Probable Vector of Crimean-Congo Hemorrhagic Fever Virus; Hyalomma aegyptium: A Systematic Review and Meta-Analysis
Abstract
Introduction
Methods
Search strategy
Eligibility criteria
Data extraction
Quality assessment
Statistical analysis
Results
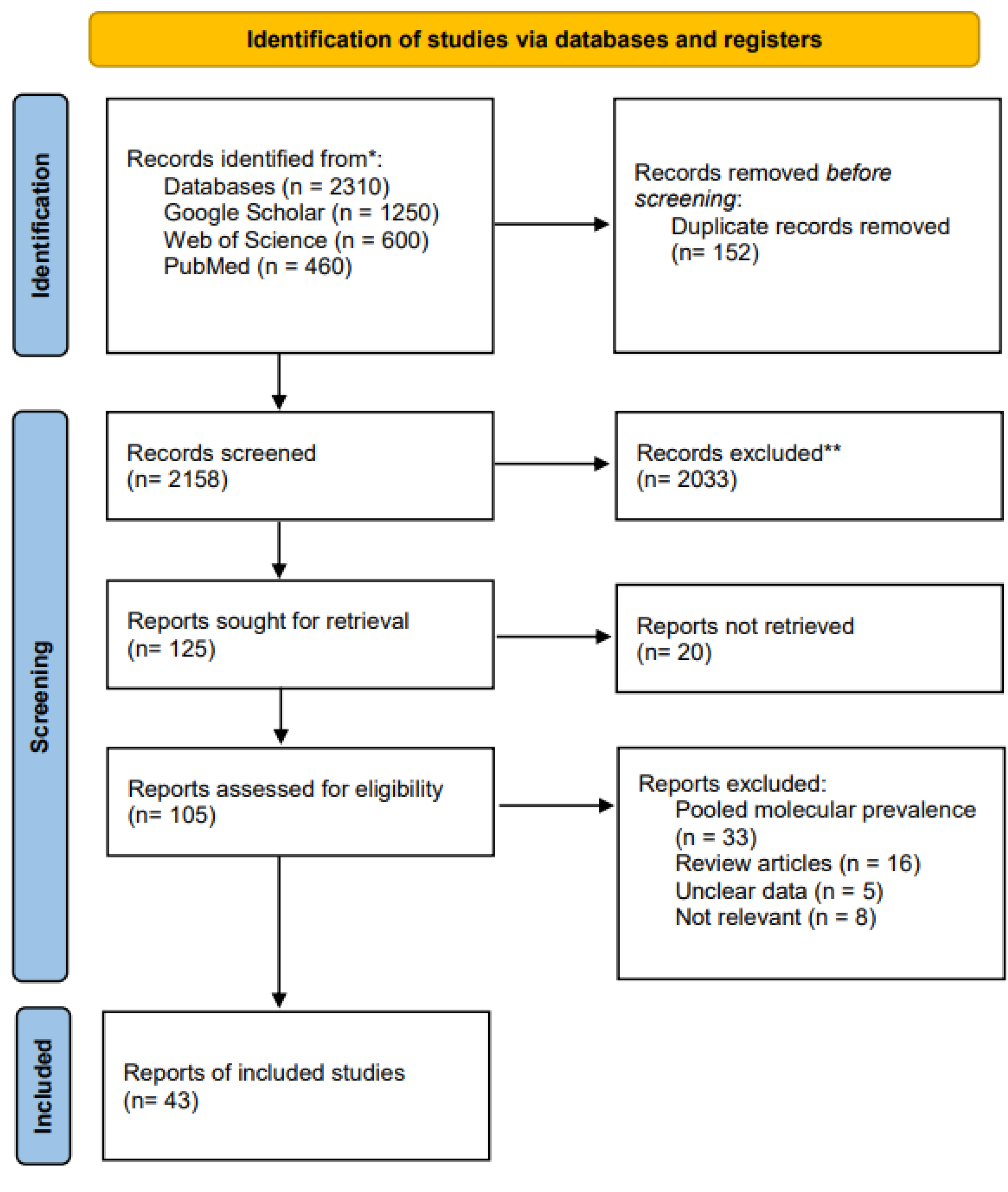
Literature search, selection and data extraction
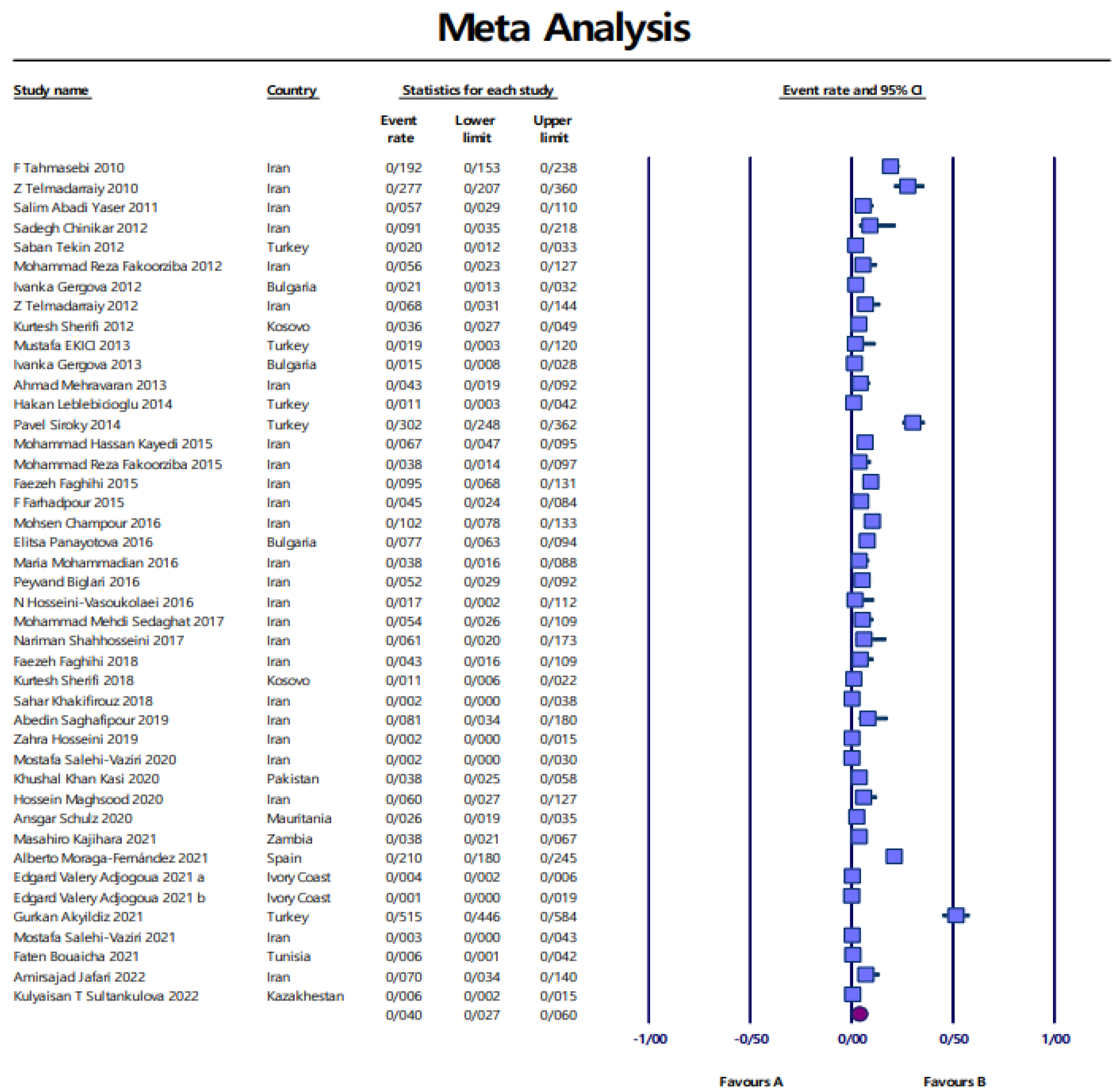
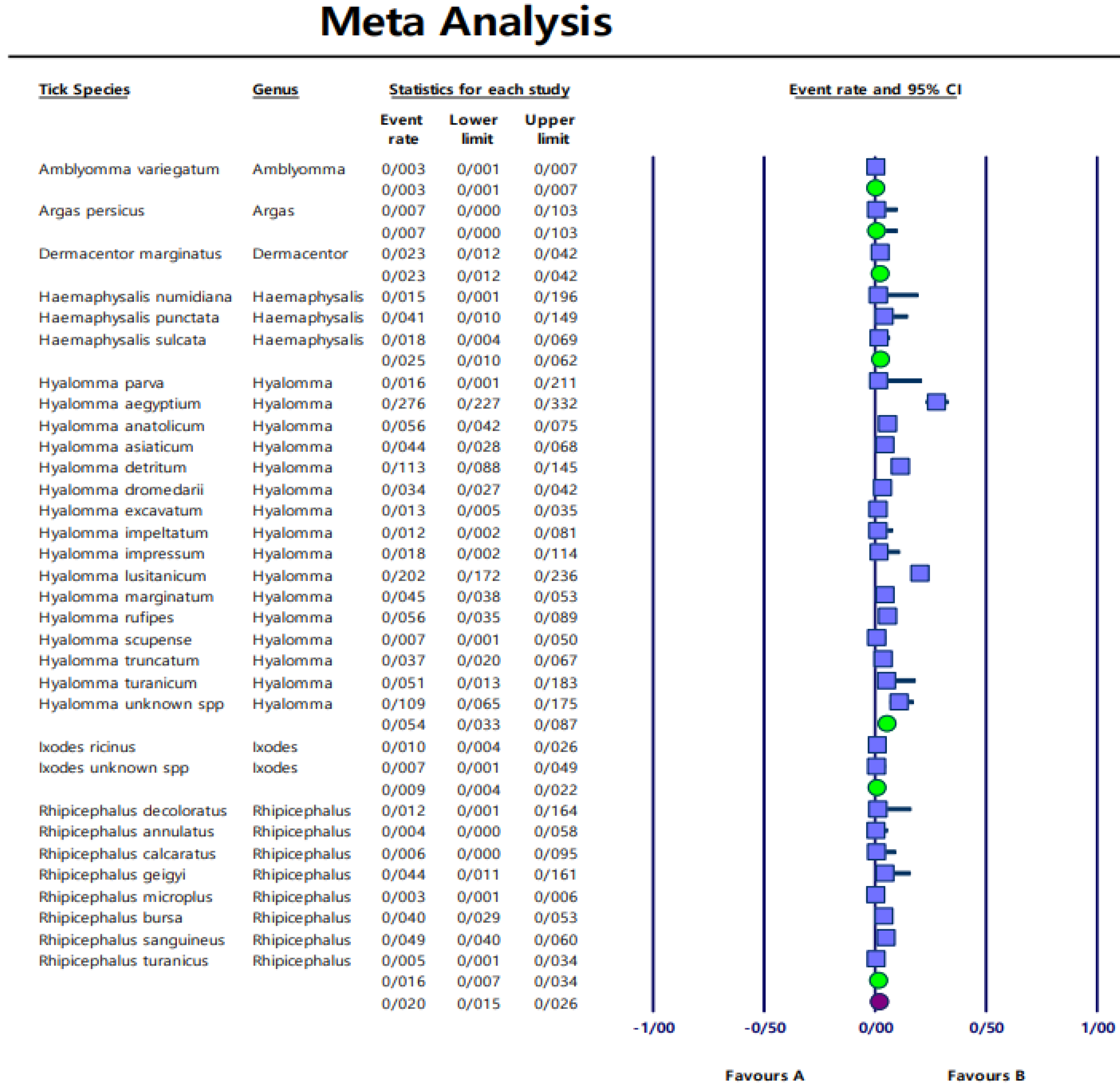
Pooled prevalence
Publication bias
Discussion
Strengths and limitations
Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of interest
References
- Sultankulova, K.T.; Shynybekova, G.O.; Kozhabergenov, N.S.; et al. The prevalence and genetic variants of the CCHF virus circulating among ticks in the southern regions of Kazakhstan. Pathogens. 2022, 11, 841. [Google Scholar] [CrossRef]
- Sadeghi, H.; Nikkhahi, F.; Maleki, M.R.; Simiari, A.; Bakht, M.; Gholamzadeh Khoei, S. Status of Crimean-Congo haemorrhagic fever virus in ticks in Iran: A systematic review with meta-analysis. Microb Pathog. 2023, 106153. [Google Scholar] [CrossRef]
- Ergönül, O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004, 64, 145–160. [Google Scholar] [CrossRef]
- Fatemian, Z.; Salehzadeh, A.; Sedaghat, M.M.; Telmadarraiy, Z.; Hanafi-Bojd, A.A.; Zahirnia, A.H. Hard tick (Acari: Ixodidae) species of livestock and their seasonal activity in Boyer-Ahmad and Dena cities of Kohgiluyeh and Boyer-Ahmad Province, Southwest of Iran. Vet World. 2018, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013, 100, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Bergeron, É.; Rollin, P.E. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. 2016, 10, e0004210. [Google Scholar] [CrossRef]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antiviral Res. 2017, 144, 93–119. [Google Scholar] [CrossRef]
