.; Piţigoi, D.; Bacruban, R. Management of accidental exposure to HCV, HBV and HIV in healthcare workers in Romania. Germs 2012, 2, 137–141. [Google Scholar] [CrossRef]
- Sukriti Pati, N.T.; Sethi, A.; et al. Low levels of awareness, vaccine coverage, and the need for boosters among health care workers in tertiary care hospitals in India. J Gastroenterol Hepatol 2008, 23, 1710–1715. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Rapiti, E.; Hutin, Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med 2005, 48, 482–490. [Google Scholar] [CrossRef]
- Leuridan, E.; Van Damme, P. Hepatitis B and the need for a booster dose. Clin Infect Dis 2011, 53, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.; Albarran, B.; Salmen, S.; et al. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology 2004, 326, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Filippelli, M.; Lionetti, E.; Gennaro, A.; et al. Hepatitis B vaccine by intradermal route in non-responder patients: An update. World J Gastroenterol 2014, 20, 10383–10394. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, X.; Liang, Z.; et al. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine 2014, 32, 5316–5322. [Google Scholar] [CrossRef] [PubMed]
- Yucesoy, B.; Johnson, V.J.; Fluharty, K.; et al. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine 2009, 27, 6991–6997. [Google Scholar] [CrossRef]
- Larsen, C.E.; Xu, J.; Lee, S.; et al. Complex cytokine responses to hepatitis B surface antigen and tetanus toxoid in responders, nonresponders and subjects naive to hepatitis B surface antigen. Vaccine 2000, 18, 3021–3030. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Z.; Lu, F.; et al. Toll-like receptors and cytokines/cytokine receptors polymorphisms associate with non-response to hepatitis B vaccine. Vaccine 2011, 29, 706–711. [Google Scholar] [CrossRef]
- Lindenstrøm, T.; Woodworth, J.; Dietrich, J.; Aagaard, C.; Andersen, P.; Agger, E.M. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun 2012, 80, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Nemoto, Y.; Kamada, N.; et al. Homeostatic (IL-7) and effector (IL-17) cytokines as distinct but complementary target for an optimal therapeutic strategy in inflammatory bowel disease. Curr Opin Gastroenterol 2009, 25, 306–313. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T cells: Differentiation and functions. Clin Dev Immunol 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, S.K.; Lam, B.; Lau, K.; Larkin, J. , 3rd. Th17 cells in immunity and autoimmunity. Clin Dev Immunol 2013, 2013, 986789. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Xu, M.; Mizoguchi, I.; et al. Pivotal roles of T- helper 17- related cytokines, IL-17, IL-22, and IL-23, in inflammatory disease. Clin Dev Immunol 2013, 2013, 968549. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Xu, K. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int J Mol Med 2011, 27, 385–392. [Google Scholar]
- Zhang, J.Y.; Song, C.H.; Shi, F.; Zhang, Z.; Fu, J.L.; Wang, F.S. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS ONE 2010, 5, e13869. [Google Scholar] [CrossRef]
- Shibui, A.; Shimura, E.; Nambu, A.; et al. Th17 cell derived IL-17 is dispensable for B cell antibody production. Cytokine 2012, 59, 108–114. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Flavell, R.A. Recent advances in IL-22 biology. Int Immunol 2011, 23, 159–163. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, Z.; Luan, Y.; et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014, 59, 1331–1342. [Google Scholar] [CrossRef]
- Djeriri, K.; Laurichesse, H.; Merle, J.L.; et al. Hepatitis B in Moroccan health care workers. Occup Med 2008, 58, 419–424. [Google Scholar] [CrossRef]
- Schmidt-Weber, C.B.; Akdis, M.; Akdis, C.A. TH17 cells in the big picture of immunology. J Allergy Clin Immunol 2007, 120, 247–254. [Google Scholar]
- Higgins, S.C.; Jarnicki, A.G.; Lavelle, E.C.; Mills, K.H. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: Role of IL-17-producing T cells. J Immunol 2006, 177, 7980–7989. [Google Scholar] [CrossRef] [PubMed]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.P.; Fan, Y.C.; Zhang, J.J.; Zhao, J.; Wang, K. IL-17A but not IL-22 suppresses the replication of hepatitis B virus mediated by over-expression of MxA and OAS mRNA in the HepG2.2.15 cell line. Antiviral Res 2013, 97, 285–292. [Google Scholar]
