Perspectives on Passive Antibody Therapy and Peptide-Based Vaccines Against Emerging Pathogens Like SARS-CoV-2
Abstract
Introduction
Methods
Literature Search
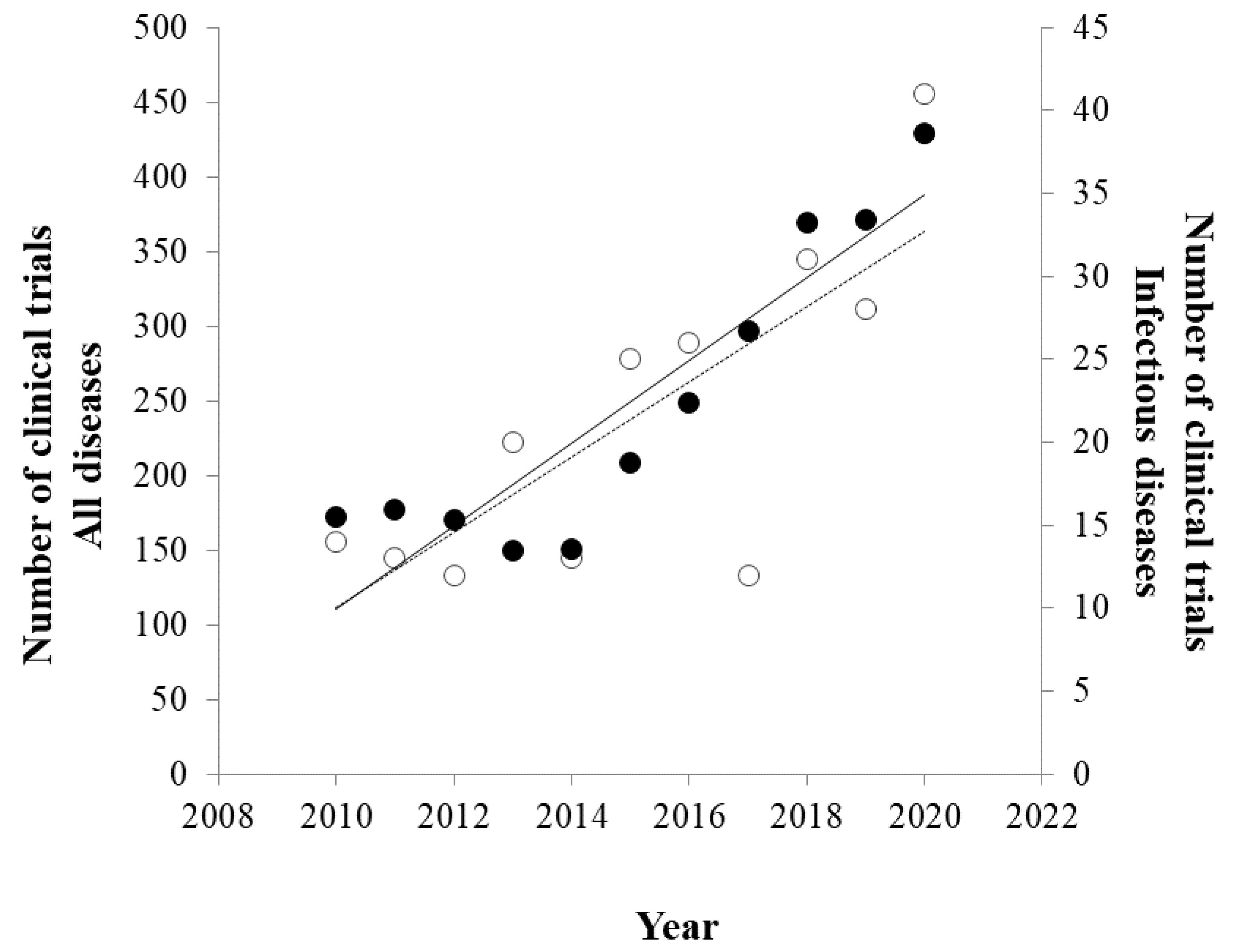
2.2. Clinical Trials Search
Literature Review
Emerging Pathogens
Antibody Response
Passive and Active Immunization
Convalescent Plasma
Passive Antibody Therapy
Immune Phage Display Libraries
| Vaccine | Target | Pathogen | Phases | NCT Number | Start Year |
|---|---|---|---|---|---|
| CMVPepVax (CMVpp65-A*0201) | HLA A*0201 restricted pp65 CD8 T-cell peptide epitope fused with the P2 peptide epitope of tetanus toxin, and mixed with a Toll-like receptor (TLR) 9 agonist | Cytomegalovirus | I, II | NCT01588015 | 2012 |
| II | NCT02396134 | 2015 | |||
| Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | Modified Vaccinia Ankara (MVA) viral vector encoding three herpes virus cytomegalovirus (CMV) tumor-associated antigens (TAAs), including UL83 (pp65), UL123 (IE1) and UL122 (IE2), with potential immunostimulating activity | Cytomegalovirus | II | NCT02506933 | 2015 |
| I, II | NCT03354728 | 2018 | |||
| II | NCT04060277 | 2019 | |||
| CENV3 | Synthetic peptide vaccine derived from HCV E1 and HCV E2 | Hepatitis C virus | I, II | NCT01718834 | 2011 |
| Multimeric 001 (M-001) | 9 conserved peptides from influenza A and B | Influenza H5N1 | II | NCT02691130 | 2015 |
| DC-HIV04 | DC-HIV vaccine with HIV peptides | HIV infection | I | NCT03758625 | 2018 |
| Multipeptide cocktail (pVAC) | CoVac-1 (SARS-CoV-2 HLA-DR peptide) | COVID-19 vaccine | I | NCT04546841 | 2020 |
| StreptInCor | 55 amino acid residues of the C-terminal portion of the M protein | Rheumatic fever, Streptococcus pyogenes | I | NCT03998592 | 2021 |
| Drug Code | Sponsors | Source | Techniques | Approval | Ref. |
|---|---|---|---|---|---|
| Regdanvimab (CT-P59) | Celltrion | B cells from convalescent patients | Phage display | EU (Mar 2021), South Korea (Feb 2021) | Kim et al., 2021 [40] |
| LY-Co555 + LY-CoV016 | AbCellera/EliLilly and Company | B cells from convalescent patients | Flow cytometry | US (Feb 2021), EU (Mar 2021) | Shi et al., 2020 [37] |
| Casirivimab/imdevimab (REGN-COV2) (REGN10987+REGN10933) | Regeneron | From mice + B cells from convalescent patients | Next-generation sequencing | US (No. 2020), EU (Feb 2021) | Hansen et al., 2020 [38] |
