Abstract
Introduction: SARS-CoV-2 represents one of the most extensively researched pathogens in the last decade due to its major impact on humanity. Not only does this viral infection cause respiratory disturbances, but it also generates cardiovascular injury. Cardiac arrhythmias represent one of the main consequences of SARS-CoV-2 infection, but they can also occur in the context of antiviral treatment. Furthermore, arrhythmias do not always seem to be correlated with the severity of the lung injury. However, they represent a poor prognostic factor in terms of mortality, increasing the need for intensive care and the length of hospitalization. Methods: In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 Statement, from September 2022 to October 2023, we conducted this study by examining the literature through the PubMed database using the following keywords: COVID-19, cardiac arrhythmias, and, in terms of study design, observational studies. Results: We initially identified 266 studies across PubMed. After applying the inclusion/exclusion criteria, we managed to include 22 studies in our review. Conclusions: Deducing the pathophysiological mechanisms behind SARS-CoV-2’s ability to disrupt the electrical activity of the heart, as well as identifying associated risk factors in patients with SARS-CoV-2 infection, could allow targeted therapeutic interventions to decrease the risk of mortality in hospitalized patients.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents one of the most extensively researched pathogens over the last decade due to the major impact it has had on humanity. It is now well known that this virus produces a multitude of effects on various organs and systems, both during the acute infection (known as coronavirus disease or COVID-19) and also following this phase (the so-called long COVID, post-COVID-19 condition, or post-COVID-19 syndrome). Perhaps some of the most damaging effects of SARS-CoV-2 are cardiovascular complications and arrhythmias, particularly because of their potentially fatal outcome [1,2].
The viral structure is crucial to understanding SARS-CoV-2’s affinity not only to the cardiovascular system but also to other organs. Apart from being a member of the family Coronaviridae and the genus Betacoronavirus, this single-stranded RNA virus is enveloped and presents a structural spike protein that enables its binding to the host’s cell receptors—the angiotensin-converting enzyme 2 (ACE2)—which is highly represented in organs such as the heart, the blood vessels, the respiratory tract, the kidneys, and the gastrointestinal tract, explaining COVID-19’s clinical manifestations at these levels [1,3]− [5] The ligand-receptor coupling requires the action of the host’s protease, the transmembrane protease serine 2 (TMPRSS2), which further cleaves the spike protein, activating it and initiating the virus’s endocytosis, finishing with its replication inside the host cell [1,4,5]. It appears that the ACE2 receptor is highly expressed in the heart, especially by the cardiomyocytes and the pericytes [1,4]. Furthermore, Nicin et al. compared the expression of ACE2 in different types of cardiac cells within two groups, one with structural heart diseases and one healthy control group, and the expression was higher in the pathological group, suggesting a susceptibility for cardiac complications in patients with heart disease and concomitant SARS-CoV-2 infection [6]. D. Bojkova et al. demonstrated the in vitro infection of cardiomyocytes with SARS-CoV-2 in an ACE2-dependent manner [7]. Moreover, Lindner et al.managed to detect the virus in autopsied hearts from patients who had tested positive for the infection [8]. All of this suggests that there might be a direct correlation between SARS-CoV-2 and cardiovascular effects such as arrhythmias.
Given the potential sequelae that come with various types of cardiac arrhythmias, we conducted a systematic review to emphasize the pathophysiology behind developing disturbances in heart rhythm in COVID-19. Moreover, through our study, we aim to clarify some of the responsible mechanisms already proposed in the literature that lacked a pathophysiological explanation.
Methods
Search strategy
In accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 Statement, from September 2022 to October 2023, we conducted this study by examining the literature through the PubMed database. The search was performed around three main subjects: COVID-19, cardiac arrhythmias, and, in terms of study design, observational studies. The exact search formula is reported below:
<< ((“COVID-19” [Mesh] OR “SARS-CoV-2” OR “coronavirus disease 2019”) AND (“Arrhythmias, Cardiac” [Mesh] OR “cardiac rhythm disorder*” OR “heart rhythm disorder*”)) AND (“Longitudinal Studies” [Mesh] OR “longitudinal” OR “cross-sectional studies” [Mesh] OR “cross-sectional” OR “Cohort Studies” [Mesh] OR “cohort” OR “Case-Control Studies” [Mesh] OR “case–control” OR “Observational Study” [Publication Type] OR “observational”) >>.
Inclusion/exclusion criteria
The studies included in our review were written in English, were conducted on humans, and were freely available in full text format. Other inclusion criteria were the study design (observational studies, respectively, including cross-sectional, case–control, and cohort studies) and the inclusion of patients with an ongoing SARS-CoV-2 infection, or a history of it, confirmed by a positive reverse transcription polymerase chain reaction (RT-PCR) test. The exclusion criteria were: languages other than English; any other species but humans; unavailable free full text; study design other than observational studies; and unconfirmed SARS-CoV-2 infection.
Data analysis
A narrative synthesis of each study was conducted in order to summarize the most frequent types of cardiac arrhythmias observed during COVID-19, along with clinical and laboratory characteristics of the study participants that might explain a pathophysiological substrate behind them.
