Coronin-1A Serves as a Serum Biomarker for Supportive Diagnosis of Mycobacterium tuberculosis Infection
Abstract
Introduction
Methods
Retrieval of proteomics data
Bioinformatics analysis
Criteria for selection of candidate biomarkers
Recruitment of subjects and ethics
Collection of serum samples
Enzyme-linked immunosorbent assay (ELISA)
Statistical analysis
Results
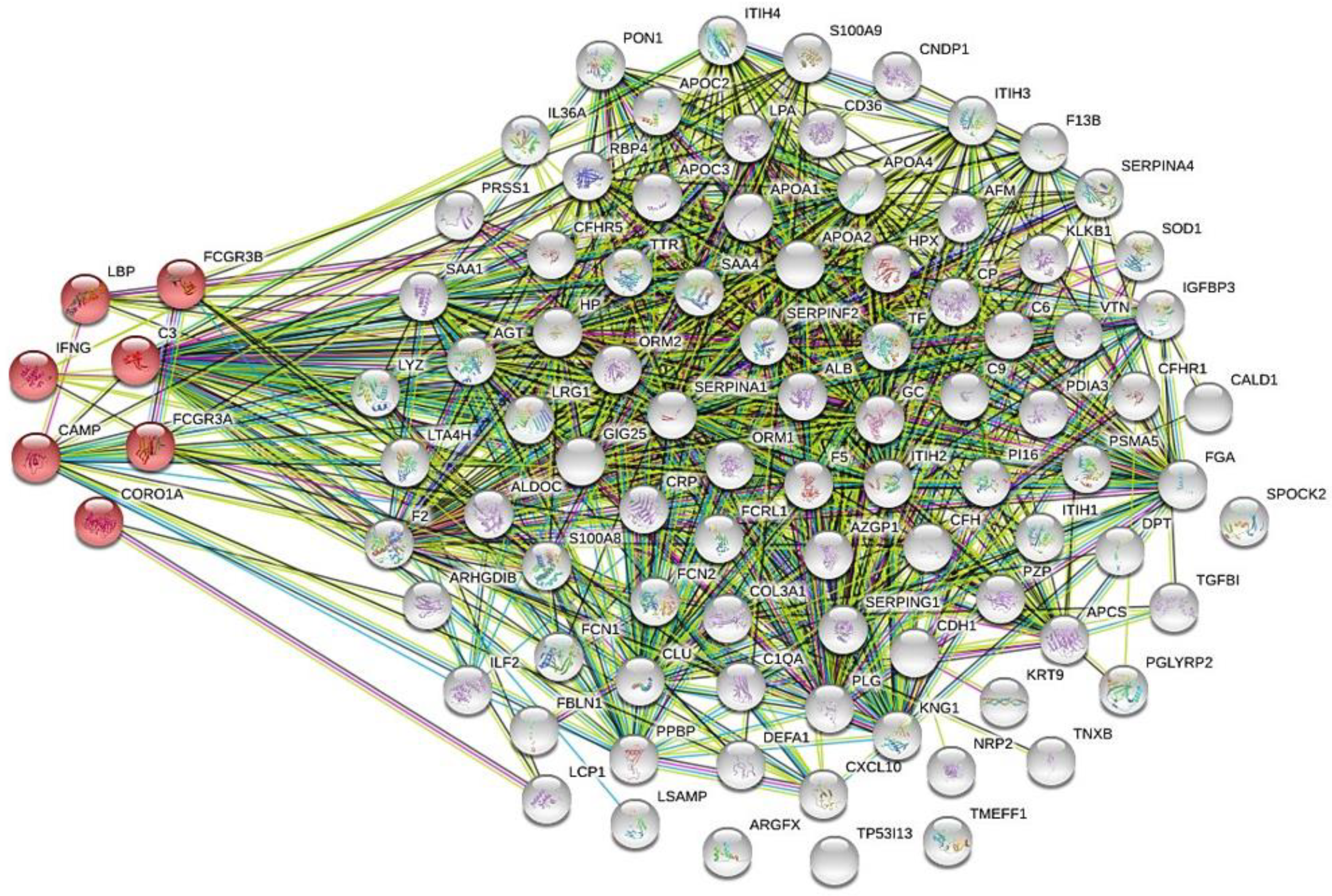
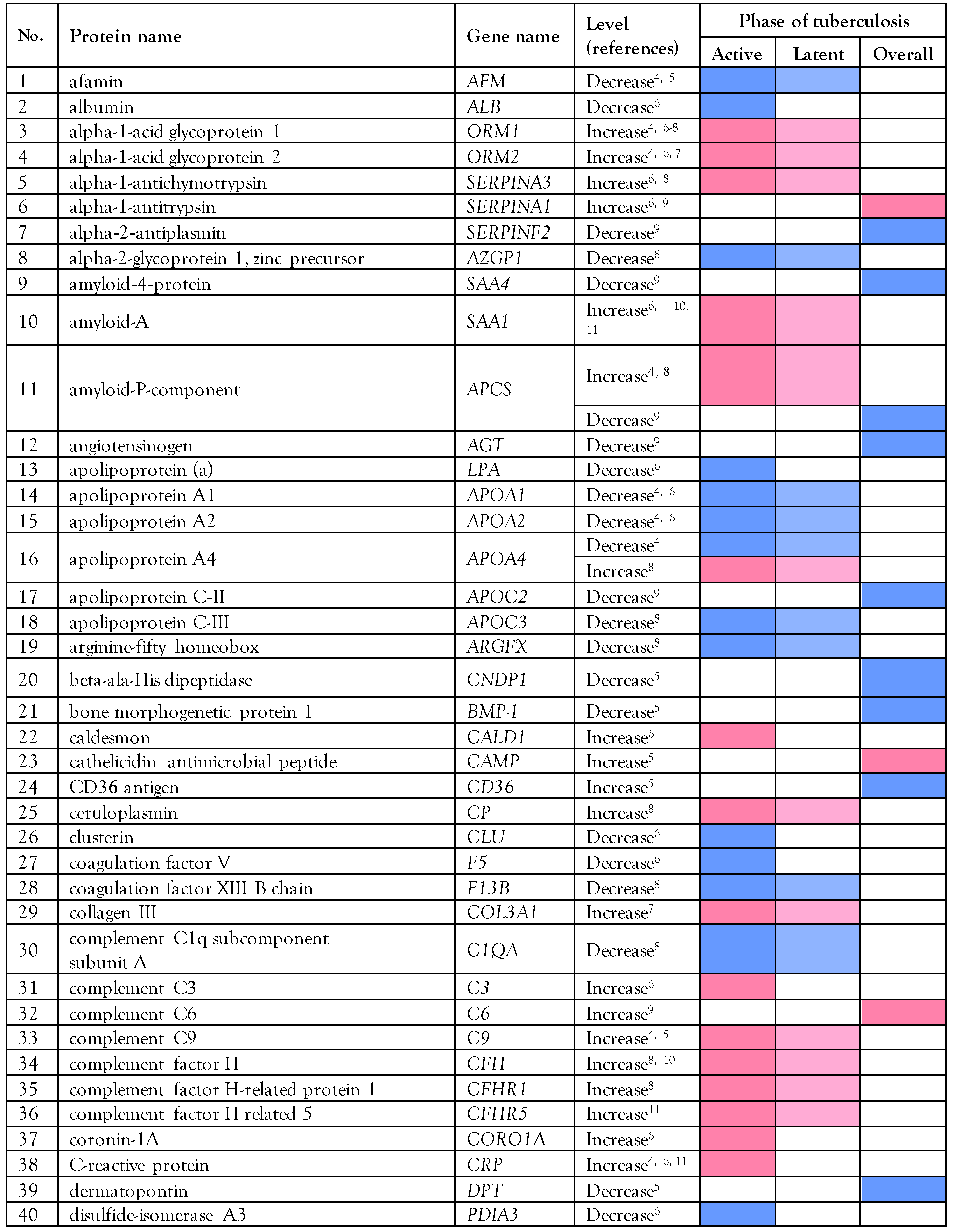
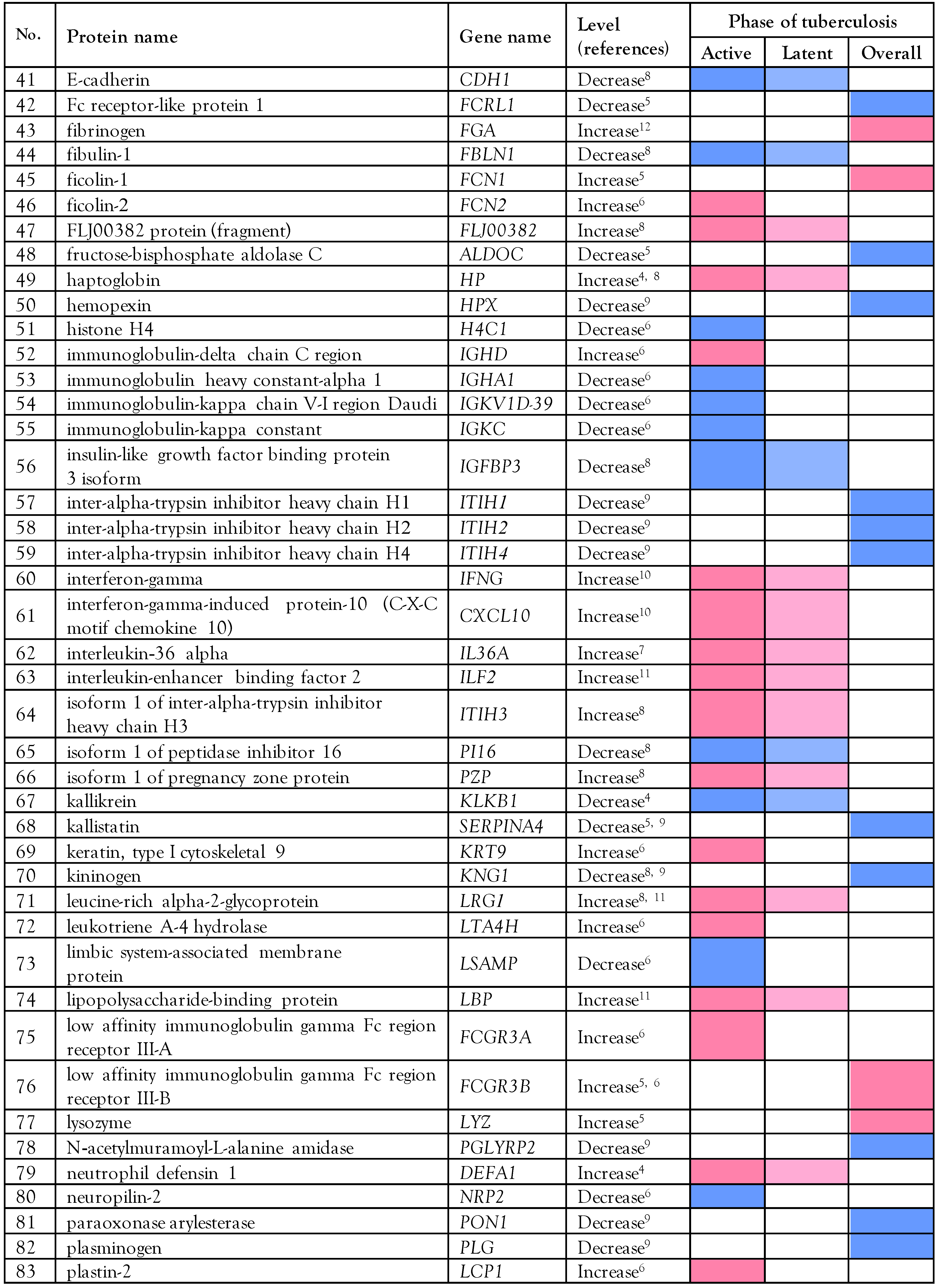
Proteomics data in tuberculosis patients
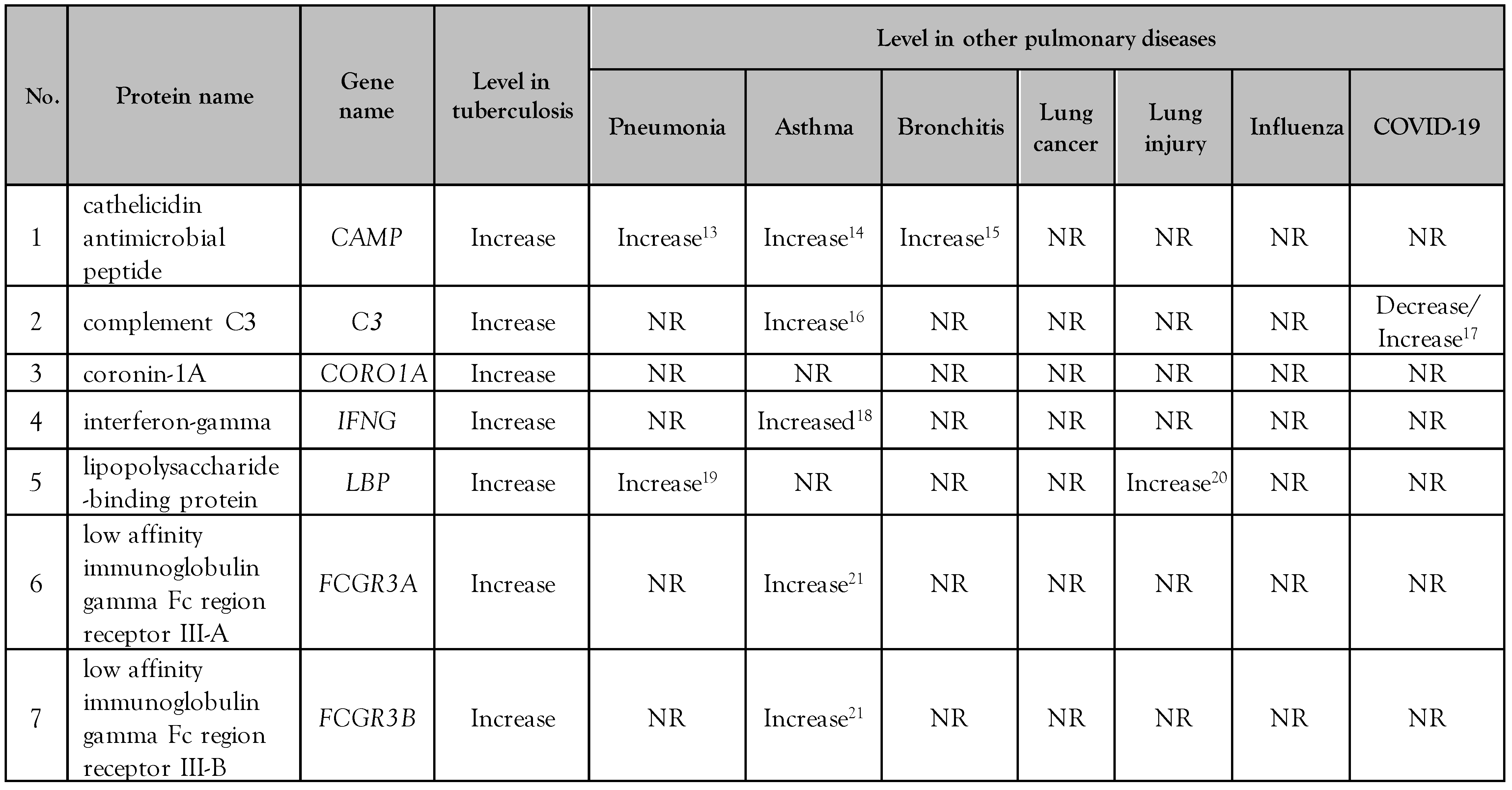
Biological analysis of the altered serum/plasma proteins
Selection of serum/plasma proteins as candidate biomarkers
Demographic data of the patients
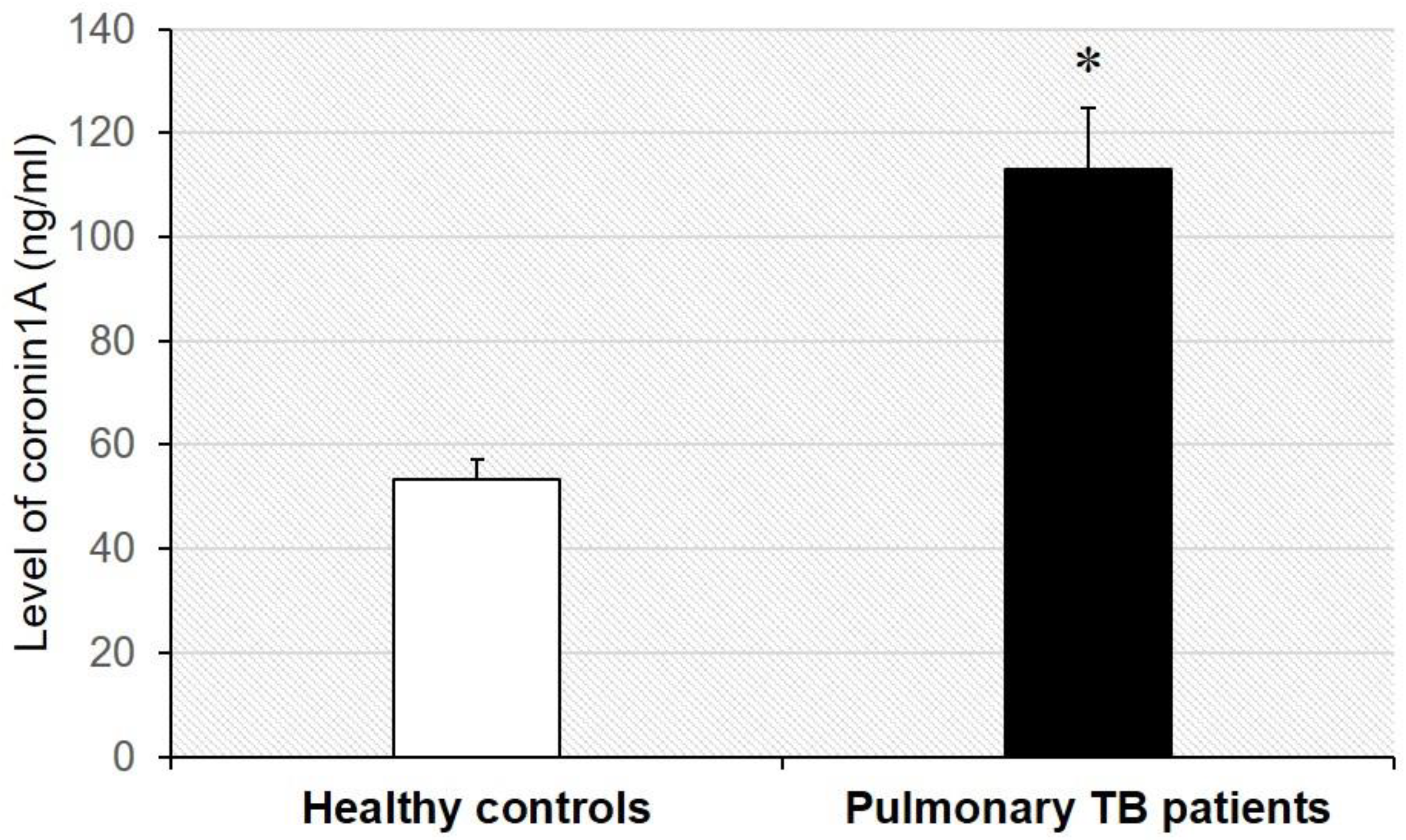
Validation of serum coronin-1A level
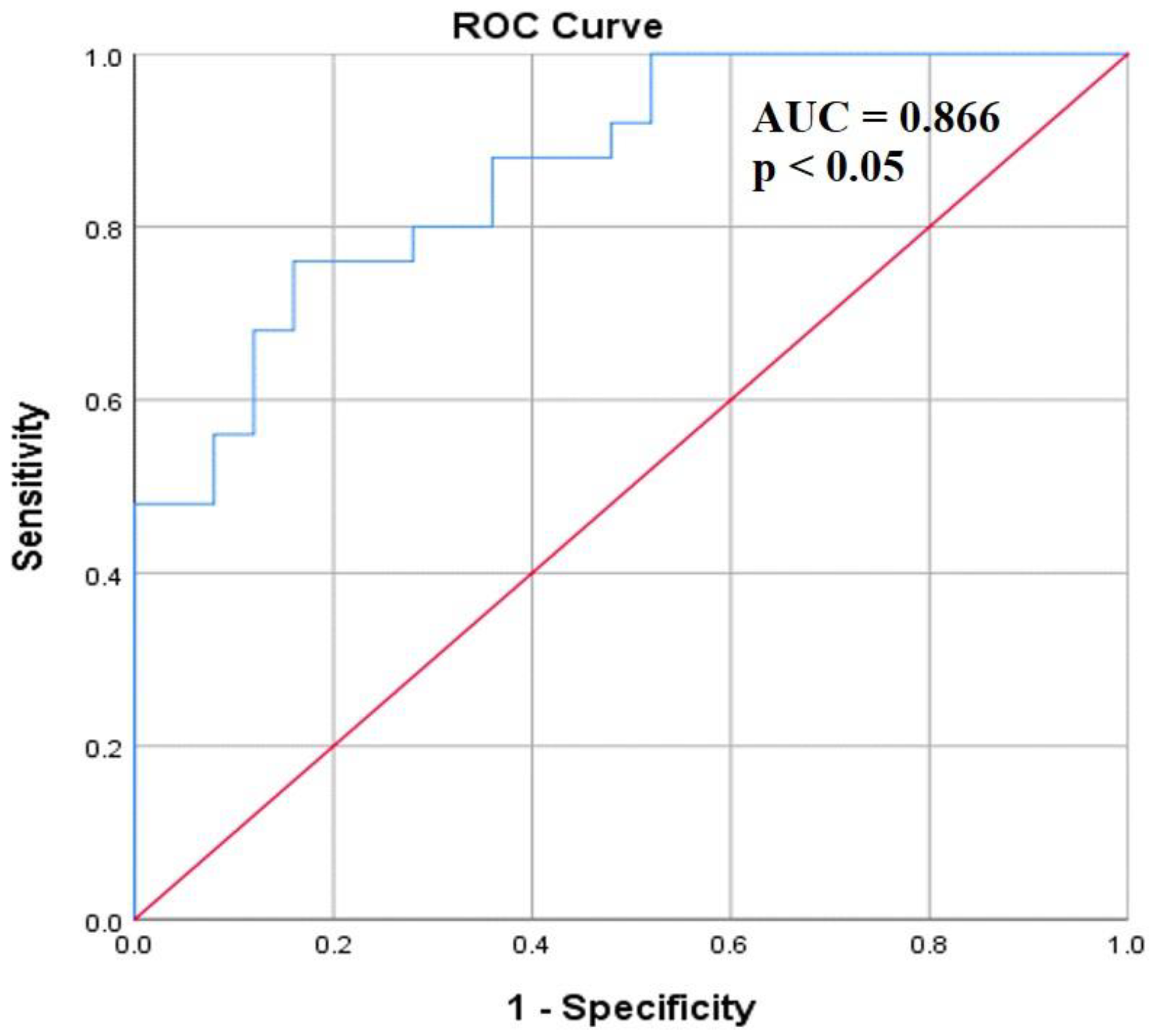
Diagnostic ability of serum coronin-1A level