- Hoogstraal, H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef]
- Spengler, J.R.; Estrada-Peña, A.; Garrison, A.R.; et al. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res. 2016, 135, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Kalvatchev, N.; Christova, I. One step RT-PCR for rapid detection of Crimean-Congo haemorrhagic fever virus. Biotechnol Biotechnolog Equip. 2008, 22, 864–866. [Google Scholar] [CrossRef][Green Version]
- Sas, M.A.; Vina-Rodriguez, A.; Mertens, M.; et al. A one-step multiplex real-time RT-PCR for the universal detection of all currently known CCHFV genotypes. J Virol Methods. 2018, 255, 38–43. [Google Scholar] [CrossRef]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013, 13, 154. [Google Scholar] [CrossRef]
- Quek, T.T.; Tam, W.W.; Tran, B.X.; et al. The Global prevalence of anxiety among medical students: A meta-analysis. Inf J Environ Res Public Health. 2019, 16, 2735. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Gholamian-Shahabad, M.R.; Azizi, K.; Asgari, Q.; Kalantari, M.; Moemenbellah-Fard, M.D. Sandflies species composition, activity, and natural infection with Leishmania, parasite identity in lesion isolates of cutaneous leishmaniasis, central Iran. J Parasit Dis. 2018, 42, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Davari, B.; Hassanvand, A.E.; Nasirian, H.; Ghiasian, S.A.; Salehzadeh, A.; Nazari, M. Comparison of cockroach fungal contamination in the clinical and non-clinical environments from Iran. J Entomol Acarol Res. 2017, 49, 6758. