Since December 2019 humanity worldwide has faced one of the worse pandemics of the modern world caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). One of the major structural proteins, abundantly expressed on the SARS-CoV-2 surface, is the spike (S) glycoprotein which mediates the virus invasion into the host cell and represents an important target for antibody production [
1]. The S1 subunit of the spike glycoprotein and its receptor binding domain (RBD) were widely used in the serological assays developed for research and diagnostic purposes during this pandemic [
2].
Serological tests are easy to perform and represent an efficient tool to assess antibody response post-infection and after vaccination. Specific antibodies are produced by the B cells of the immune system as a response to antigens. First, IgM antibodies are produced, which are characterized by a short half-life. In contrast, the IgG antibodies, detectable almost one week later, usually remain stable for months, are produced in large amounts and provide a longer-lasting immunity [
2].
We conducted a prospective study to evaluate the SARS-CoV-2 antibody response in a cohort of infection-naïve healthcare workers (HCW) following first and second vaccination dose using the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine. Our subjects comprised physicians, nurses, administrative and technical staff working in our hospital. Healthy individuals, with no comorbidities, were invited to participate in this study, at the initiation of the hospital’s staff vaccination plan. Before the first vaccine dose, a blood sample was obtained to exclude possible prior COVID-19 infection by assessing the presence of specific anti-SARS-CoV-2 IgG antibodies. Two volunteers tested positive for the aforementioned antibodies and these subjects were not included in this study. Moreover, during the study period, 3 subjects tested positive for COVID-19 and refused to further participate in the study. Testing for COVID-19 was mandatory for all HCW every two weeks, using a real-time RT-PCR test, the Cepheid Xpert Xpress SARS-CoV-2 assay performed on GeneXpert Instrument Systems (Cepheid, USA).
Thus, 142 healthy adults [45 males (31.7%), 97 females (68.3%) with a median age of 48 years±11 years, IQR=16 years (39-54 years)] entered our study. They were categorized in four age-groups: G1, 21-38 years: <25th percentile, 36 participants; G2, 39-48 years: 25-50th percentile, 38 participants; G3, 49-54 years: 50th-75th percentile, 40 participants and G4, 55-67 years: >75th percentile, 28 participants. Written informed consent statement was obtained from all participants and the study was approved by the Ethical Committee of Aretaieio University Hospital (Nr. 305/7-1-2021).
In total, four blood samples were obtained from each participant: before vaccination, 3 weeks after the first vaccine dose, 3 weeks after the second vaccine dose and 4 months after the second vaccine dose. At each time point, blood samples were centrifuged and the resulting serum samples were processed in the same run to ensure optimal performance, in concordance to manufacturer’s instructions and the qualitative control requirements. Participants’ data was anonymized, thus laboratory assays were performed in a blinded fashion. SARS-CoV-2 antibody levels were determined using SARS-CoV-2 IgG II Quant chemiluminescent microparticle assay (Abbott, Ireland) on ARCHITECT i1000SR analyser (Abbott, USA). This assay identified IgG antibodies to the receptor binding domain of the S1 subunit of the spike protein of SARS-CoV-2, had a detection limit of 6.8 AU/mL, an analytical measuring interval ranging from 21.0-40,000.0 AU/mL, while levels <50.0 AU/mL were considered negative and ≥ 50.0 AU/mL, positive.
Data were represented as mean values for continuous variables, and frequency and percentage values for qualitative variables. In group comparisons, one-way analysis of variance or paired samples t-test were used for continuous variables and Chi-square test for qualitative variables. Statistical analysis was performed using SPSS 26.0 (ΙΒΜ Corp, USA) and significance was set at p<0.050.