Discussion
Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukunaga, R.; Glaziou, P.; Harris, J.B.; Date, A.; Floyd, K.; Kasaeva, T. Epidemiology of tuberculosis and progress toward meeting global targets-worldwide, 2019. MMWR Morb Mortal Wkly Rep 2021, 70, 427–430. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu Rev Immunol 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Azadi, D.; Motallebirad, T.; Ghaffari, K.; Shojaei, H. Mycobacteriosis and tuberculosis: Laboratory diagnosis. Open Microbiol J 2018, 12, 41–58. [Google Scholar] [CrossRef]
- Mateos, J.; Estevez, O.; Gonzalez-Fernandez, A.; et al. Serum proteomics of active tuberculosis patients and contacts reveals unique processes activated during Mycobacterium tuberculosis infection. Sci Rep 2020, 10, 3844. [Google Scholar] [CrossRef]
- De Groote, M.A.; Sterling, D.G.; Hraha, T.; et al. Discovery and validation of a six-marker serum protein signature for the diagnosis of active pulmonary tuberculosis. J Clin Microbiol 2017, 55, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, L.L.; Shi, L.Y.; et al. Screening and identification of five serum proteins as novel potential biomarkers for cured pulmonary tuberculosis. Sci Rep 2015, 5, 15615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, L.; Han, F.; et al. Proteomic profiling for plasma biomarkers of tuberculosis progression. Mol Med Rep 2018, 18, 1551–1559. [Google Scholar] [CrossRef]
- Sun, H.; Pan, L.; Jia, H.; et al. Label-free quantitative proteomics identifies novel plasma biomarkers for distinguishing pulmonary tuberculosis and latent infection. Front Microbiol 2018, 9, 1267. [Google Scholar] [CrossRef]
