Prevalence of and Risk Factors for Pulmonary Tuberculosis Among Newly Diagnosed HIV-1 Infected Nigerian Children
Abstract
Introduction
Methods
Study design
Study subjects
Study setting
Source of data
Study procedure
Operational definitions
Statistical analysis
Ethical approval
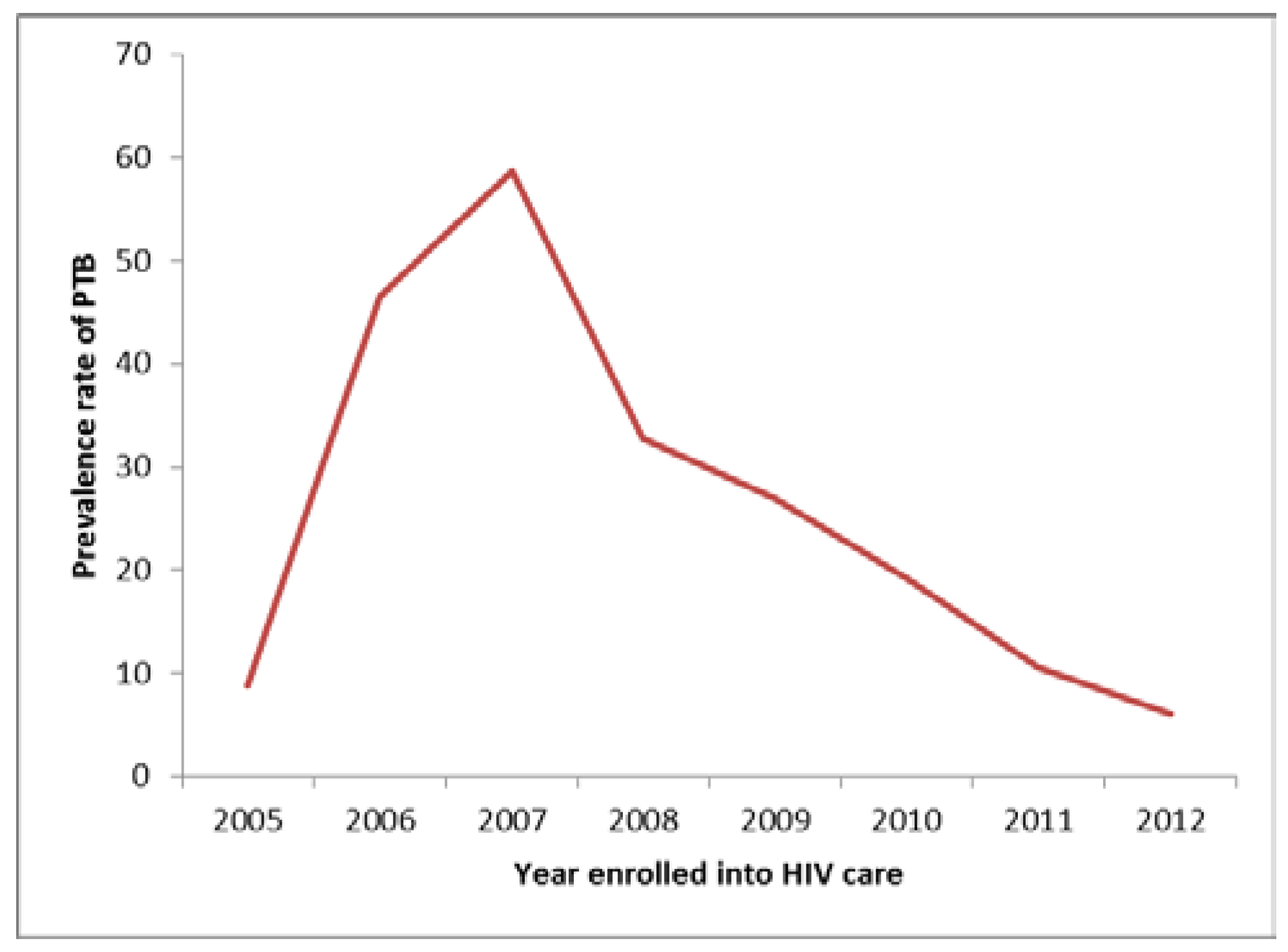
Results


Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USAID. The Twin Epidemics: HIV and TB Co-Infection; United States Agency for International Development: Washington, DC, USA, 2014. Available online: https://www.usaid.gov/news-information/fact-sheets/twin-epidemics-hiv-and-tb-co-infection (accessed on 8 September 2015).[Green Version]
- Thomas, P.; Bornschlegel, K.; Singh, T.P.; et al. Tuberculosis in human immunodeficiency virus-infected and human immunodeficiency virus-exposed children in New York City. The New York City Pediatric Spectrum of HIV Disease Consortium. Pediatr Infect Dis J 2000, 19, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.; Cotton, M.F.; Rabie, H.; Schaaf, H.S.; Walters, L.O.; Marais, B.J. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Auld, A.F.; Tuho, M.Z.; Ekra, K.A.; et al. Tuberculosis in human immunodeficiency virus-infected children starting antiretroviral therapy in Côte d’Ivoire. Int J Tuberc Lung Dis 2014, 18, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Bakeera-Kitaka, S.; Conesa-Botella, A.; Dhabangi, A.; et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis 2011, 15, 1082–1086. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okechukwu, A.A.; Okechukwu, O.I. Clinical correlate of tuberculosis in HIV co-infected children at the University of Abuja Teaching Hospital, Gwagwalada, Nigeria. Niger J Clin Pract 2011, 14, 206–211. [Google Scholar] [CrossRef] [PubMed]
- National Population Commission. Population Distribution by Sex, State, LGA & Senatorial District. 2006 Population and Housing Census: Priority Table Volume III. National Population Commission, of Abuja, Nigeria. Available online: http://catalog.ihsn.org/index.php/catalog/3340/download/48521 (accessed on 26 August 2015).
- Federal Ministry of Health. National Guidelines for Paediatric HIV and AIDS Treatment and Care; Federal Ministry of Health: Abuja, Nigeria, 2010; Available online: http://preventcrypto.org/wp-content/uploads/2015/10/NigeriaPaediatricARTguidelines20101369045239.pdf (accessed on 28 February 2016).