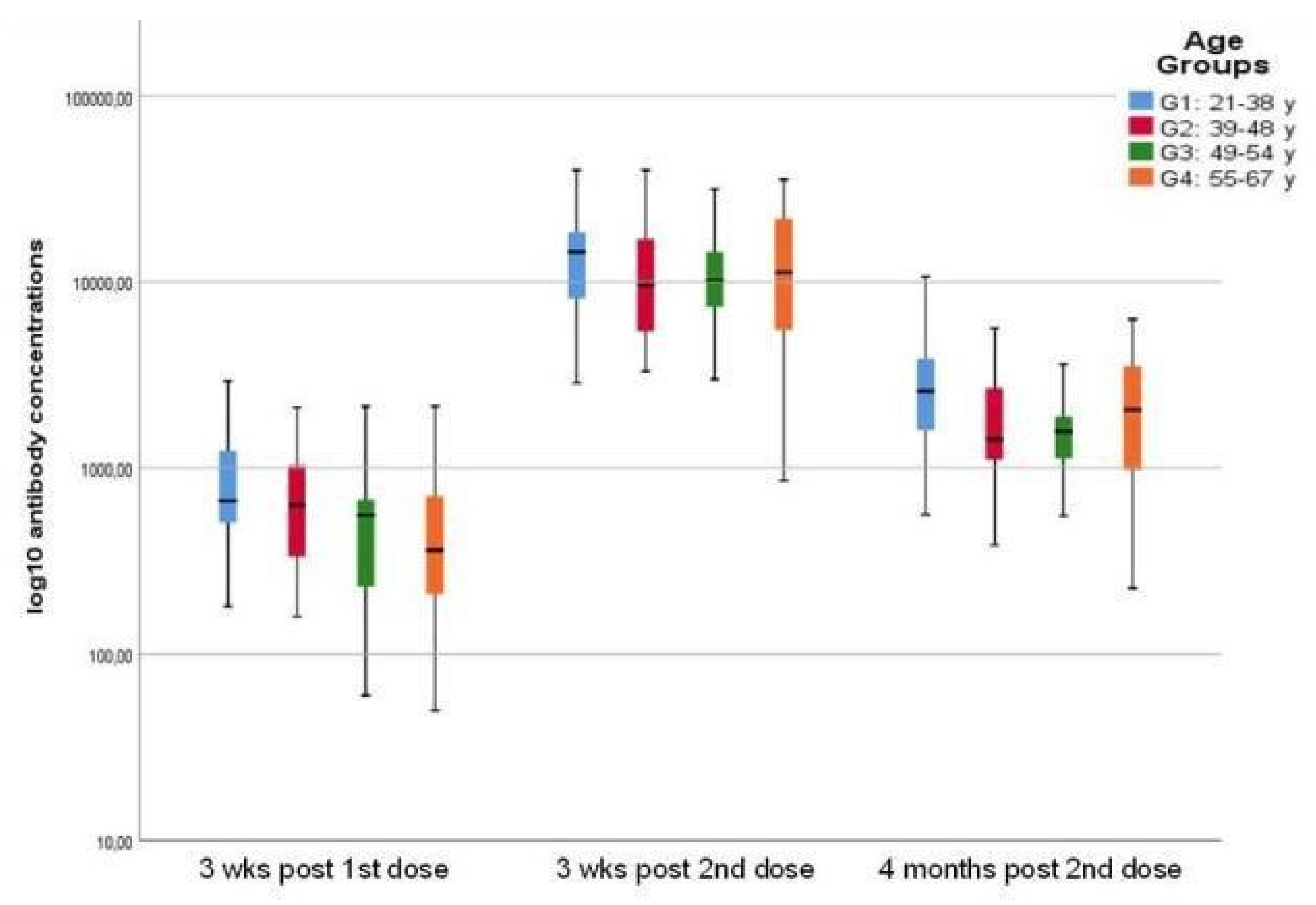
All subjects were seronegative before vaccination. Following the first vaccine dose, the G1 group had a significantly better antibody response than G3 (p=0.004) and G4 (p=0.010) –
Table 1. After the second vaccine dose, total mean antibody levels significantly increased in all age-groups (p<0.001), with an impressive fold increase trend [F(3,138)=3.056; p=0.031, ANOVA], statistically significant for G3 (28.5-fold; p=0.022) and G4 (31.9-fold; p=0.006) compared to G1 group (18.3-fold), resulting in almost similar antibody levels between the groups G1 and G4.
Four months following the second dose, total mean antibody levels decreased significantly (p<0.001) although remaining higher than the levels reached after the first dose (p<0.001), in all age groups [F(3,138)=3.391; p=0.020, ANOVA] –
Figure 1. The G1 group displayed significantly higher antibody levels compared to G2 (p=0.006) and G3 (p=0.007), while comparable with the G4 group. Notably, the G4 group, with the lowest antibody response after the first dose, retained a significantly higher fold rate compared to the G1 group [F(3,138)=5.5; CI: 0.3–3.7; p=0.022, ANOVA]. No statistical difference in antibody levels was observed between men and women.
The introduction of vaccines against SARS-CoV-2 represented not only a challenge to cope with the COVID-19 pandemic, but also the hope that human race was awaiting to end this pandemic and return to normality [
3]. In this study, we specifically focused on the anti-SARS-CoV-2 IgG antibody response in COVID-19 infection-naïve HCW, following Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccination. As demonstrated by others, [
4] comparable antibody levels were observed between men and women, although decreasing with age [
4,
5]. Consistent with previous work, [
5] the youngest group exhibited significantly increased antibody levels following the administration of the first vaccine dose, compared to the other groups. Humoral immune response to vaccination in older adults is often decreased [
6] and has been attributed to adaptive immunosenescence.
