Introduction
Vancomycin is a tricyclic glycopeptide discovered in the 1950s. [
1] It is one of the most important agents for the treatment of serious infections caused by methicillin-resistant
Staphylococcus aureus (MRSA) and methicillin- resistant
Enterococcus (MRE). [
2] The proportion of methicillin-resistant organisms in India has increased from 29% in 2009 to 47% in 2014, [
3] and the trend is persisting. [
4] Alongside, the minimum inhibitory concentration (MIC) of vancomycin to susceptible pathogens is also increasing. [
5] Further, inappropriate use of antimicrobials increases the probability of antimicrobial resistance, the length of hospital stays, treatment cost, morbidity, and mortality. [
6] Other than choosing an appropriate antimicrobial, the correct dose of the antimicrobial is important for improving patient outcomes. [
7]
Intensive care units (ICUs) are well known to harbor multidrug-resistant (MDR) pathogens causing nosocomial infections, forcing clinicians to use reserve class of antimicrobials such as carbapenems and or vancomycin. National, international, and institutional guidelines recommend using vancomycin along with beta- lactam antibiotics or carbapenems for empiric or culture-proven treatment of sepsis. [
8] The recommended dose of vancomycin is a loading dose 25 to 30 mg/kg, intravenous (IV), followed by 15 to 20 mg/kg IV every 8 to 12 hours. [
9] An AUC/MIC ratio of ≥400 is the current accepted critical PK/PD “efficacy”target of vancomycin activity. [
10] However, in some hospitals, a standard dose of 1 gram IV twice daily of vancomycin is administered to the majority of patients. [
11] In patients with acute kidney injury (AKI), 1 gram every 72 hrs is given.
Sepsis is a state of systemic inflammation. This inflammatory state causes several changes in the body, namely increased endothelial permeability (capillary leakage syndrome) vasodilation and increased renal blood flow. The increased renal blood flow increases the glomerular filtration rate (GFR), leading to augmented renal clearance (ARC). [
12] Plasma proteins are also often deranged in these patients. Additionally, an increase in extracellular body water occurs, leading to a high volume of drug distribution. These changes are likely to influence the pharmacokinetic parameters of hydrophilic drugs such as vancomycin and may result in an uncertainty of pharmacotherapeutic outcomes. Nevertheless, despite being in clinical use for several decades, the pharmacokinetics data of vancomycin in critically ill patients are limited. [
6,
13] In our healthcare setups, 1 gram/IV/twice daily is the standard dose of vancomycin, which is often given for patients with suspected Grampositive infections with or without sepsis. [
14] Considering the influence of sepsis on the pharmacokinetic parameters of vancomycin, the aim of the study was to ascertain the adequacy of the administered dose in the patients and conduct a prediction population pharmacokinetic study in patients with sepsis.
Methods
Study design and duration
This was a prospective study conducted over the period of 15 months from May 2019 to July 2020 in adult patients with sepsis admitted to the emergency medical outpatient ward, medical and surgical intensive care units of a tertiary care center. The study was initiated after obtaining permission from the Institutional Ethics Committee (IEC) (NK/4928/MD/566), and written informed consent of eligible patients or their legal guardian was obtained prior to the start of the study.
Every consecutive adult patient from the units involved in the study and diagnosed with sepsis, admitted in the hospital, being treated empirically or definitively (lab-based) with vancomycin with or without other antimicrobials, was screened for potential eligibility. Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. [
15] Patients who were neutropenic, on immunosuppressants, on interventions like hemodialysis, with any contraindication to the use of vancomycin, and those who themselves or their legally accepted representatives denied the consent to participate in the study were excluded.
Study procedure
Each patient’s demographic characteristics and comorbidities were recorded on admission. For each patient, the severity of illness was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II score, given that the patient was enrolled within 24 hours of admission. Additionally, Sequential Organ Failure Assessment (SOFA) and/or quick SOFA scores were also recorded. Creatinine clearance (CrCL) at 24 hours of therapy or on the first day of enrolment was determined based on urinary creatinine clearance (uCrCL [mL/min]), which is calculated using the following formula:
uCrCL(mL/min) = {urinary creatinine concentration (mg/dL) × volume(mL)} / {serum creatinine concentration (mg/dL) × duration of urine collection (minutes)}.
The serum creatinine concentration was measured on Roche Cobas 8000 (spectrophotometry based, Roche Diagnostics, USA).
