Management of Accidental Exposure to HCV, HBV and HIV in Healthcare Workers in Romania
Abstract
Introduction
Methods
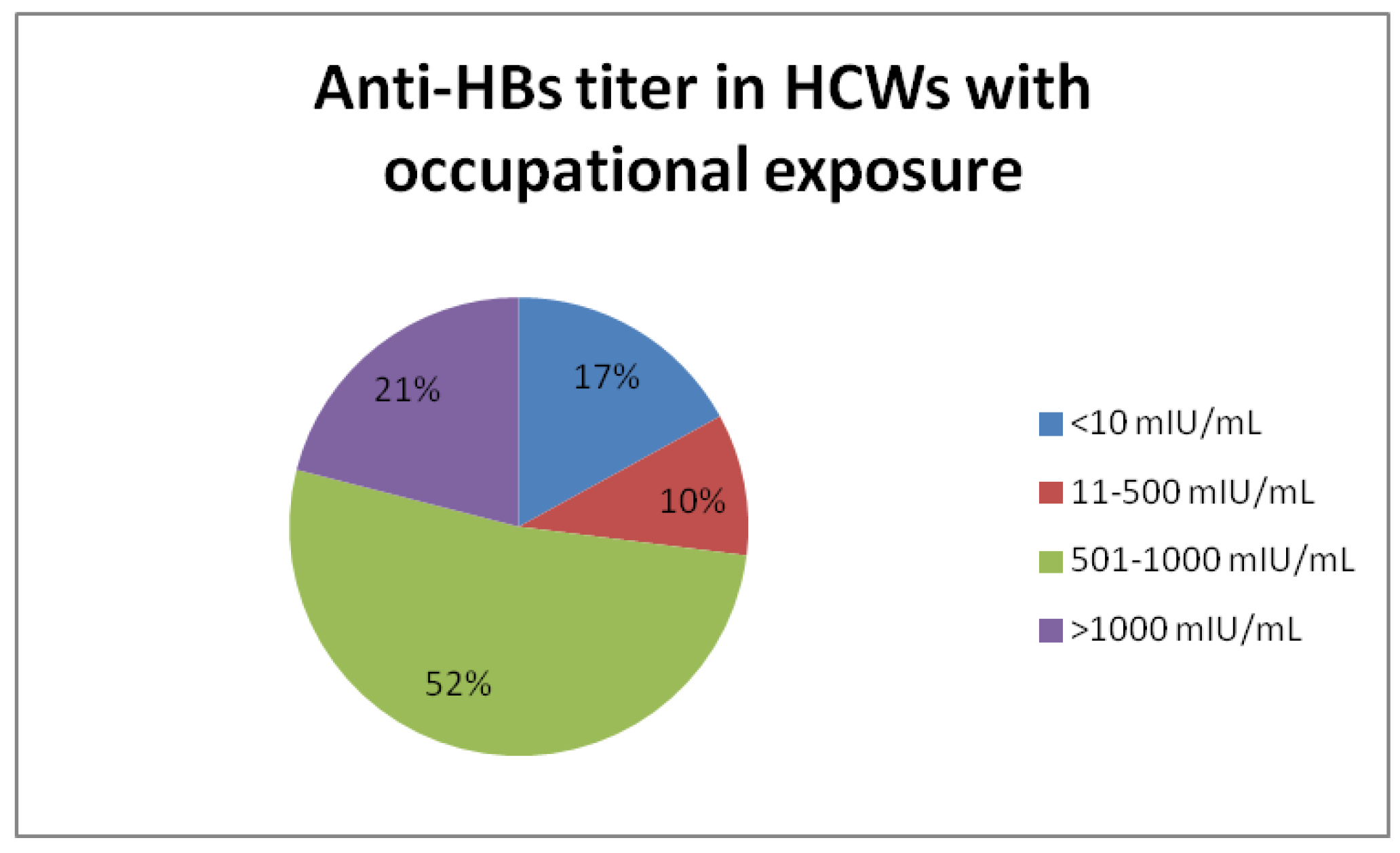
Results
Discussion
Author Contributions
Conflicts of interest
References
- Streinu-Cercel, A. Hepatitis B in the spotlight. GERMS 2011, 1, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Căruntu, F.A.; Streinu-Cercel, A.; Gheorghe, L.S.; et al. Efficacy and safety of peginterferon alpha-2a (40KD) in HBeAg-positive chronic hepatitis B patients. J. Gastrointestin Liver Dis. 2009, 18, 425–431. [Google Scholar] [PubMed]
- Cui, Q.; Zhang, Y.; Su, J.; et al. The association between the genetic polymorphisms of LMP2/LMP7 and the outcomes of HCV infection among drug users. J. Biomed. Res. 2010, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- CDC The STOP STICKS Campaign. Available online: http://www.cdc.gov/ niosh/stopsticks (accessed on 1 October 2012).
