Introduction
The presence of antibodies-mediated humoral response to SARS-CoV-2 has been demonstrated in patients with COVID-19. Most antibodies against SARS-CoV-2 target the spike and nucleocapsid antigens of the virus. Although the first detected antibodies in COVID-19 infection are SARS-CoV-2 IgM class antibodies, it has been shown that the conversion from IgM to IgG is rapid during acute infection. The mean detection time of SARS-CoV-2 IgG is between 7 and 14 days after disease symptoms [
1,
2]. The presence of SARS-CoV-2 specific antibodies indicates exposure to the agent or post-vaccination immunization.
Different vaccines based on inactivated whole virion, mRNA and vectors have been developed for SARS-CoV-2 [
3]. It has been determined that the COVID-19 vaccines that have been put into use associate significantly lower risk of severe and critical disease and a varying degree of protection against infection. Therefore, widespread community vaccination is very important in breaking the transmission chain of SARS-CoV-2 infections and ending the pandemic.
CoronaVac
® (Sinovac, China) is an inactivated whole virus vaccine. It is administered in two doses 14 or 28 days apart. Phase 3 studies of CoronaVac
® (Sinovac, China), an inactivated whole virion SARS-CoV-2 vaccine, were conducted in four countries, including Turkey. In Chile phase 3 study results of CoronaVac
®, the efficacy in preventing SARS-CoV-2 infection among fully vaccinated people was 65.9%, the efficacy in preventing hospitalization was reported as 87.5%, and in Turkey phase 3 results, the efficacy of the vaccine was reported as 83.5% after the second dose [
4,
5]. The inactivated CoronaVac
® vaccine was approved for emergency use on January 13, 2021 by the Turkish Ministry of Health. After the CoronaVac
® vaccine was given emergency use permission in Turkey, SARS-CoV-2 vaccination was started primarily in healthcare workers (HCWs) and in high-risk groups on January 14, 2021. Emergency use of the CoronaVac
® vaccine has also been approved by the World Health Organization (WHO) [
6].
Individual factors and vaccine-related factors such as vaccine type, presence of adjuvant and dose range are important in the immune response to the vaccine. Antibodies positivity does not always imply full protection against a disease, nor does the absence of demonstrated antibodies positivity necessarily imply disease susceptibility. However, the presence of post-vaccine antibodies positivity is used as a parameter in the evaluation of the vaccine response, as it is one of the indicators of immune response [
7].
In this study, we aimed to evaluate post-vaccine serum antibody levels against SARS-CoV-2 in HCWs vaccinated with two doses of inactivated CoronaVac® vaccine (vaccinated without having had the disease and vaccinated after having had COVID-19). At the start of this study, only the CoronaVac® vaccine had been given an emergency use permit in our country.
Methods
This prospective cohort study was conducted between February 2021 and July 2021.
This study was carried out with the approval of the Ministry of Health of the Republic of Turkey, General Directorate of Health Services (Date and No: 30.01.2021/2021-01-26T14_25_49) and Pamukkale University Faculty of Medicine, Non-Interventional Clinical Research Ethics Committee (Date and No: 02.02.2021/03). Written informed consent was obtained from all participants.
Working groups
Healthcare workers of Pamukkale University Hospitals, Turkey were included in the study. The study was planned to be carried out in two group of individuals, without history of prior documented COVID-19 (Group 1) and with history of prior documented COVID 19 before vaccination (Group 2). The study was explained by visiting each clinical unit and a participation form was prepared for individuals who volunteered to participate in the study. Demographic information was collected face-to-face through a questionnaire, and informed consent was obtained from the participants.
A questionnaire was filled out for each participant (whether they have been diagnosed with COVID-19 with SARS-CoV-2 RT-PCR, whether they work actively in areas with a medium/high risk of COVID-19, whether they provide COVID-19 positive patient care, whether they are constantly taking medication etc.) The severity of the disease in healthcare workers who were diagnosed with COVID-19 before vaccination was reported as asymptomatic, mild illness, or requiring hospitalization. Asymptomatic disease was defined as not having any symptoms with a positive PCR test after a history of contact with an individual with a diagnosis of COVID-19 or during periodic screening. Mild disease criteria were defined as symptoms such as fever/cough/muscle joint pain/weakness without pneumonia findings or mild pneumonia that did not require hospitalization.