Sampling
Sample collection for pharmacokinetic analysis was started before the steady-state (i.e., five doses) for vancomycin was expected to be reached. On the first day, the blood samples (2 mL in a heparinized vacutainer) were obtained at 5, 10, and 30 minutes and thereafter at 2 and 6 hrs, following the completion of the vancomycin infusion. One trough sample was also obtained just prior to the next dose. On subsequent days, only a trough sample was obtained each day until the patient was administered vancomycin or was discharged from the unit or died, whichever was earlier. The plasma was separated and stored at − 80 °C until further analysis.
Population pharmacokinetic modeling
Population pharmacokinetic analysis was conducted using non-linear mixed-effects modeling (NLME, Phoenix 8.3.2.166, Certara USA, Inc., USA). Different structural models along with different residual error models were tested to develop a base model. An exponential model was used for inter-patient variability. The data were analysed using the first-order estimation method (FO). Different covariates, i.e., weight, uCrCl and CrCl were tested to account for the observed variability. The influence of each covariate was evaluated by the difference in objective function value (OBJ) between the base model and the model, including the covariate by “stepwise forward inclusion and backward elimination methods”. A p-value less than 0.05 (ΔOBJ>3.841 with one degree of freedom assuming a Chi-squared distribution) in the forward inclusion method and 0.005 (ΔOBJ>7.88) in the backward elimination were considered statistically significant. The final model was evaluated using the nonparametric bootstrap and visual predictive check options in NLME.
Statistical analysis
Appropriate descriptive statistics were used. For age [median (IQR)] is used. For variables like body weight, APACHE II score, SOFA score, qSOFA score, urinary creatinine clearance (uCrCL) and PK parameters, [mean (SD)] were used. The significant difference in the AUC0-last between the two outcome groups and the significant difference in the Cmax between the two outcome groups were assessed by independent sample t test. The significant difference in the AUC0-∞ between the two outcome groups and the significant difference in the mean trough concentrations between the two outcome groups were assessed by Mann-Whitney U test. All statistical analyses were performed on Statistical Package for the Social Sciences (SPSS) software version 25 (IBM, USA).