- Chen, Y.; Fang, J.; Chen, X.; Pan, C.; Liu, X.; Liu, J. Effects of the Treg/Th17 cell balance and their associated cytokines in patients with hepatitis B infection. Exp Ther Med 2015, 9, 573–578. [Google Scholar] [CrossRef]
- Barbosa, R.R.; Silva, S.P.; Silva, S.L.; et al. Primary B-cell deficiencies reveal a link between human IL-17-producing CD4 T-cell homeostasis and B-cell differentiation. PLoS ONE 2011, 6, e22848. [Google Scholar] [CrossRef]
- Du, W.J.; Zhen, J.H.; Zeng, Z.Q.; et al. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection: IL-17 in HBV infection. Diagn Pathol 2013, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, W.J.; Qin, L.Y.; Xing, Z.Z.; Qin, X.H.; Chen, S.J. Expression of interleukin-17 in hepatitis B related liver fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2009, 25, 133–135. [Google Scholar]
- Zhang, Y.; Cobleigh, M.A.; Lian, J.Q.; et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 2011, 141, 1897–1906. [Google Scholar] [CrossRef]
- Pan, C.X.; Tang, J.; Wang, X.Y.; Wu, F.R.; Ge, J.F.; Chen, F.H. Role of interleukin-22 in liver diseases. Inflamm Res 2014, 63, 519–525. [Google Scholar] [CrossRef]
- Guo, X.; Barroso, L.; Lyerly, D.M.; Petri, W.A., Jr.; Houpt, E.R. CD4+ and CD8+ T cell- and IL-17-mediated protection against Entamoeba histolytica induced by a recombinant vaccine. Vaccine 2011, 29, 772–777. [Google Scholar] [CrossRef]
- Wu, B.; Zou, Q.; Hu, Y.; Wang, B. Interleukin-22 as a molecular adjuvant facilitates IL-17-producing CD8+ T cell responses against a HBV DNA vaccine in mice. Hum Vaccin Immunother 2013, 9, 2133–2141. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.; Li, Z.; et al. IL17A gene polymorphisms, serum IL-17A and IgE levels, and hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. Mol Carcinog 2014, 53, 447–457. [Google Scholar] [CrossRef]
- Xi, X.E.; Liu, Y.; Lu, Y.; Huang, L.; Qin, X.; Li, S. Interleukin-17A and interleukin-17F gene polymorphisms and hepatitis B virus-related hepatocellular carcinoma risk in a Chinese population. Med Oncol 2015, 32, 355. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.J.; Frodsham, A.J.; Hellier, S.; et al. Influence of IL-10RA and IL-22 polymorphisms on outcome of hepatitis C virus infection. Liver Int 2007, 27, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Chen, L.; et al. Impact of IL-22 gene polymorphism on human immunodeficiency virus infection in Han Chinese patients. J Microbiol Immunol Infect 2016, 49, 872–878. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2016.
Share and Cite
Borzooy, Z.; Streinu-Cercel, A.; Mirshafiey, A.; Khamseh, A.; Mahmoudie, M.K.; Navabi, S.S.; Nosrati, M.; Najafi, Z.; Hosseini, M.; Jazayeri, S.M. IL-17 and IL-22 Genetic Polymorphisms in HBV Vaccine Non- and Low-Responders Among Healthcare Workers. GERMS 2016, 6, 14-20. https://doi.org/10.11599/germs.2016.1084
Borzooy Z, Streinu-Cercel A, Mirshafiey A, Khamseh A, Mahmoudie MK, Navabi SS, Nosrati M, Najafi Z, Hosseini M, Jazayeri SM. IL-17 and IL-22 Genetic Polymorphisms in HBV Vaccine Non- and Low-Responders Among Healthcare Workers. GERMS. 2016; 6(1):14-20. https://doi.org/10.11599/germs.2016.1084
Chicago/Turabian StyleBorzooy, Zohreh, Adrian Streinu-Cercel, Abbass Mirshafiey, Azam Khamseh, Masoud Karkhaneh Mahmoudie, Shadi Sadat Navabi, Marjan Nosrati, Zahra Najafi, Mostafa Hosseini, and Seyed Mohammad Jazayeri. 2016. "IL-17 and IL-22 Genetic Polymorphisms in HBV Vaccine Non- and Low-Responders Among Healthcare Workers" GERMS 6, no. 1: 14-20. https://doi.org/10.11599/germs.2016.1084
APA StyleBorzooy, Z., Streinu-Cercel, A., Mirshafiey, A., Khamseh, A., Mahmoudie, M. K., Navabi, S. S., Nosrati, M., Najafi, Z., Hosseini, M., & Jazayeri, S. M. (2016). IL-17 and IL-22 Genetic Polymorphisms in HBV Vaccine Non- and Low-Responders Among Healthcare Workers. GERMS, 6(1), 14-20. https://doi.org/10.11599/germs.2016.1084