| Antibody | Pathogen | Target | Approval (Year) |
|---|---|---|---|
| Ansuvimab | Ebola virus | Receptor-binding domain | US (2020) |
| Antibody cocktail: atoltivimab, maftivimab, and odesivimab-ebgn | Ebola virus | Glycoprotein on the surface of Ebola virus | US (2020) |
| Obiltoxaximab | Bacillus anthracis | B. anthracis exotoxin | EU (2020), US (2016) |
| RabiMabs (antibody cocktail) | Rabies virus | Site II and III on G protein of rabies virus envelope | India (2019) |
| Ibalizumab | HIV | CD4 | EU (2019), US (2018) |
| HyperRAB (HRIG*) | Rabies virus | Rabies virus | US (2018) |
| Bezlotoxumab | Clostridioides difficile | C. difficile enterotoxin B | EU (2017), US (2016) |
| KamRAB/KedRAB (HRIG*) | Rabies virus | Rabies virus | US (2017) |
| Rmab | Rabies virus | Amino acids 336–342 of the GP (antigenic site III) | India (2016) |
| Raxibacumab | Bacillus anthracis | B. anthracis protective antigen (PA) | US (2012) |
| Palivizumab | Respiratory syncytial virus | Antigenic site II region of F protein | EU (1999), US (1998) |
| Imogam (HRIG) | Rabies virus | Rabies virus | US (1984) |
| Vaccine Type | Vaccine Name (Sponsors) | Origin | Approval |
|---|---|---|---|
| Inactivated virus vaccines | CoronaVac (Sinovac) | China | China, Brazil |
| BBIBP-CorV (Sinopharm) | China | China | |
| WIBP-CorV (Sinopharm) | China | China | |
| Covaxin (Bharat Biotech) | India | India, Mexico | |
| CoviVac | Russia | Russia | |
| mRNA-based vaccines | BNT162b2 (Pfizer-BioNTech) | Multinational | US, EU, UK |
| mRNA-1273 (Moderna) | US | US, EU, UK | |
| Non-replicating vector vaccines | Convidicea (CanSino) | China | China, Hungary, Mexico, Chile |
| AZD1222 (AstraZeneca/Oxford) | UK | EU, UK | |
| Janssen (Johnson & Johnson) | The Netherlands, US | US, EU, Canada | |
| Protein-based vaccines | ZF2001 (Anhui Zhifei Longcom) | China, Uzbekistan | China, Uzbekistan |
| Peptide vaccine | EpiVacCorona (Federal Budgetary Research Institution) | Russia | Belarus, Russia, Turkmenistan |
Active Immunization with Peptides
Peptide Libraries
Discussion
Conclusions
Funding
Conflicts of Interest
Acknowledgments
Ethics Approval
References
- Morse, S.S. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int/?gclid=EAIaIQobChMIxYXludnP6gIVVIXVCh1zuQ8iEAAYASAAEgK00fD_BwE (accessed on 23 April 2021).