Services provided to patients with COVID-19 (such as anesthesia, intensive care unit, infectious diseases, lung diseases), COVID-19 outpatient clinic, COVID-19 sampling area, emergency room and COVID-19 PCR laboratory were evaluated as medium/high risk areas.
Serum samples were collected at four blood sampling time points: immediately before the second vaccine dose and at the 1
st and 3
rd month and 141-150 days after the second dose of vaccination. The working flow chart is shown in
Figure 1.
Investigation of serum antibody presence
At the blood sampling time points for SARS-CoV-2 antibodies positivity monitoring, 5-10 mL of venous blood samples were taken from the participants and the serum was separated by centrifugation. Serum samples were stored in the refrigerator at 2-8 °C under appropriate conditions until the time to be tested in the study respecting the kit content recommendations. Serum samples were tested for IgG antibodies to receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 with SARS-CoV-2 IgG II Quant Assay, on Architect analyzer (Abbott Diagnostics, Ireland). For anti-RBD IgG, results ≥50 AU/mL were considered positive according to the recommendation of the manufacturer. The analytical detection range of the test was 21 to 40 000 AU/mL. IgG class antibodies against SARS-CoV-2 nucleocapsid (NC) protein were investigated using qualitative chemiluminescent microparticle immunoassay (Architect, Abbott Diagnostics, Ireland) kit and anti-nucleocapsid IgG <1.40 S/C results were negative, ≥1.40 S/C results were considered positive.
Statistical analysis
Descriptive statistics were calculated for demographic data and given as numbers and percentages. Categorical variables were compared using the Chi-square test. Conformity of continuous variables to normal distribution was tested using the Kolmogorov-Smirnov test. Mann Whitney U test was used to compare quantitative antibody levels. Friedman test was used for repeated measurements to examine the change in antibody levels over time. The significance level was set at 0.05. A p value of less than 0.05 was considered statistically significant. SPSS 21.0 program (IBM Corp., USA) was used to evaluate the data.
Results
A total of 242 volunteer HCWs participated in the study; 193 of them were in Group 1 and 49 people were in Group 2. Of the participants, 162 (66.9%) were female and 80 (33.1%) were male. Of the participants in Group 2, 20.4% had been asymptomatic, 71.4% had had mild disease, and 8.2% had required hospitalization. Of all the participants, 116 (47.9%) were working in areas at medium/high risk for SARS-CoV-2, and 126 (52.1%) were working in areas with partially low risk of SARS-CoV-2. Between two months after the second dose and the last blood sampling time, a total of eight participants in Group 1 had PCR-confirmed COVID-19. These participants who had the disease after vaccination continued to be followed in the study in terms of SARS-CoV-2 antibodies positivity, but the antibody level results of these participants were excluded from the antibody levels analysis of 3rd and 4th blood sampling time points. In addition, three participants who gave serum samples after the third dose of the vaccine were excluded from the analysis of antibody level results at the 4th blood sampling time.
In terms of demographic characteristics of the participants, no statistically significant differences were found between Group 1 and Group 2 (
Table 1).