- Federal Ministry of Health. National Tuberculosis and Leprosy Control Programme—Workers’ Manual, 5th ed.; Federal Ministry of Health: Abuja, Nigeria; Department of Public Health, 2010; Available online: http://www.who.int/hiv/pub/guidelines/nigeria_tb.pdf (accessed on 26 August 2014).
- World Health Organization (WHO). Global Database on Child Growth and Malnutrition; World Health Organization: Geneva, Switzerland; Available online: http://www.who.int/nutgrowthdb/about/introduction/en/index5.html (accessed on 20 April 2014).
- World Health Organization (WHO). Application Tools: WHO AnthroPlus Software; World Health Organization: Geneva, Switzerland; Available online: http://www.who.int/growthref/tools/en/ (accessed on 21 April 2014).
- Centers for Disease Control and Prevention (CDC). 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age; Official authorized addenda: Human immunodeficiency virus infection codes and official guidelines for coding and reporting ICD-9-CM. MMWR Morb Mortal Wkly Rep 1994, 43, 1–19. [Google Scholar]
- World Health Organization (WHO). Iron Deficiency Anaemia: Assessment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2001; Available online: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/ (accessed on 26 August 2014)A Guide for Program Managers.
- Holmes, C.B.; Hausler, H.; Nunn, P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis 1998, 2, 96–104. [Google Scholar] [PubMed]
- Bellamy, R.; Beyers, N.; McAdam, K.P.; et al. Genetic susceptibility to tuberculosis in Africans: A genome-wide scan. Proc Natl Acad Sci USA 2000, 97, 8005–8009. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Tuberculosis Control 2015; World Health Organization: Geneva, Switzerland, 2015; Available online: www.who.int/tb/publications/global_report (accessed on 8 September 2015).
- Graham, S.M.; Coulter, J.B.; Gilks, C.F. Pulmonary disease in HIV-infected African children. Int J Tuberc Lung Dis 2001, 5, 12–23. [Google Scholar] [PubMed]
- Ruiz Jiménez, M.; Guillén Martín, S.; Prieto Tato, L.M.; et al. Induced sputum versus gastric lavage for the diagnosis of pulmonary tuberculosis in children. BMC Infect Dis 2013, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Agbaji, O.; Ebonyi, A.O.; Meloni, S.T.; et al. Factors associated with pulmonary tuberculosis-HIV co-infection in treatment-naive adults in Jos, North Central Nigeria. J AIDS Clin Res 2013, 4, 1000222. [Google Scholar]
- United Nations General Assembly Special Session Country Progress Report, Nigeria. 2010. Available online: http://data.unaids.org/pub/Report/2010/nigeria_2010_country_progress_report_en.pdf (accessed on 28 February 2016).