7 Undoubtedly, the optimal humoral immune response has not been established, as yet. Emerging data provided evidence that a single mRNA vaccine dose offered only 50% protection against severe infection, [
8] while a recent investigation regarding the effectiveness of the vaccination plan in Israel, showed a preventive efficiency for SARS-CoV-2 related illnesses of 46-62%, after a single dose, which increased to 92-94%, after the second dose [
3]. In individuals with prior SARS-CoV-2 infection, after the administration of first dose, IgG anti-spike antibodies reached levels similar to those recorded in infection-naïve subjects after the second dose, suggesting a more rapid response to the vaccine [
5]. Hence, since older individuals are more prone to severe manifestations of SARS-CoV-2 infection, stronger immunization should be pursued with a second boosting vaccine dose administration.
In line with published data,
5,6 antibody levels exhibited a sustained increase after the first dose, followed by an additional significant raise after the second dose, in all age groups, generating a robust humoral immune response. In our study, subjects over the age of 55 benefited mostly from the second boosting dose, starting from low antibody levels after the first dose and reaching the highest levels, similar to those recorded in individuals 21-38 years old, after the second dose. Our data provide additional support to previous reported studies, [
5] although lower antibody levels have been reported in individuals over 80 years old, following complete vaccination [
7]. This finding could be attributed to the heterogeneity of immune responses post vaccination, which becomes more apparent with increasing age [
2]. Age-related changes in both innate and adaptive immunity are responsible for decreased vaccine responses. The constant challenging of the immune system over the individual’s lifespan results in a continuous state of activation of the innate immune system, thus exhausting the cells involved [
9]. Moreover, the alterations in T and B cells populations and functions are also significant [
10,
11]. The thymic involution, which ends around the age of 50, is responsible for the imbalance between the cells compartments of the immune system, with massive changes in T cells, which in turn are responsible for the delay in the activation of the B cells and the antibody production [
9]. Dan et al. [
10] reported that every specific cell type and antibodies exhibited distinct kinetics during the immune response. The magnitude of the antibody response against SARS-CoV-2 is highly heterogeneous in different subjects and antibody levels reflect the successful activation of B cells, short-lived plasma cells and long-lived plasma cells [
2,
10,
11]. It is possible that, although reacting later to the first vaccination dose, in this group of individuals, after the second boosting dose, the long-lived plasma cells were involved more actively and quickly in the process of antibody production, thus producing an increased level of antibodies, comparable to younger adults.
Antibody levels significantly decreased four months after the second dose, consistent with literature data [
11,
12]. However, protective immunity against SARS-CoV-2 is not restricted to humoral immunity since specific circulating memory T and B cells are involved and ultimately responsible for antibody responses [
2,
10]. In fact, memory B cells and plasma cells have independent kinetics, maintaining increased levels 6 months post-infection, with significant implications for continuing immune responses [
10,
11]. The rapid decay in anti-SARS-CoV-2 spike protein IgG antibodies in the first four months which was continued to a lesser extent over the following months was consistent with the transition from the activation of short-lived plasmablasts (derived from memory B cells) to the long-lived plasma cells generated later in the immune response, as a source of antibodies.
11Although a remarkable scientific effort has been made, the duration and the protective thresholds of the antibody levels as well as the effectiveness of cellular immunity against SARS-CoV-2 are still under investigation. The documented significant decline in the humoral response does not necessarily translate to a loss of protective immunity, [
2,
10] and further studies are warranted to document optimal antibody levels. However, monitoring antibody levels and their possible decrease over time will prove important for the subsequent interpretation of SARS-CoV-2 serology results. Clearly, vaccination has a short-lasting protective effect and since HCW are at increased risk of SARS-CoV-2 infection, adequate protective equipment use as well as strict infection prevention and control measures are imperative.
The findings of our study are limited by the use of a single vaccine in our hospital. A comparison with another vaccine would have been very helpful in comparing antibody levels. We studied only circulating antibodies and not the different cell compartments of the immune response, and this was another limitation of our study. Moreover, the study population was small, since it was limited to the HCW working in a small 100-bed tertiary-care, university-based hospital. Data from infected or convalescent patients was not available to us so our study population comprised only healthy subjects with no apparent comorbidities.
Our findings indicated that the youngest group reacted much faster to the administered first dose of the vaccine, compared to the other age groups. However, individuals 55 to 67 years old responded better to the second dose, increasing significantly their antibody levels, reaching the levels observed in the youngest group. Four months following complete vaccination, antibody levels were still present in all HCW, although significantly decreased, outreaching the antibody response after the first dose.