All participants were monitored for SARS-CoV-2 antibodies positivity and antibody levels at the four blood sampling time points after the first dose of vaccination. On the 28th day after the first dose vaccination, serum anti-RBD IgG antibodies were positive (>50 AU/mL) in 71% of the participants in Group 1 and 98% of the participants in Group 2. The mean anti-RBD IgG antibodies were 339.5 (median 99.0) AU/mL in Group 1, and 1813.9 (median 1116.3) AU/mL in Group 2. Anti-RBD IgG antibody levels in 57 people (23.6%), including one participant in Group 2 and 56 participants in Group 1, were found below 50 AU/mL 28 days after the first dose vaccination and these persons were negative for SARS CoV-2 antibodies positivity. In the first month follow-up after the second dose, serum anti-RBD IgG antibodies were found to be positive in all participants in both groups, except for one participant, and the SARS-CoV-2 antibodies positivity rate was found as 99.6% in all participants. The mean antibody levels in the second blood sampling time point were 1496.2 (median 1117.8) AU/mL in Group 1 and 2082.8 (median 1242.2) AU/mL in Group 2. In the third follow-up period, which was carried out in the third month after the second dose, 99.1% of all participants still had anti-RBD IgG antibody positivity, but a decrease in antibody levels was observed compared to the previous blood sampling time point mean 584.9 (median 336.8) AU/mL in Group 1, mean 1186.1 (median 655.0) AU/mL in Group 2). Anti-RBD IgG antibody positivity was detected in 95.6% of all participants in the fourth follow-up period and a decrease in antibody levels was observed in this period compared to the third blood sampling time point. In the fourth blood sampling time point, the mean anti-RBD IgG was 304.7 (median 183.0) AU/mL in Group 1 and 979.7 (median 476.9) in Group 2. Anti-RBD antibody levels of Group 2 were found to be significantly higher than those of Group 1 for all four blood sampling time points (
Table 2). Decrease in anti-RBD IgG antibody level with time was more evident in Group 1. Anti-RBD IgG antibody levels in the blood sampling time points are presented in
Figure 2 and
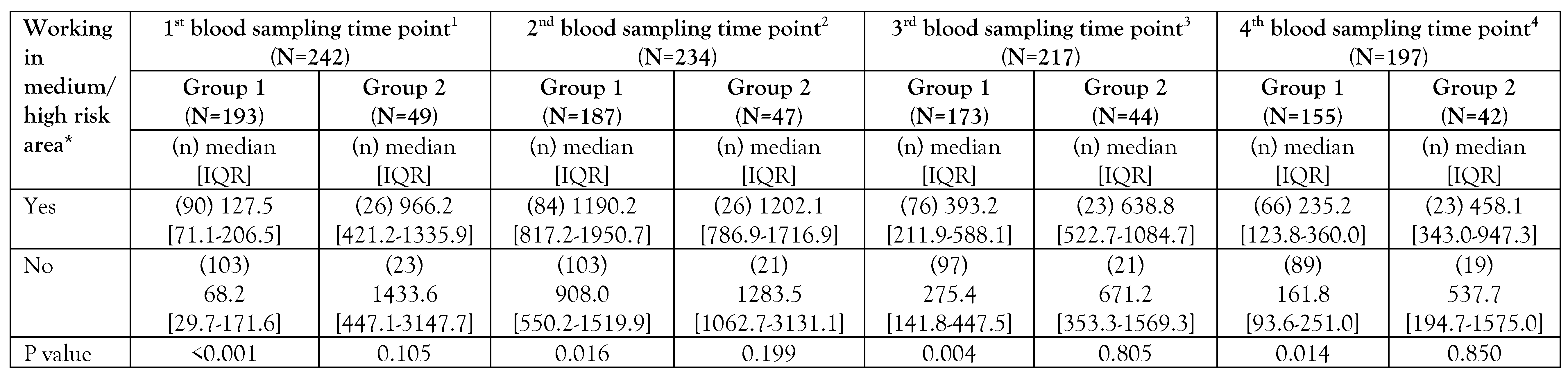
Table 2. In blood sampling time points, anti-RBD antibody levels were found to be higher in participants working in medium/high risk areas compared to participants working in areas with partially low risk in Group 1 (
Table 3).
Anti-nucleocapsid antibody was positive in 44 (18.2%) of all participants on the 28
th day after the first dose. The positivity was detected in 12 participants (6.2%) in Group 1 and 32 participants (65.3%) in Group 2. One month after the second dose, 90 (48.1%) participants in Group 1 and 36 (76.5%) participants in Group 2 were positive for anti-nucleocapsid antibodies. At the fourth blood sampling time points, anti-nucleocapsid antibody positivity was detected in a total of 20 (9.7%) subjects, including 10 (6.1%) participants in Group 1 and 10 (23.8%) participants in Group 2. In all blood sampling time points, anti-nucleocapsid positivity rate in Group 2 was found to be higher than in Group 1 (
Table 4). At the 1
st and 2
nd blood sampling time, the anti-nucleocapsid antibody positivity rate was found to be higher in participants working in medium/high risk areas compared to participants working in areas with partially low risk in Group 1 (
Table 4). In the first blood sampling time point, 10 (83.3%) of the 12 HCWs who were found to be anti-nucleocapsid antibody positive in Group 1 and 16 (50%) of the 32 HCWs who were anti-nucleocapsid antibody positive in Group 2 were working in the medium/high risk areas. At the second blood sampling time point, a total of 90 HCWs in Group 1 were found to be anti-nucleocapsid antibody positive, 51 (56.7%) were working in medium/high risk areas. In Group 2, 36 HCWs were found to be anti-nucleocapsid antibody positive, 20 (55.6%) were working in medium/high risk areas (
Table 4).