Results
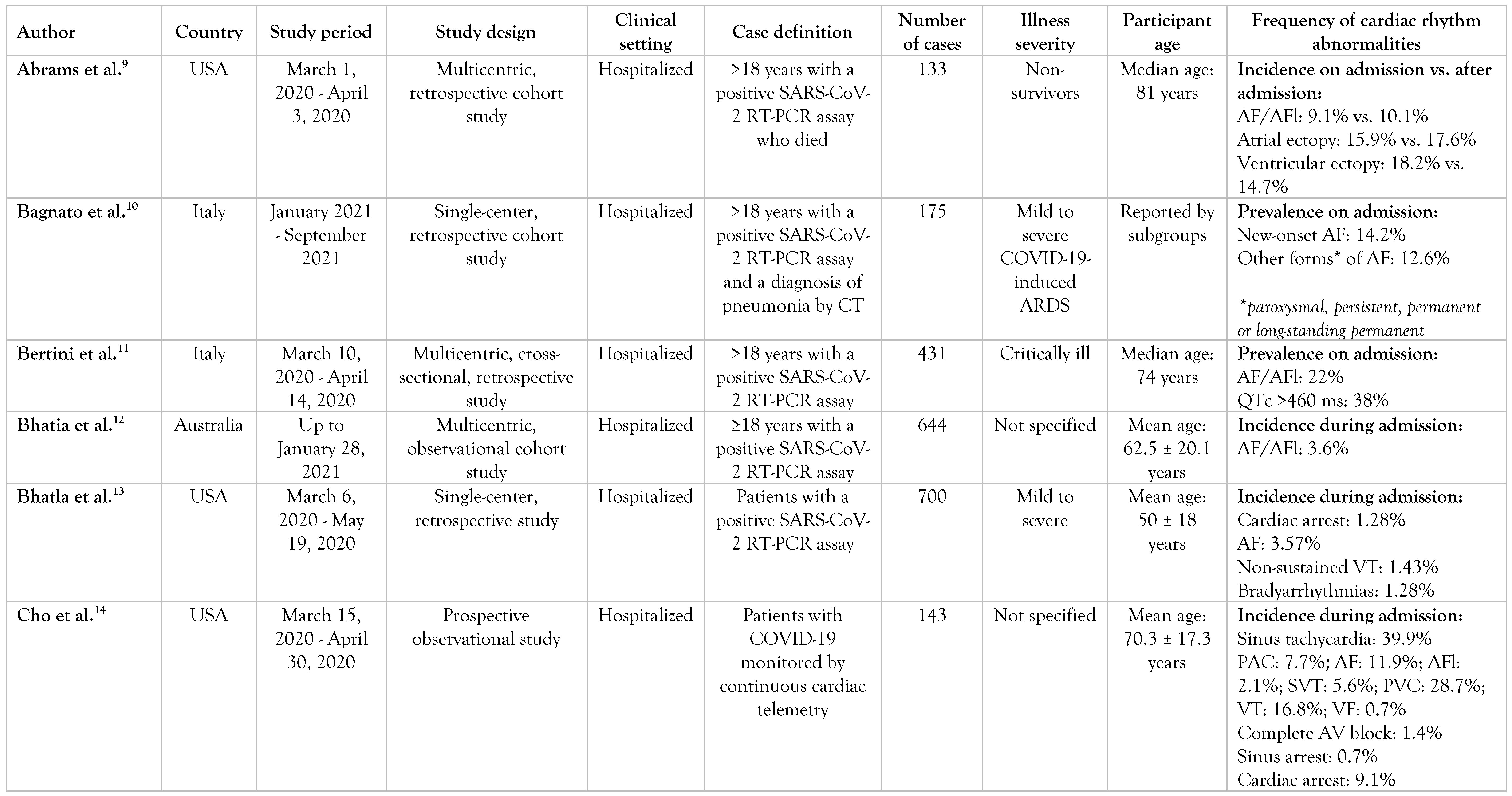
We initially identified 266 studies across PubMed, of which 2 were removed: 1 after applying the English language filter and another 1 after applying the human species filter. The remaining 264 records were screened by title and by abstract according to our objectives and our inclusion/exclusion criteria, and 162 records were excluded. Of the 102 remaining studies, 40 were not retrieved in free full text. Sixty-two potentially relevant studies were then screened with full-text reading, of which we managed to include 22 in our review (Figure 1). All 22 selected articles were reviewed by an independent reviewer to check that the interpretation was correct.
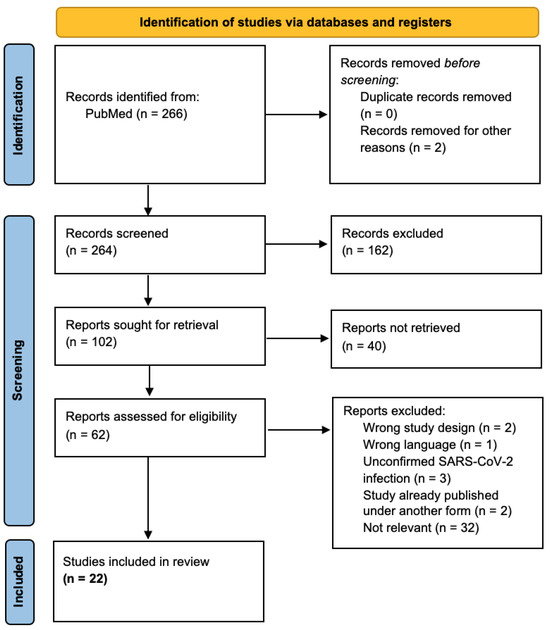
Figure 1.
PRISMA flow diagram.