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M. The discrepancy of Crimean-Congo hemorrhagic fever-related tick vectors: An urgent need for boosted surveillance. Ann Med Surg (Lond). 2022, 81, 104412. [Google Scholar] [CrossRef]
- Keikha, M.J.I.J.o.S. The impact of Crimean-Congo hemorrhagic fever on travelers: The urgency of surveillance-Correspondence. 2022; 106, 106902. [Google Scholar] [CrossRef]
- Papa, A.; Dalla, V.; Papadimitriou, E.; Kartalis, G.; Antoniadis, A. Emergence of Crimean-Congo haemorrhagic fever in Greece. Clin Microbiol Infect. 2010, 16, 843–847. [Google Scholar] [CrossRef]
- Kabir, M.; Mondal, M.; Eliyas, M.; et al. An epidemiological survey on investigation of tick infestation in cattle at Chittagong District, Bangladesh. Afr J Microbiol Res. 2011, 5, 346–352. [Google Scholar]
- Ergönül, Ö. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Tekin, S.; Bursali, A.; Mutluay, N.; Keskin, A.; Dundar, E. Crimean-Congo hemorrhagic fever virus in various ixodid tick species from a highly endemic area. Vet Parasitol 2012, 186, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Ince, Y.; Yasa, C.; Metin, M.; et al. Crimean-Congo hemorrhagic fever infections reported by ProMED. Int J Infect Dis. 2014, 26, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, H. Ticks infected with Crimean-Congo hemorrhagic fever virus (CCHFV): A decision approach systematic review and meta-analysis regarding their role as vectors. Travel Med Infect Dis. 2022, 47, 102309. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Nuttall, P. Viruses transmitted by ticks. In: Bowman AS, Nuttall PA, eds. Ticks: Biology, disease and control. Cambridge University Press; 2008, 253-280. [CrossRef]