In order to compare the changes in anti-RBD IgG antibody levels over time according to groups, a total of 188 participants, including 148 participants in Group 1 and 40 participants in Group 2, who had been fully adhered to all blood sampling time points were analyzed. During the blood sampling time points after vaccination, anti-RBD IgG antibody levels were found to decrease over time in both groups (p<0.001).
Discussion
Healthcare workers have crucial roles in maintaining healthcare during the COVID-19 pandemic. One of the biggest risks to the healthcare system is the potentially high infection rate among healthcare workers due to SARS-CoV-2. It is known that at the beginning of the COVID-19 pandemic, SARS-CoV-2 infection was more common in healthcare workers than in the general population [
8,
9]. HCWs account for less than 3% of the population in most countries and less than 2% in most low-income and middle-income countries [
10]. However, approximately 14% of COVID-19 cases reported to the WHO have occurred among HCWs [
10]. During the pandemic, it is important to constantly monitor healthcare workers, as they can transmit the virus to colleagues, hospitalized patients and family members if they become infected. Rising infection rates among healthcare workers could lead to the collapse of the healthcare system and worsening of the pandemic [
11]. For these reasons, it is of great importance to vaccinate healthcare workers, who are a high-risk group. As a matter of fact, after the emergency use approval for the CoronaVac
® vaccine in our country, healthcare workers were vaccinated as a priority group. Vaccine hesitancy and vaccine refusal are extremely complex social issues. Increasing awareness about vaccination and voluntary vaccination by providing clear and understandable information with an appropriate vaccination policy is important in reducing the public health impact of the COVID-19 pandemic [
12,
13,
14]. Vaccination of healthcare workers in our country was not compulsory, it was optional during the study period. The healthcare workers involved in this study were those who voluntarily agreed to be vaccinated.
In this study, anti-RBD-IgG positivity was detected in 99.6% in the first month after the second vaccine dose. Higher antibody levels were observed in the vaccinated group consisting of those who had had a previous COVID-19 episode compared to the vaccinated group who had not had the disease at all follow-up time points (
Figure 2).
In a study conducted in healthcare workers, it has been reported that the mean antibody level after the first dose of inactivated CoronaVac
® vaccine administration in HCWs previously exposed to COVID-19 was higher than in naive individuals after the second dose vaccination [
15]. In another study conducted in Turkey, it was reported that significantly higher antibody titrations were observed in participants with a natural history of COVID-19 before vaccination [
16].
In our study, a total of eight participants in Group 1 had PCR-confirmed SARS-CoV-2 infection in the timespan between two months after the second dose of vaccine and the last follow-up time point. Some other studies have also reported cases of breakthrough COVID-19 among healthcare workers [
17,
18]. It has been suggested that the risk of COVID-19 in healthcare workers persists despite vaccination, therefore, regular testing for COVID-19 among medical personnel is recommended [
18].
In our study, the last blood sampling time point was planned at the sixth month after the second dose. However, due to the decision of the Ministry of Health to administer the third dose on July 1, 2021, the last follow-up sampling was carried out before the third (booster) dose (141-150 days after the second dose). Because the third dose decision taken by the Ministry of Health coincided with the summer vacation period, and because of the vaccine indecision for the third dose, the number of our participants decreased in this follow-up period compared to other follow-up periods. After this decision of the Ministry, serum samples were taken from the participants who intended to be vaccinated within ten days, for the last follow-up time point before the third dose of vaccination. In our study it was observed that serum antibody levels decreased in both groups (
Figure 2) in the third and last follow-up time. In a study investigating the antibody levels after the second dose vaccination with the inactivated CoronaVac
® vaccine in healthcare workers in Turkey, it was reported that although the seropositivity rate was quite high, like our findings, there was a significant decrease in antibody levels within three months [
19]. In another study investigating anti-SARS-CoV-2 IgG spike protein antibody responses in Turkish HCWs, four and a half months after a two-dose vaccination regimen with an inactive vaccine CoronaVac, they reported relatively low anti-SARS-CoV-2 IgG S antibodies compared with previously reported levels [
20].