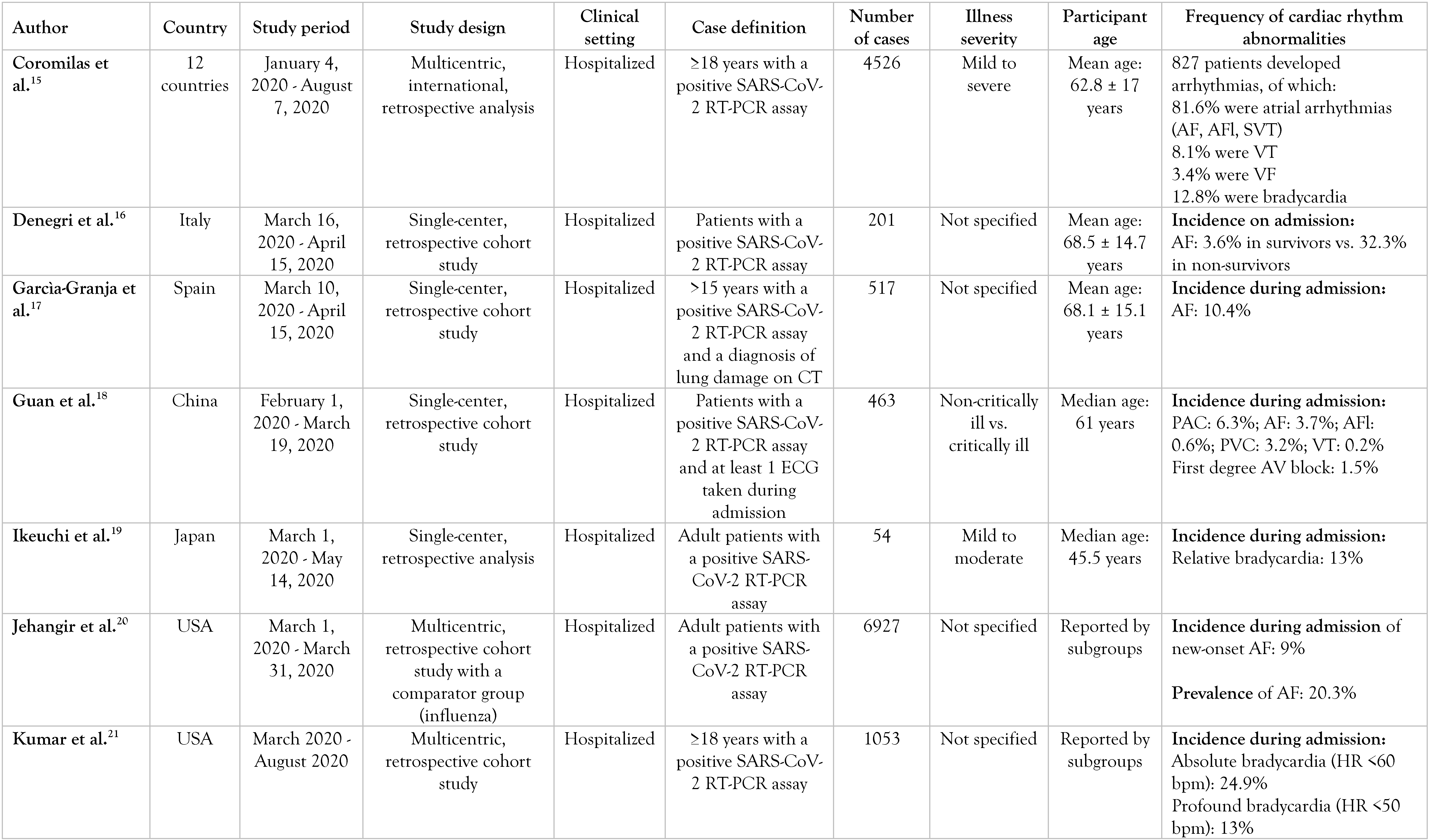
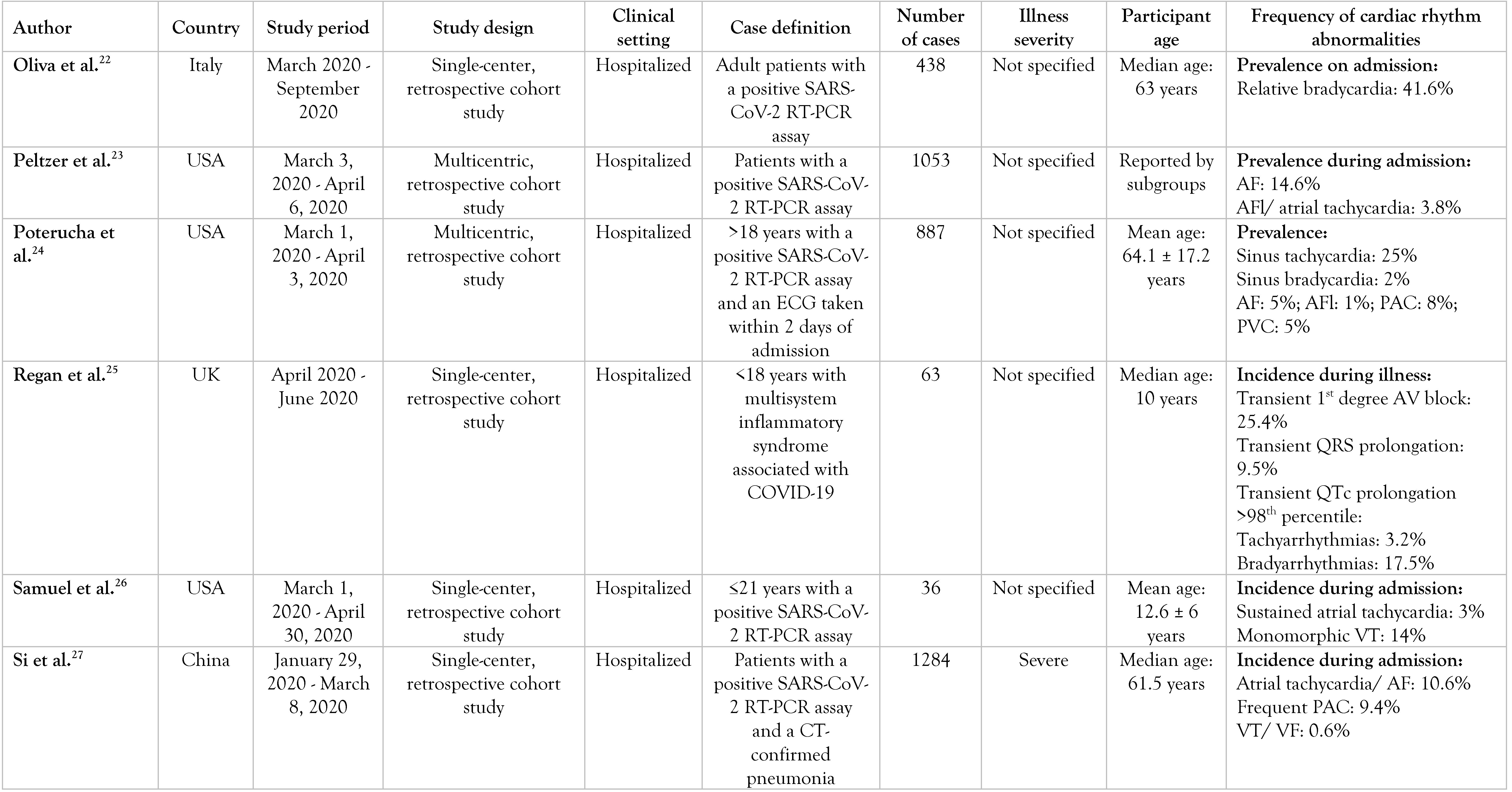
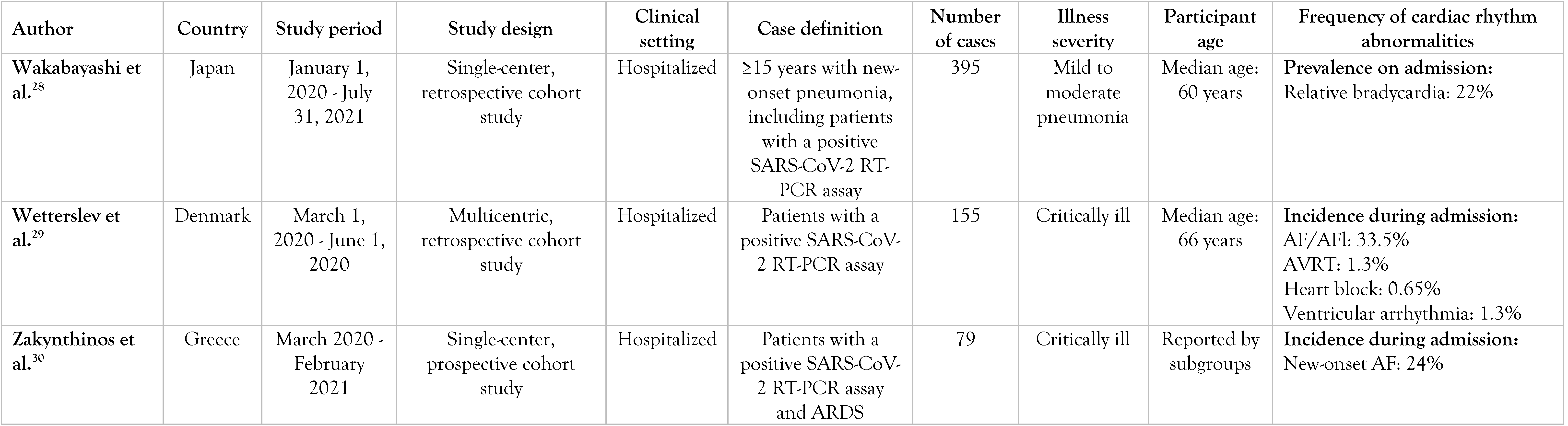
A summary of them can be found in Table 1.

Table 1.
Selected articles.
General findings in COVID-19 studies
Abrams et al. investigated the characteristics of a cohort of 133 COVID-19 non-survivors by dividing it into an arrhythmic (n = 11) and a non-arrhythmic (n = 122) group based on the cause of death [9]. They compared baseline ECGs (taken within 2 years of admission), ECGs on admission, and follow-up ECGs, as well as patients’ clinical characteristics and laboratory findings. In the total cohort, 97% had preexisting comorbidities, of which coronary artery disease (CAD), diabetes mellitus (DM), and asthma were significantly more frequent in the arrhythmic deaths compared to the non-arrhythmic ones. Moreover, the arrhythmic death group had a longer corrected QT interval (QTc) and more ventricular ectopias on admission. Of the 8.3% of patients who had died of an arrhythmic cause, eight deaths were attributable to ventricular tachycardia (VT)/ventricular fibrillation (VF), one was attributable to bradycardia, and another two were sudden deaths without an identified cause. Cardiac markers such as high-sensitivity troponin T (hs-TnT) and N-terminal pro-B type natriuretic peptide (NT-pro-BNP), as well as inflammatory markers like interleukin 6 (IL-6), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) or ferritin, were elevated on admission in most deceased patients.
Bhatia et al. studied the incidence of cardiac complications during COVID-19 hospitalization and found that patients without preexisting cardiac diseases had lower rates of cardiac sequelae [12]. While 9.6% of the patients had a history of atrial fibrillation (AF)/atrial flutter (AFl), only 3.6% newly acquired it during admission, especially those patients who were at least 65 years old.
Garcìa-Granja et al. found that 10.4% of their COVID-19 patients developed AF during hospitalization, of whom 83% had a history of AF and only 17% developed it de novo [17].
By contrast, Coromilas et al. conducted a worldwide survey and observed that most patients who developed arrhythmias during COVID-19 did not have preexisting heart rhythm disturbances [15]. While tachyarrhythmias such as atrial fibrillation (AF)/atrial flutter (AFl) were frequent in Europe and North and South America, Asian patients had a higher incidence of bradyarrhythmias and AV blocks during SARS-CoV-2 infection. The Asian cohort was also healthier overall, with a lower prevalence of hypertension, diabetes, CAD, or AF/AFl and a lower mean body mass index (BMI). This might suggest that preexisting inflammatory conditions such as metabolic syndrome could enhance the acute inflammatory state caused by SARS-CoV-2.