Results
Demographic characteristics
A total of 15 patients were enrolled (8 males and 7 females) with a median (IQR) age of 34 (31) years and a mean (SD) weight of 69.67 (10.26) kg. The mean (SD) age was 42.4 (17.25) years. The mean (SD) APACHE II score, SOFA score, and qSOFA score were 14.67 (7.28), 7.6 (3.85), and 1.5 (0.85), respectively. The mean (SD) CrCL was 66.22 (43.1) mL/minute. While one patient received vancomycin for proven
Enterococcus faecium infection, all other 14 patients received vancomycin for suspected Gram-positive bacterial infection. The demographic characteristics of individual study participants and their clinical diagnosis are presented in Supplementary
Table 1.
Pharmacokinetic parameters
We observed that there was wide inter- individual variability in pharmacokinetic parameters. The mean (SD) value of maximum (C
max) and minimum concentration (C
min) after a single dose were 38.69 (15.08) mg/L and 11.37 (7.76) mg/L, respectively. The mean (SD) elimination half-life (t
1/2) after a single dose was 19.81 (33.24) hours. The mean (SD) AUC
0-last and AUC
0-∞ after single dose were 215.65 (128.08) mg*hr/L and 593.15 (676.22) mg*hr/L, respectively. The mean (SD) volume of distribution (V
d) and clearance after a single dose were 1 (0.9) L and 1.27 (1.40) mL/min, respectively (
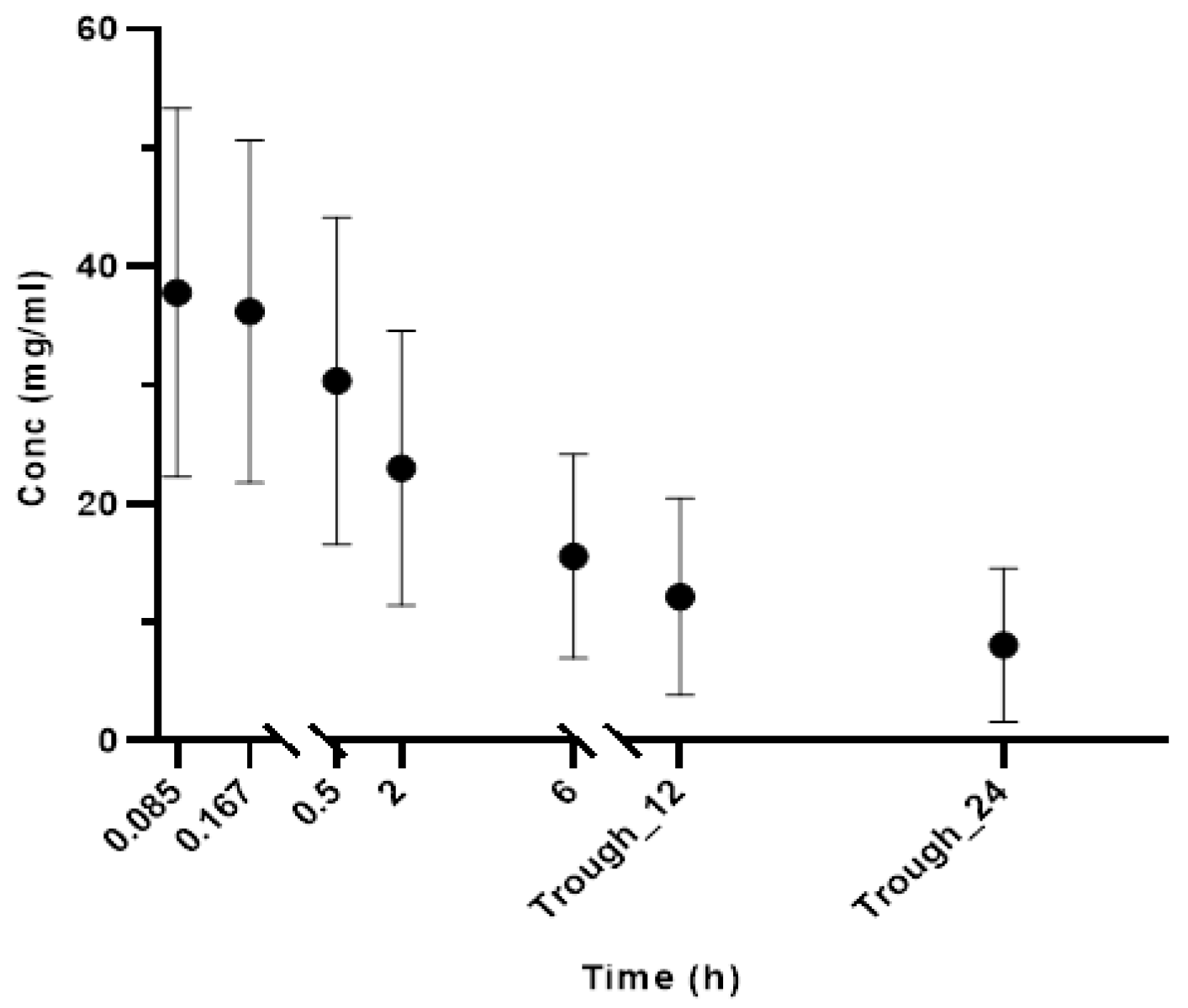
Table 1). The mean (SD) concentrations following single dose at various time points are shown in
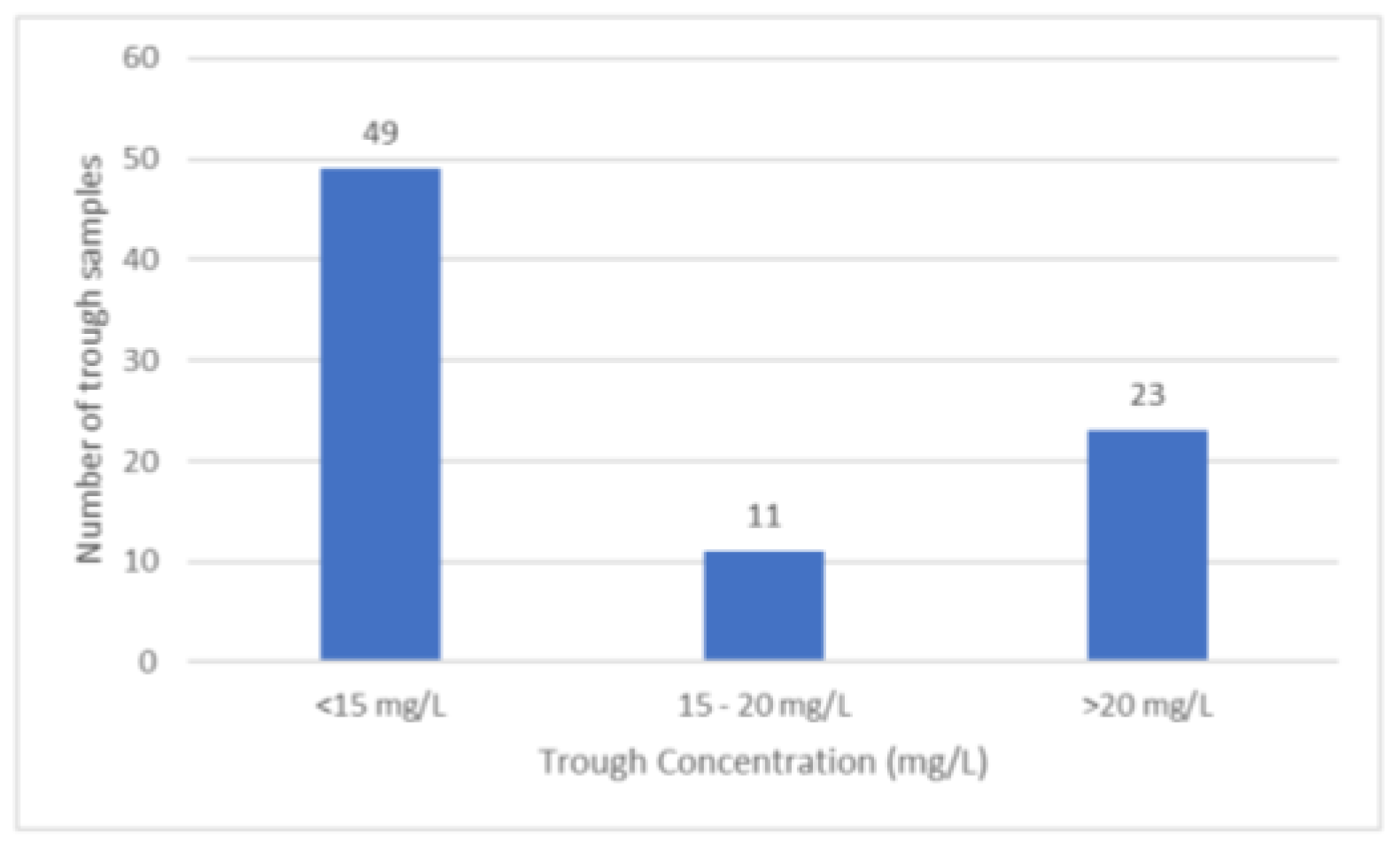
Figure 1. Forty-nine trough samples out of 83 samples were below the recommended therapeutic concentration (
Figure 2).
Pharmacokinetic model
A total of 83 vancomycin samples were best described by a two-compartment linear model. The goodness-of-fit plots showed that the final covariate pharmacokinetic model adequately described the observed vancomycin concentrations (