- Song, S.H.; Han, M.; Choi, Y.S.; et al. Proteomic profiling of serum from patients with tuberculosis. Ann Lab Med 2014, 34, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.C.; Hoggart, C.J.; Chegou, N.N.; et al. Evaluation of host serum protein biomarkers of tuberculosis in sub-saharan Africa. Front Immunol 2021, 12, 639174. [Google Scholar] [CrossRef]
- Garay-Baquero, D.J.; White, C.H.; Walker, N.F.; et al. Comprehensive plasma proteomic profiling reveals biomarkers for active tuberculosis. JCI Insight 2020, 5, e137427. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, T.; Wei, L.; et al. The discovery and identification of a candidate proteomic biomarker of active tuberculosis. BMC Infect Dis 2013, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Majewski, K.; Kozłowska, E.; Żelechowska, P.; Brzezińska-Błaszczyk, E. Serum concentrations of antimicrobial peptide cathelicidin LL-37 in patients with bacterial lung infections. Cent Eur J Immunol 2018, 43, 453–457. [Google Scholar] [CrossRef]
- Liu, M.C.; Xiao, H.Q.; Brown, A.J.; Ritter, C.S.; Schroeder, J. Association of vitamin D and antimicrobial peptide production during late-phase allergic responses in the lung. Clin Exp Allergy 2012, 42, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Gedik, A.H.; Cakir, E.; Gokdemir, Y.; et al. Cathelicidin (LL-37) and human beta2-defensin levels of children with post-infectious bronchiolitis obliterans. Clin Respir J 2017, 11, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, M.; El Baz, M.; Sherif, A.; Adel, A. Complement components (C3, C4) as inflammatory markers in asthma. Indian J Pediatr 2010, 77, 771–773. [Google Scholar] [CrossRef]
- Bagherimoghaddam, A.; Rafatpanah, H.; Mansouritorghabeh, H. Elevated levels of C3, C4, and CH50 of the complement system in ICU and non-ICU patients with COVID-19. Health Sci Rep 2022, 5, e519. [Google Scholar] [CrossRef]