Peltzer et al. found a prevalence of 15.8% of atrial arrhythmias such as AF, AFl, and atrial tachycardia among their COVID-19 hospitalized patients, with more than 60% of them without a known history of these heart rhythm disorders [23]. Atrial fibrillation was the most frequently found arrhythmia. Those with atrial arrhythmias had higher peak levels of myocardial injury biomarkers (TnI) and inflammatory markers (CRP, ESR, ferritin) than patients without arrhythmias (p<0.001 for all).
Jehangir et al. divided their COVID-19 cohort into Group 1 (patients with normal sinus rhythm), Group 2 (patients with new-onset AF/AFl), and Group 3 (patients with a history of AF/AFl who remained with a normal sinus rhythm/developed AF/AFl) [20]. Group 2 had higher peak levels of troponin I and inflammatory markers than Group 1 and Group 3. Patients with new-onset AF/AFl were also more frequently hypoxic than those from Group 1 or 3. When compared to a matched influenza cohort, the incidence of new-onset AF/AFl in COVID-19 was higher than that in influenza (9% vs. 2.5%). This might support the idea that SARS-CoV-2 promotes inflammation more intensively than influenza viruses. Bhatla et al. observed that COVID-19 patients admitted to the Intensive Care Unit (ICU) had higher chances of developing arrhythmias during hospitalization than did patients admitted to a non-ICU ward [13]. The former more frequently presented a history of CAD, hypertension, heart failure, DM, chronic obstructive pulmonary disease (COPD), liver disease, and CKD and showed higher levels of BNP, high-sensitivity CRP, and D-dimer concentration on admission. After multivariable adjustment, the ICU status was independently associated with AF and non-sustained VT. Severe systemic illness, reflected by the ICU status, appeared to be a substrate for arrhythmogenesi
Denegri et al. also found AF on admission to have been more frequent in COVID-19 non-survivors than in survivors [16].
Wetterslev et al. discovered an incidence of arrhythmias equal to 37% among their critically ill COVID-19 patients, of which the most common was new-onset AF/AFl [29].
Bertini et al. also included critically ill COVID-19 patients in their study, dividing them into a subgroup aged under 74 and another one above this age [11]. They found electrocardiographic abnormalities on admission in 93% of the patients. However, what struck them the most was the high prevalence of AF/AFl, which was more frequent in patients over 74 years, as well as the high prevalence of ECG signs of acute right ventricular pressure overload (RVPO) such as an S1Q3T3 pattern or a right bundle branch block.
Cho et al. compared the incidence of different arrhythmias between COVID-19 patients with elevated troponin levels and patients with normal levels [14]. It was found that non-sustained VT was significantly more frequent in the former than in the latter and the same thing was seen in the incidence of new-onset AF. Moreover, all malignant ventricular arrhythmias, as well as complete heart blocks and sinus arrests, were developed only by patients with elevated troponin levels.
Si et al. found that 14.7% of their COVID-19 participants with a cTnI level measured on initial presentation were positive for cardiac injury and episodes of atrial/ventricular arrhythmias [27]. Of these patients, the peak cTnI level was significantly higher in non-survivors than in those discharged alive from the hospital.
Guan et al. conducted a retrospective study and discovered that 18.4% of 463 COVID-19 patients developed arrhythmias [18]. In 328 patients they analyzed the relationship between the serum levels of inflammatory cytokines and the various types of arrhythmias developed, and found a strong association between atrial arrhythmias and high levels of IL-6.
Bagnato et al. categorized their COVID-19 cohort into three groups: new-onset AF, other AF (paroxysmal, persistent, permanent, or long-standing permanent), and no AF [10]. They observed that IL-6, creatinine, and urea serum levels were significantly higher on admission in the new-onset AF group than the others. However, a history of CKD was also more frequent in the AF groups than in patients without AF. The question would be if these three serum values were increased in this new-onset AF group 1) as a result of a preexisting renal condition (as elevated IL-6 was shown to be associated with CKD), 2) due to acute inflammation or possible acute kidney injury caused by SARS-CoV-2, or 3) because of a mixture of these two situations.
Studies conducted on pediatric patients with COVID-19
Regan et al. investigated the electrocardiographic changes in a pediatric cohort with systemic inflammatory response syndrome (SIRS) induced by SARS-CoV-2 infection [25]. Of the 21% of children who developed arrhythmias during their illness, bradyarrhythmias were more frequent than tachyarrhythmias. A transient first-degree AV block was present in 25.4% of children, while a transient QTc prolongation of the >98th percentile for age was present in 22.2% of patients. These abnormal ECG findings reflect the toxic effects of systemic inflammation within the heart, especially on the cardiac conduction system.
Samuel et al. excluded pediatric patients with a history of known arrhythmias, inherited arrhythmia syndromes, or hemodynamically significant congenital heart diseases from their study and found an incidence rate of 17% for significant arrhythmias (mainly non-sustained monomorphic VT) [26]. This is a surprisingly elevated proportion considering that the authors chose a COVID-19 cohort with structurally normal hearts.
Studies about absolute and relative bradycardia in COVID-19
Kumar et al. investigated the incidence of bradycardia in a COVID-19 cohort and found that 24.9% presented a heart rate (HR) of <60 beats/min (defined as absolute bradycardia) and 13% had a HR of <50 beats/min (defined as profound bradycardia) [21]. Even though some patients had received medications such as remdesivir, dexamethasone, or tocilizumab and developed bradycardia during hospitalization, there was still a proportion of patients with bradycardic events who had not been treated with these drugs. The lack of obvious causes of bradycardia suggests that SARS-CoV-2 might exert a direct toxicity on the cardiac conduction system.