Figure 3). Inclusion of weight and urinary creatinine clearance in the final model refined the model (OFV of 1133 in base model vs 1109 in final covariate model). We replaced urinary creatinine clearance with creatinine clearance by Cockcroft-Gault formula in the final model which resulted in OFV of 1133 in base model vs 1110 in final covariate model. Although there was similar reduction in OFV, urinary creatinine clearance was slighty better so we used it in the final model. Final estimates are as shown in Supplementary Table S2. The performance of the model was validated using 1000 replicates generated from the original dataset to evaluate the stability of the final model. The mean values of the original dataset were within the 95% CI of the bootstrap values, indicating that all pharmacokinetic parameters can be estimated with acceptable precision (Supplementary Table S2). The visual predictive checks show that the observed concentrations lie within the 90% prediction intervals of the 5th, 50th, and 95th percentiles, calculated from 1000 simulated datasets as shown in Supplementary Figure S1.
Relation between dependent variables and clinical outcome
A total of 12 patients were included in the analysis. The clinical outcome was not known for three patients as they left against medical advice. Four patients improved while eight patients died. The mean AUC0-last and AUC0-∞ in patients who improved and died were (AUC(0-last)=293 (152.97); AUC(0-∞)=535.14 (353.67) and (AUC(0-last)=137.19 (51.37); AUC(0-∞)=582.12 (1036.09) respectively, the difference between the two outcome groups was not statistically significant (p=0.104). Among the patients who died, one patient had augmented renal clearance (Cr.Cl=134.41 mL/min). The average trough concentration for this patient was 6.723 µg/mL. The criteria of AUC0-24/MIC ≥400 was met in two (13.3%) patients. None of the study participants developed any adverse reactions related to vancomycin.
Multiple linear regression
A multiple linear regression analysis was carried out between the dependent variable AUC0-last and independent variables namely age, approximate body weight, serum albumin, and uCrCL. A similar analysis was carried out by replacing uCrCL with CrCL by Cockcroft-Gault formula. Similarly, the analysis was also employed for other dependent variables namely AUC0-∞, Cmax, and mean trough concentrations. None of the correlations were statistically significant except for the correlation between mean trough concentrations and uCrCL (p=0.038).