- Davoodi, P.; Mahesh, P.A.; Holla, A.D.; et al. Serum levels of interleukin-13 and interferon-gamma from adult patients with asthma in Mysore. Cytokine 2012, 60, 431–437. [Google Scholar] [CrossRef]
- Hopstaken, R.M.; Cals, J.W.; Dinant, G.J. Accuracy of lipopolysaccharide-binding protein (LBP) and fibrinogen compared to C-reactive protein (CRP) in differentiating pneumonia from acute bronchitis in primary care. Prim Care Respir J 2009, 18, 227–230. [Google Scholar] [CrossRef]
- Villar, J.; Pérez-Méndez, L.; Espinosa, E.; et al. Serum lipopolysaccharide binding protein levels predict severity of lung injury and mortality in patients with severe sepsis. PLoS ONE 2009, 4, e6818. [Google Scholar] [CrossRef]
- Wu, J.; Lin, R.; Huang, J.; et al. Functional Fcgamma receptor polymorphisms are associated with human allergy. PLoS ONE 2014, 9, e89196. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Di Ciano-Oliveira, C.; Grinstein, S.; Trimble, W.S. Coronin function is required for chemotaxis and phagocytosis in human neutrophils. J Immunol 2007, 178, 5769–5778. [Google Scholar] [CrossRef]
- Seto, S.; Tsujimura, K.; Koide, Y. Coronin-1a inhibits autophagosome formation around Mycobacterium tuberculosis-containing phagosomes and assists mycobacterial survival in macrophages. Cell Microbiol 2012, 14, 710–727. [Google Scholar] [CrossRef]
- Chong, W.; Kaiqi, H.; Weiqiang, R.; Junge, Z.; Maybank, S. Large-scale weakly supervised object localization via latent category learning. IEEE Trans Image Process 2015, 24, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Gupta, G.; Biswas, S.; et al. Coronin-1 levels in patients with tuberculosis. Indian J Med Res 2021, 154, 866–870. [Google Scholar] [CrossRef] [PubMed]


© GERMS 2023.
Share and Cite
Khamchun, S.; Pongtussanahem, O. Coronin-1A Serves as a Serum Biomarker for Supportive Diagnosis of Mycobacterium tuberculosis Infection. GERMS 2023, 13, 20-31. https://doi.org/10.18683/germs.2023.1363
Khamchun S, Pongtussanahem O. Coronin-1A Serves as a Serum Biomarker for Supportive Diagnosis of Mycobacterium tuberculosis Infection. GERMS. 2023; 13(1):20-31. https://doi.org/10.18683/germs.2023.1363
Chicago/Turabian StyleKhamchun, Supaporn, and Orathai Pongtussanahem. 2023. "Coronin-1A Serves as a Serum Biomarker for Supportive Diagnosis of Mycobacterium tuberculosis Infection" GERMS 13, no. 1: 20-31. https://doi.org/10.18683/germs.2023.1363
APA StyleKhamchun, S., & Pongtussanahem, O. (2023). Coronin-1A Serves as a Serum Biomarker for Supportive Diagnosis of Mycobacterium tuberculosis Infection. GERMS, 13(1), 20-31. https://doi.org/10.18683/germs.2023.1363