- Kar, S.; Rodriguez, S.E.; Akyildiz, G.; et al. Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in East Thrace, Turkey: Potential of a cryptic transmission cycle. Parasit Vectors. 2020, 13, 201. [Google Scholar] [CrossRef]
© GERMS 2024.
Share and Cite
Sadeghi, H.; Khoei, S.G.; Shahsavari, S.; Aslanimehr, M.; Nikkhahi, F.; Babaei, A.; Gheibi, N.; Bizhani, B. Probable Vector of Crimean-Congo Hemorrhagic Fever Virus; Hyalomma aegyptium: A Systematic Review and Meta-Analysis. Germs 2024, 14, 45-62. https://doi.org/10.18683/germs.2024.1417
Sadeghi H, Khoei SG, Shahsavari S, Aslanimehr M, Nikkhahi F, Babaei A, Gheibi N, Bizhani B. Probable Vector of Crimean-Congo Hemorrhagic Fever Virus; Hyalomma aegyptium: A Systematic Review and Meta-Analysis. Germs. 2024; 14(1):45-62. https://doi.org/10.18683/germs.2024.1417
Chicago/Turabian StyleSadeghi, Hamid, Saeideh Gholamzadeh Khoei, Sara Shahsavari, Masoumeh Aslanimehr, Farhad Nikkhahi, Abouzar Babaei, Nematollah Gheibi, and Behzad Bizhani. 2024. "Probable Vector of Crimean-Congo Hemorrhagic Fever Virus; Hyalomma aegyptium: A Systematic Review and Meta-Analysis" Germs 14, no. 1: 45-62. https://doi.org/10.18683/germs.2024.1417
APA StyleSadeghi, H., Khoei, S. G., Shahsavari, S., Aslanimehr, M., Nikkhahi, F., Babaei, A., Gheibi, N., & Bizhani, B. (2024). Probable Vector of Crimean-Congo Hemorrhagic Fever Virus; Hyalomma aegyptium: A Systematic Review and Meta-Analysis. Germs, 14(1), 45-62. https://doi.org/10.18683/germs.2024.1417