- UNAIDS. HIV and AIDS Estimates; UNAIDS: Geneva, Switzerland, 2014; Available online: http://www.unaids.org/en/regionscountries/countries/nigeria/ (accessed on 21 November 2015).
- Sagay, A.S.; Ebonyi, A.O.; Meloni, S.T.; et al. Mother-to-child transmission outcomes of HIV-exposed infants followed up in Jos, North-Central Nigeria. Curr HIV Res 2015, 13, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Braitstein, P.; Nyandiko, W.; Vreeman, R.; et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J 2009, 28, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Geldmacher, C.; Zumla, A.; Hoelscher, M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Curr Opin HIV AIDS 2012, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Naing, C.; Mak, J.W.; Maung, M.; Wong, S.F.; Kassim, A.I. Meta-analysis: The association between HIV infection and extrapulmonary tuberculosis. Lung 2013, 191, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ebonyi, A.O.; Oguche, S.; Ejeliogu, E.U.; et al. Risk factors for first-line antiretroviral treatment failure in HIV-1 infected children attending Jos University Teaching Hospital, Jos, North Central Nigeria. Br J Med Med Res 2014, 4, 2983–2994. [Google Scholar] [CrossRef][Green Version]
- Bolton-Moore, C.; Mubiana-Mbewe, M.; Cantrell, R.A.; et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007, 298, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Raguenaud, M.E.; Isaakidis, P.; Zachariah, R.; et al. Excellent outcomes among HIV+ children on ART, but unacceptably high pre-ART mortality and losses to follow-up: A cohort study from Cambodia. BMC Pediatr 2009, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Yotebieng, M.; Van Rie, A.; Moultrie, H.; et al. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS 2010, 24, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Buck, W.C.; Olson, D.; Kabue, M.M.; et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuber Lung Dis 2013, 17, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2016.
Share and Cite
Ebonyi, A.O.; Oguche, S.; Ejeliogu, E.U.; Agbaji, O.O.; Shehu, N.Y.; Abah, I.O.; Sagay, A.S.; Ugoagwu, P.O.; Okonkwo, P.I.; Idoko, J.A.; et al. Prevalence of and Risk Factors for Pulmonary Tuberculosis Among Newly Diagnosed HIV-1 Infected Nigerian Children. Germs 2016, 6, 21-28. https://doi.org/10.11599/germs.2016.1085
Ebonyi AO, Oguche S, Ejeliogu EU, Agbaji OO, Shehu NY, Abah IO, Sagay AS, Ugoagwu PO, Okonkwo PI, Idoko JA, et al. Prevalence of and Risk Factors for Pulmonary Tuberculosis Among Newly Diagnosed HIV-1 Infected Nigerian Children. Germs. 2016; 6(1):21-28. https://doi.org/10.11599/germs.2016.1085
Chicago/Turabian StyleEbonyi, Augustine O., Stephen Oguche, Emeka U. Ejeliogu, Oche O. Agbaji, Nathan Y. Shehu, Isaac O. Abah, Atiene S. Sagay, Placid O. Ugoagwu, Prosper I. Okonkwo, John A. Idoko, and et al. 2016. "Prevalence of and Risk Factors for Pulmonary Tuberculosis Among Newly Diagnosed HIV-1 Infected Nigerian Children" Germs 6, no. 1: 21-28. https://doi.org/10.11599/germs.2016.1085
APA StyleEbonyi, A. O., Oguche, S., Ejeliogu, E. U., Agbaji, O. O., Shehu, N. Y., Abah, I. O., Sagay, A. S., Ugoagwu, P. O., Okonkwo, P. I., Idoko, J. A., & Kanki, P. J. (2016). Prevalence of and Risk Factors for Pulmonary Tuberculosis Among Newly Diagnosed HIV-1 Infected Nigerian Children. Germs, 6(1), 21-28. https://doi.org/10.11599/germs.2016.1085