Ikeuchi et al. studied the incidence of relative bradycardia in mildly to moderately ill COVID-19 patients [19]. After measuring the body temperature and the pulse rate on admission, the median values were 37.2 °C and 84 beats/min, respectively. During hospitalization, 13% of the patients had criteria compatible with relative bradycardia: high fever (>38.9 °C) with a pulse rate <120 beats/min.
Oliva et al. found that relative bradycardia, defined in this study as a HR <90 bpm with a body temperature of at least 38.3 °C, was present in 41.6% of the patients with COVID-19 on hospital admission [22]. Median age in subjects with relative bradycardia was higher than in febrile patients without it and median body temperature was also more elevated in the former.
Wakabayashi et al. investigated the utility of relative bradycardia on initial presentation in diagnosing COVID-19 pneumonia from a cohort with new-onset infectious pneumonia [28]. They suggested that relative bradycardia might be a finding in patients with infectious pneumonia and a high inflammatory state regardless of the incriminating pathogen, as they found higher levels of CRP and ferritin in patients with pneumonia and relative bradycardia than in those without relative bradycardia (p<0.001 for both).
Discussion
In these included studies, the arrhythmias most frequently found during COVID-19 were atrial arrhythmias such as AF and AFl, followed by premature ventricular contractions. Sinus tachycardia is also a common finding in COVID-19, as it represents a normal response to infections. A particular clinical finding was relative bradycardia, usually reported by Japanese studies.
A couple of studies found an association between comorbidities and the development of arrhythmias during SARS-CoV-2 infection [9,10,12,13,15]. Some authors investigated the relationship between heart rhythm disturbances and cardiac injury/dysfunction as reflected by an increase in troponin/NT-pro-BNP or BNP serum levels [9,10,13,14,20,23,26,27]. Other studies proved a correlation between inflammation in COVID-19 and cardiac arrhythmias [13,18,20,23,25,28]. Some authors found ECG abnormalities in patients with laboratory signs of disruption of the coagulation cascade [11,13]. Other studies showed that hypoxia in COVID-19 might induce atrial arrhythmias [20,23]. A direct toxicity of SARS-CoV-2 on the heart was reflected by studies reporting bradycardia [19,21].
Therefore, the pathophysiology of cardiac arrhythmias in COVID-19 (see Figure 2) is complex, and it seems that this complication could be caused by a primary event or could by the decompensation of preexisting diseases, cardiovascular or not. Among the primary events that could generate arrhythmias in COVID-19, we found myocardial injury, ventricular dysfunction, use of drugs with arrhythmic side effects, water-electrolyte imbalances, and pain/anxiety. Myocardial injury could be 1) due to SARS-CoV-2’s ability to directly invade heart cells and to insult them or, 2) secondary to a COVID-19 complication such as hypoxia, SIRS, sepsis, pulmonary embolism, or disseminated intravascular coagulation (DIC) [1,31,32,33].
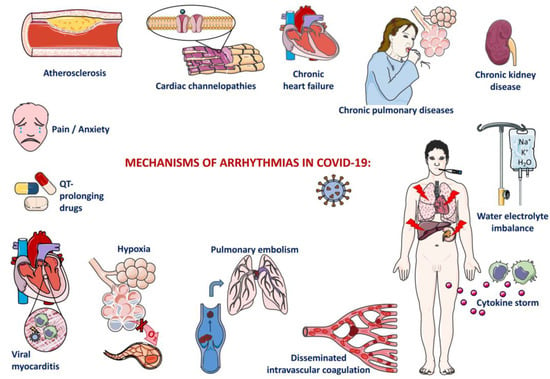
Figure 2.
Mechanisms of cardiac arrhythmia.
Although in the majority of cases, SARS-CoV-2 infection is localized at the level of the upper respiratory tract, when the virus reaches the lung parenchyma, it generates an interstitial pneumonia; this is usually mild/moderate but could eventually be complicated with acute respiratory distress syndrome and, therefore, respiratory failure with consequent hypoxia [34]. This generates hypoxemia, which further activates peripheral chemoreceptors from carotid and aortic bodies, stimulating the sympathetic nervous system with cardiac effects such as sinus tachycardia. Nevertheless, hypoxia was also found to be associated with AF, AFl, and atrial tachycardia in 2 of our analyzed studies, [20,23]. probably through cardiac ischemia.
Myocarditis in COVID-19 can be associated with sinus tachycardia as a result of the fever that accompanies it or, on the contrary, with sinus bradycardia as a consequence of sinus node dysfunction [31,36]. Another cause of bradycardia in myocarditis could be an AV block due to AV nodes’ involvement in the inflammatory process [37]. Atrial fibrillation and atrial flutter are also frequently encountered in myocarditis but could also be precipitated by the cardiac fibrosis that follows the episode of myocarditis, as well as other reentry arrhythmias [38].
Myocardial ischemia results in the cardiomyocyte’s switch to anaerobic metabolism with intracellular acidosis; intracellular calcium overload could be a source of delayed afterdepolarizations and hence a source of premature contractions or even ventricular tachycardia. In addition, intracellular acidosis can alter gap junctions, diminishing the electrical coupling between cardiomyocytes [38]. If untreated, myocardial ischemia can progress to myocardial infarction and subsequent fibrotic scars that act as ideal sites for reentry arrhythmias such as AF, AFl, or VT [31].