Discussion
This study was undertaken to determine and understand the appropriateness of vancomycin dosing regimen in critically ill patients in a tertiary care unit in a low middle income country (LMIC). A practice of standard dose of 1 gm IV BD is followed for the majority of patients, even in critically ill patients. In our study also, the majority of patients were hemodynamically unstable and required either fluid or inotropic support.
We observed a large variance in PK parameters, particularly AUC
0-∞ and elimination half-life following a single dose. After adjusting for an outlier, whose AUC
0-∞ and elimination half-life values after single dose were much higher (3.15 times SD) as compared to other subjects, the mean (SD) AUC
0-∞ and elimination half-life were 441.06 (344.64) mg*hr/L and 11.44 (7.73) hours, respectively. Four patients received renally modified vancomycin dose, which could also be a possible explanation for high variability in PK parameters. We also observed a substantial degree of variance in both V
d and clearance, which again could be attributed to the hydrophilic nature of vancomycin, inadequate perfusion of eliminating organs, capillary leakage, need for fluid and inotropic support. [
16]
The goodness of fit plots showed that having covariates in the model significantly improved the predicted vancomycin concentrations. Multiple linear regression analysis showed a statistically significant correlation between mean trough concentrations and uCrCL. However, a similar analysis performed using CrCL by Cockcroft-Gault formula instead of uCrCL failed to show a statistically significant correlation with mean trough concentrations. It may be suggested that uCrCL is a better predictor of GFR and renal function than CrCL as it is calculated by the Cockcroft-Gault formula. This finding falls in line with observations in other studies where it has been observed that estimation of GFR using Cockcroft-Gault formula significantly underestimated renal function in those with ARC and overestimating it in those with normal or decreased 8 hour-CrCL. [
17,
18] At the same time, it must be noted that measurement of urinary creatinine clearance is a cumbersome process as it involves collecting urine for 24 hours. In our study, urinary creatinine clearance could not be measured for two patients since both died before the completion of 24-hour urine collection.
Standard dose with adjustments for creatinine clearance is a norm in healthcare setups in India. Generally, the main reasons for deviation from recommended protocols of dose adjustment are the non-availability of provisions for measuring trough concentrations, inability to make correct assessment of weights in recumbent positions, and availability of other agents to handle MRSA. Paucity of weight calibrated beds is an important limitation in most healthcare setups in LMIC.
For vancomycin which is used for methicillin- resistant Gram-positive organisms, AUC
0-24/MIC ratio of ≥400, is associated with desirable clinical outcome. [
19,
20,
21] In this study, only two patients achieved this value with the administered standard dose. For routine clinical practice, calculation of AUC
0-24/MIC ratio is cumbersome since it requires rich sampling scheme. Therefore, trough concentrations are recommended as a surrogate marker for AUC
0-24. [
10] In our study, we observed that nearly 60% of trough samples were below the recommended range of 15 to 20 mg/L, and only 13.25% achieved the target range with the current dosing schedule. Despite the extensive use of vancomycin and several studies being conducted on its pharmacokinetics in critically ill patients so far, there exists no consensus for an optimal dosing regimen. Three similar studies conducted in critically ill patients also report that the majority of patients had sub- therapeutic vancomycin concentrations. [
22,
23]
Although all of the patients included in the study were critically ill, differences between them were found regarding cardiorespiratory and renal support. Fourteen patients received vancomycin empirically, and for one patient, it was a culture- proven prescription. The clinical outcomes cannot be correlated to vancomycin alone since multiple other factors could have affected the outcomes besides appropriate PK-PD correlate of vancomycin.
Limitations
Our study has highlighted the issue of inappropriate dosing of vancomycin in critically ill patients; however, it has certain limitations. The most important of these was the limited sample size. Another limitation was the non- inclusion of patients who were on renal replacement therapy. During this period only one patient with augmented creatinine clearance was included in the study compromising the generalizability of the findings.
Conclusions
In conclusion, wide interindividual variability was observed in pharmacokinetic parameters of vancomycin in critically ill patients with sepsis. Recommended trough concentrations and AUC0- 24/MIC ≥400 were not archived in the majority of patients with the vancomycin dosing schedule of 1 gram/IV/twice daily. The finding of the study was that including covariates such as urinary creatinine clearance and weight in the pharmacokinetic model helped predict drug concentrations more accurately and based on that finding it could be stated that taking into account those covariates can help modify dosing regimens or achieve PK/PD targets more accurately. However, further studies are required to demonstrate efficacy regarding applying this strategy.