In our study, we also aimed to monitor the course of anti-nucleocapsid antibodies after vaccination. Since CoronaVac
® vaccine is an inactivated whole virion vaccine, antibody response is expected against nucleocapsid and other viral proteins besides the spike protein. The nucleocapsid protein of coronaviruses, which is also immunogenic, has been noted to be smaller than the spike protein and to induce antibodies sooner than the spike protein during infection [
21]. It has been reported that SARS-CoV-2 anti-nucleocapsid antibodies decrease earlier than anti-spike antibodies in plasma in individuals who have previously had the disease [
22]. In our study, the rate of anti-nucleocapsid antibody positivity was found to be higher in Group 2 than in Group 1. Anti-nucleocapsid IgG positivity was 48.1% in the first month after the second vaccine dose of the vaccinated participants who had not had the disease, while it was 76.5% in the participants who had had the disease before and were subsequently vaccinated. In the 1
st and 2
nd blood sampling time, the anti-nucleocapsid antibody positivity rate was found to be higher in participants working in medium/high risk areas compared to participants working in areas with partially low risk in Group 1. However, in the last blood sampling time point, most of the participants in both groups were found to have negative anti-nucleocapsid antibodies.
In a recently published article, investigating the total antibody response to the nucleocapsid protein of the virus in healthcare workers with symptomatic and asymptomatic COVID-19 infection, the seroconversion rate in symptomatic and asymptomatic healthcare workers was detected 78.3% and 26.7%, respectively [
23]. In another study conducted in our country, serum total anti-nucleocapsid antibody levels were evaluated in healthcare workers who had had the disease. In that study, it was reported that SARS-CoV-2-specific anti-nucleocapsid total antibodies reached the peak level between 30-45 days and remained positive for six months in most of the cases, but the antibody level decreased over time. In the same study, it was stated that these antibodies retain their detectable levels for a longer period of time in those working in high-risk areas [
24].
In our study, the rate of anti-nucleocapsid IgG antibody positivity and levels of anti-RBD IgG antibody was found to be higher in the participants working in medium/high risk areas in Group 1 compared to the participants working in partially low-risk areas. This may be because some of those working in medium/high risk areas may have had a subclinical infection without a definitive diagnosis prior to vaccination or may have developed memory cells against SARS-CoV-2 due to close contact with no detectable infection. Therefore, the antibody response may be more strongly induced after vaccination. In a study, it was shown that close contacts can acquire T-cell immunity against SARS-CoV-2 despite the absence of detectable infection [
25]. During the study period, personal protective equipment such as disposable gowns, surgical and N95 masks, visors, gloves and overalls required in medium/high risk areas were provided by the hospital management. In addition, although it has not been evaluated with a scaled study, it has been observed that the compliance of health workers with the use of personal protective equipment was mostly high.
Our study has certain limitations for different reasons. One of these was the inability to control the baseline reactivity for anti-spike and anti-nucleocapsid antibody levels of the participants before the first dose of vaccine. There are several reasons for this. 1- Before the start of the study, it was unclear whether the official authorities would approve the use of vaccines in our country; 2- It was not predicted when the vaccination would start; 3- There was a delay in obtaining the ethics committee approval and financial support. Therefore, the first blood sampling time point was started 28 days after the first vaccination date and antibody levels could be measured just before the second vaccination dose. Despite this, antibody levels of healthcare workers were monitored after the first dose of vaccine and then at three separate times, so that post-vaccine antibody response could be compared between groups.
Another limitation of our study is that the follow-up was carried out at four different time points, which caused compliance problems in the participants. Despite this, although the number of participants decreased in the blood sampling time points, 85.1% participation was achieved even in the last blood sampling time point. One of the important limitations of our study is that the last blood sampling time point had to be different from the planned one. On July 1, 2021, the Ministry of Health decided to vaccinate with the third dose people aged 50 and over and healthcare workers who had received two doses. Although the last blood sampling time point was planned at the 6th month after the second dose, the last blood sampling time point had to be carried out before the third dose (141-150 days after the second dose) due to the decision of the Ministry of Health to start administering the third vaccine dose to healthcare workers on 1 July 2021. Because of this decision, although we evaluated the change in antibody levels over time, sampling HCWs between 141-150 days, not after 180 days as intended in our initial study plan, we think that sufficient data were obtained to evaluate the trend of change in antibody levels during the blood sampling time points.