The increased expression of ACE2 by the endothelium could lead to its own injury during COVID-19, as SARS-CoV-2 could penetrate it and thus disrupt the coagulation cascade, initiating a DIC [1]. Endothelial damage could precipitate a deep-vein thrombosis with consequent pulmonary embolism, or it could generate in situpulmonary arterial thrombosis [1]. Patients admitted to the ICU have a higher chance of developing venous thrombosis as a consequence of being bedridden and/or elderly (it was observed that advanced age is a risk factor for severe forms of COVID-19) [39]. Pulmonary embolism leads to an increase in the pulmonary artery pressure and thus produces a ventilation–perfusion mismatch, causing hypoxia and sinus tachycardia. Pulmonary hypertension increases the right ventricle afterload with right ventricular dilation and secondary tricuspid regurgitation, causing a rise in the right atrial pressure that could possibly lead to atrial tachyarrhythmias. The increased right ventricular parietal stress generates ventricular ischemia (a source of VT), which could be aggravated by an eventual right coronary artery compression as an effect of the right ventricle’s dilation. Another effect of the right ventricular dilation could be a reduced left ventricular filling due to interventricular interdependence with consequent reduced cardiac output and, therefore, arterial hypotension. The latter induces sinus tachycardia but could also contribute to myocardial ischemia and thus cause ventricular arrhythmias [40].
Another mechanism that could lead to arrhythmias in COVID-19 is represented by an abnormal immune response (SIRS) characterized by a cytokine storm: a rise in IL-1, IL-6, and tumoral necrosis factor alfa (TNF-a) plasmatic levels [1,31]. This cytokine storm could directly injure the sinoatrial node, causing sinus bradycardia, or could lead to sinus tachycardia as a result of their pyretic effects [36,37]. Additionally, in the context of SIRS, the sympathetic nervous system could be excessively activated, causing the destabilization of a vulnerable atherosclerotic plaque with consecutive acute coronary syndrome and myocardial ischemia (and hence a source of arrhythmias); [31]. and it could prolong the QT interval with risk of ventricular arrhythmias, including torsade de pointes [31,32]. Furthermore, the cytokine storm possesses direct cardiotoxicity that could manifest as AF or as myocarditis [1,31]. The acute inflammation during SARS-CoV-2 infection might be exacerbated by preexisting inflammatory conditions such as obesity or metabolic syndrome, as already observed by Coromilas et al. [15].
Drugs such as hydroxychloroquine, chloroquine, and azithromycin used to be considered a cause of ventricular arrhythmias like torsade de pointes, VF, or even sudden cardiac death at the beginning of the coronavirus pandemic [31,33,36]. Nowadays, the World Health Organization (WHO) no longer recommends their use in COVID-19 treatment and mentions their QT-prolonging effects [31].
Inherited arrhythmia syndromes could also be a cause of heart rhythm disturbances in SARS-CoV-2 infection [33]. Long QT syndrome is associated with a high risk of malignant arrhythmias [33]. This could be triggered by the administration of QT-prolonging drugs in COVID-19 patients with this genetic condition. Another syndrome associated with VF and sudden cardiac death is Brugada syndrome, characterized by a mutation in genes encoding sodium channels in cardiomyocytes. As it is already known that fever represents a trigger for arrhythmias in this condition, it is mandatory to treat patients with Brugada syndrome and concomitant SARS-CoV-2 infection with antipyretics, preferably acetaminophen [33].
Water-electrolyte imbalances could also disturb the heart rhythm. As the gastrointestinal tract represents a possible target of SARS-CoV-2 infection due to ACE2’s localization at this level, COVID-19 can manifest with diarrhea, consecutive hypokalemia, and the elongation of the QT interval, causing premature ventricular contractions or even torsade de pointes and VF [33]. Gastrointestinal manifestations could also generate dehydration and, thus, could lead to sinus tachycardia.
Conclusions
Knowledge of the arrhythmogenic effects of SARS-CoV-2 infection can ensure careful follow-up and prompt intervention to decrease the mortality risk of hospitalized patients.
The direct cytotoxicity of SARS-CoV-2 on cardiomyocytes causes myocarditis, and a sustained inflammatory response predisposes patients to heart rhythm disorders. Patients with severe systemic illness who require hospitalization must be monitored for hemodynamic status, hydroelectrolytic imbalances, adverse drug reactions (QTc interval prolongation), inflammatory status, and comorbidities.
Additional studies are needed to clarify the pathophysiological mechanisms and the role of associated risk factors in patients with SARS-CoV-2 infection to ensure effective prevention and prompt therapeutic intervention for a favorable evolution of these patients.
Author Contributions
MAJ contributed to research conceptualization, design, literature review, and writing. EB and LMS contributed to the review and revision of the manuscript. DAI contributed to research conceptualization, design, and approval of the final manuscript. All authors made equal contributions. All authors have read and agreed to the final version of the manuscript.
Funding
None to declare.
Data Availability Statement
The data supporting the findings of this study are available within the article.
Conflicts of interest
All authors—none to declare.