- Updated, U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR Recomm. Rep. 2001, 50, 1–52. [Google Scholar]
- Teshale, E. Infectious Diseases Related To Travel. Hepatitis B. In CDC Health Information for International Travel The Yellow Book; B. In CDC Health Information for International Travel The Yellow Book; Brunette, G., Ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Streinu-Cercel, O.; Streinu-Cercel, A.; Preoţescu, L.; Streinu-Cercel, A. Entecavir as specific antiviral therapy in selected cases of severe acute hepatitis B. GERMS 2012, 2, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Piţigoi, D.; Rafila, A.; Pistol, A.; Aramă, V.; Molagic, V.; Streinu-Cercel, A. Trends in hepatitis B incidence in Romania, 1989-2005. Euro Surveill. 2008, 13, 8012–8012. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, M.; Iliescu, L.; Ionescu, C.; et al. A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania. J. Gastrointestin. Liver Dis. 2010, 19, 43–48. [Google Scholar] [PubMed]
- Gheorghe, L.; Csiki, I.E.; Iacob, S.; Gheorghe, C.; Smira, G.; Regep, L. The prevalence and risk factors of hepatitis C virus infection in adult population in Romania: A nationwide survey 2006–2008. J. Gastrointestin. Liver Dis. 2010, 19, 373–379. [Google Scholar] [PubMed]
- Gheorghe, L.; Iacob, S.; Csiki, I.E. Prevalence of hepatitis C in Romania: Different from European rates? J Hepatol 2008, 49, 661–662. [Google Scholar] [PubMed][Green Version]
- UNAIDS report on the global AIDS epidemic 2010. 2010. Available online: http://issuu.com/unaids/docs/unaids_globalreport_2010 (accessed on 13 September 2012).
- Deuffic-Burban, S.; Delarocque-Astagneau, E.; Abiteboul, D.; Bouvet, E.; Yazdanpanah, Y. Blood-borne viruses in health care workers: Prevention and management. J. Clin. Virol. 2011, 52, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Management of healthcare workers exposed to hepatitis B virus or hepatitis C virus UpToDate, 2012. Available online: http://www.uptodate.com/contents/management-of-healthcare-workers-exposed-to-hepatitis-b-virus-or-hepatitis-c-virus/contributors?utdPopup=true (accessed on 16 September 2012).
- Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Recomm. Rep. 2012, 61, 1–12.
- Mahoney, F.J.; Stewart, K.; Hu, H.; Coleman, P.; Alter, M.J. Progress toward the elimination of hepatitis B virus transmission among health care workers in the United States. Arch. Intern. Med. 1997, 157, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Streinu-Cercel, O. AIDS and Sexually Transmitted Infections in Africa. GERMS 2012, 2, 5. [Google Scholar] [CrossRef][Green Version]
- Bai, H.; Huan, X.; Tang, W.; et al. A survey of HIV infection and related high-risk factors among men who have sex with men in Suzhou, Jiangsu, China. J. Biomed. Res. 2011, 25, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernandes, L. Human immunodeficiency virus and cancer: A population of HIV-infected patients at Hospital de Santa Maria and predictors of cancer. GERMS 2012, 2, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Mihăilescu, R.; Aramă, V.; Paraschiv, S.; et al. Impact of highly active antiretroviral therapy on cytomegalovirus viraemia in the absence of specific anti-cytomegalovirus therapy. Rom. J. Intern. Med. 2008, 46, 305–311. [Google Scholar]
- Panlilio, A.L.; Cardo, D.M.; Grohskopf, L.A.; Heneine, W.; Ross, C.S. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm. Rep. 2005, 54, 1–17. [Google Scholar] [PubMed]
- Puvacic, S.; Ravlija, J.; Puvacic, Z.; Curic, I. Long term protection after hepatitis B vaccination. Bosn. J. Basic. Med. Sci. 2005, 5, 50–53. [Google Scholar] [PubMed]
- Platkov, E.; Shlyakhov, E.; Glick, Y.; Khalemsky, S.; Fischbein, A. Immunologic evaluation of hepatitis B vaccine application in hospital staff. Int. J. Occup. Med. Environ. Health 2003, 16, 249–253. [Google Scholar] [PubMed]

| Age ± standard deviation | 36 ± 10 years | ||
| Gender | Males | Females | Total |
| 6 (10%) | 54 (90%) | 60 (100%) | |
| Occupation | Nurses | Doctors | Assisting staff |
| 48 (80%) | 7 (12%) | 5 (8%) | |
| Exposure | Percutaneous | Mucosal/corneal | |
| 49 (82%) | 11 (18%) |
© GERMS 2012.
Share and Cite
Malka, E.; Streinu-Cercel, A.; PiŢIgoi, D.; Bacruban, R. Management of Accidental Exposure to HCV, HBV and HIV in Healthcare Workers in Romania. Germs 2012, 2, 137-141. https://doi.org/10.11599/germs.2012.1025
Malka E, Streinu-Cercel A, PiŢIgoi D, Bacruban R. Management of Accidental Exposure to HCV, HBV and HIV in Healthcare Workers in Romania. Germs. 2012; 2(4):137-141. https://doi.org/10.11599/germs.2012.1025
Chicago/Turabian StyleMalka, Eyal, Anca Streinu-Cercel, Daniela PiŢIgoi, and Rodica Bacruban. 2012. "Management of Accidental Exposure to HCV, HBV and HIV in Healthcare Workers in Romania" Germs 2, no. 4: 137-141. https://doi.org/10.11599/germs.2012.1025
APA StyleMalka, E., Streinu-Cercel, A., PiŢIgoi, D., & Bacruban, R. (2012). Management of Accidental Exposure to HCV, HBV and HIV in Healthcare Workers in Romania. Germs, 2(4), 137-141. https://doi.org/10.11599/germs.2012.1025