References
- Magadum, A.; Kishore, R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020, 9, 2508. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; et al. COVID-19 and cardiovascular disease. Circulation. 2020, 141, 1648–1655. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Thum, T. SARS-CoV-2 receptor ACE2 expression in the human heart: Cause of a post-pandemic wave of heart failure? Eur Heart J. 2020, 41, 1807–1809. [Google Scholar] [CrossRef]
- Bojkova, D.; Wagner, J.U.G.; Shumliakivska, M.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.P.; Wan, E.Y.; Waase, M.P.; et al. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City Cohort. J Cardiovasc Electrophysiol. 2020, 31, 3086–3096. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, G.; Imbalzano, E.; Aragona, C.O.; et al. New-onset atrial fibrillation and early mortality rate in COVID-19 patients: Association with IL-6 serum levels and respiratory distress. Medicina (Kaunas). 2022, 58, 530. [Google Scholar] [CrossRef]
- Bertini, M.; Ferrari, R.; Guardigli, G.; et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: An insight into the mechanisms of cardiac involvement. Europace. 2020, 22, 1848–1854. [Google Scholar] [CrossRef]
- Bhatia, K.S.; Sritharan, H.P.; Chia, J.; et al. Cardiac complications in patients hospitalised with COVID-19 in Australia. Heart Lung Circ. 2021, 30, 1834–1840. [Google Scholar] [CrossRef]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Cho, J.H.; Namazi, A.; Shelton, R.; et al. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLoS ONE. 2020, 15, e0244533. [Google Scholar] [CrossRef] [PubMed]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; et al. Worldwide Survey of COVID-19-associated arrhythmias. Circ Arrhythm Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef] [PubMed]
- Denegri, A.; Pezzuto, G.; D’Arienzo, M.; et al. Clinical and electrocardiographic characteristics at admission of COVID-19/SARS-CoV2 pneumonia infection. Intern Emerg Med. 2021, 16, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- García-Granja, P.E.; Veras, C.; Aparisi, Á.; et al. Atrial fibrillation in patients with SARS-CoV-2 infection. Med Clin (Barc). 2021, 157, 58–63. [Google Scholar] [CrossRef]
- Guan, H.; Liu, J.; Ding, J.; et al. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Incidences and implications. J Electrocardiol. 2021, 65, 96–101. [Google Scholar] [CrossRef]
- Ikeuchi, K.; Saito, M.; Yamamoto, S.; Nagai, H.; Adachi, E. Relative bradycardia in patients with mild-to-moderate coronavirus disease, Japan. Emerg Infect Dis. 2020, 26, 2504–2506. [Google Scholar] [CrossRef]
- Jehangir, Q.; Lee, Y.; Latack, K.; et al. Incidence, mortality, and imaging outcomes of atrial arrhythmias in COVID-19. Am J Cardiol. 2022, 173, 64–72. [Google Scholar] [CrossRef]
- Kumar, S.; Arcuri, C.; Chaudhuri, S.; et al. A novel study on SARS-COV-2 virus associated bradycardia as a predictor of mortality-retrospective multicenter analysis. Clin Cardiol. 2021, 44, 857–862. [Google Scholar] [CrossRef]
- Oliva, A.; Franchi, C.; Gatto, M.C.; Galardo, G.; Pugliese, F.; Mastroianni, C. Prevalence and clinical significance of relative bradycardia at hospital admission in patients with coronavirus disease 2019 (COVID-19). Clin Microbiol Infect. 2021, 27, 1185–1187. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020, 31, 3077–3085. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Elias, P.; Jain, S.S.; et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc. 2021, 10, e018476. [Google Scholar] [CrossRef] [PubMed]
- Regan, W.; O’Byrne, L.; Stewart, K.; et al. Electrocardiographic changes in children with multisystem inflammation associated with COVID-19: Associated with coronavirus disease 2019. J Pediatr. 2021, 234, 27–32.e2. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Friedman, R.A.; Sharma, C.; et al. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR-positive SARS-CoV-2 infection, including drug-induced changes in the corrected QT interval. Heart Rhythm. 2020, 17, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Si, D.; Du, B.; Ni, L.; et al. Death, discharge and arrhythmias among patients with COVID-19 and cardiac injury. CMAJ. 2020, 192, E791–E798. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Iwata, H. The clinical utility of relative bradycardia for identifying cases of coronavirus disease 2019 pneumonia: A retrospective pneumonia cohort study. Intern Med. 2023, 62, 1931–1938. [Google Scholar] [CrossRef]
- Wetterslev, M.; Jacobsen, P.K.; Hassager, C.; et al. Cardiac arrhythmias in critically ill patients with coronavirus disease 2019: A retrospective population-based cohort study. Acta Anaesthesiol Scand. 2021, 65, 770–777. [Google Scholar] [CrossRef]
- Zakynthinos, G.E.; Tsolaki, V.; Karavidas, N.; et al. Secondary bacterial infections are a leading factor triggering New Onset Atrial Fibrillation in intubated ICU Covid-19 ARDS patients. J Infect Public Health. 2022, 15, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Duckheim, M.; Schreieck, J. COVID-19 and cardiac arrhythmias. Hamostaseologie. 2021, 41, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Malaty, M.; Kayes, T.; Amarasekera, A.T.; Kodsi, M.; MacIntyre, C.R.; Tan, T.C. Incidence and treatment of arrhythmias secondary to coronavirus infection in humans: A systematic review. Eur J Clin Invest. 2021, 51, e13428. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.I.; Postema, P.G.; Arbelo, E.; et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020, 17, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, evaluation, and treatment of Coronavirus (COVID-19). 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Patel, N.H.; Rutland, J.; Tecson, K.M. Arrhythmias and intraventricular conduction disturbances in patients hospitalized with coronavirus disease 2019. Am J Cardiol. 2022, 162, 111–115. [Google Scholar] [CrossRef]
- Manolis, A.S.; Manolis, A.A.; Manolis, T.A.; Apostolopoulos, E.J.; Papatheou, D.; Melita, H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020, 30, 451–460. [Google Scholar] [CrossRef]
- Kasper, D.L.; Fauci, A.S.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. Harrison’s principles of internal medicine. 19th Edition. McGraw-Hill Education; 2015.
- Dherange, P.; Lang, J.; Qian, P.; et al. Arrhythmias and COVID-19: A review. JACC Clin Electrophysiol. 2020, 6, 1193–1204. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open. 2021, 11, e044640. [Google Scholar] [CrossRef] [PubMed]
- Bryce, Y.C.; Perez-Johnston, R.; Bryce, E.B.; Homayoon, B.; Santos-Martin, E.G. Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension: A pictorial essay for the interventional radiologist. Insights Imaging. 2019, 10, 18. [Google Scholar] [CrossRef]
© GERMS 2024.