- Antibody Society. COVID-19 Biologics Tracker-Clinical Studies Evaluating Anti-SARS-CoV-2 Monoclonal Antibodies. 2021. Available online: https://www.antibodysociety.org/covid-19-biologics-tracker/ (accessed on 19 April 2021).
- Regulatory Affairs Professionals Society. COVID-19 Vaccine Tracker. 2021. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (accessed on 19 April 2021).
- Woolhouse, M.E.J.; Howey, R.; Gaunt, E.; Reilly, L.; Chase-Topping, M.; Savill, N. Temporal trends in the discovery of human viruses. Proc. R. Soc. Biol. Sci. 2008, 275, 2111–2115. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.-M.; Chan, K.-H.; Tsoi, H.-W.; Huang, Y.I.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.-K.; et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Prosser, A.T.; LeBreton, M.; Mpoudi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. USA 2005, 102, 7994–7999. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, L.; Pyrc, K.; Berkhout, B. Human coronavirus NL63, a new respiratory virus. Fems Microbiol. Rev. 2006, 30, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Oberste, M.S.; Monroe, S.S.; Nix, W.A.; Campagnoli, R.; Icenogle, J.P.; Peñaranda, S.; Bankamp, B.; Maher, K.; Chen, M.-H.; et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003, 300, 1394–1399. [Google Scholar] [CrossRef]
- Hamelin, M.; Abed, Y.; Boivin, G. Human metapneumovirus: A new player among respiratory viruses. Clin. Infect. Dis. 2004, 38, 983–990. [Google Scholar] [CrossRef]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- Zhang, G.; Nie, S.; Zhang, Z.; Zhang, Z. Longitudinal change of severe acute respiratory syndrome coronavirus 2 antibodies in patients with coronavirus disease 2019. J. Infect. Dis. 2020, 222, 183–188. [Google Scholar] [CrossRef]
- Zohar, T.; Loos, C.; Fischinger, S.; Atyeo, C.; Wang, C.; Slein, M.D.; Burke, J.; Yu, J.; Feldman, J.; Hauser, B.M.; et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell 2020, 183, 1508–1519.e12. [Google Scholar] [CrossRef]
- Van Griensven, J.; Edwards, T.; De Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.W.; Raoul, H.; Magassouba, N. ’f.; Antierens, A.; et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef] [PubMed]
- The United States Food and Drug Administration. FDA Issues Emergency Use Authorization for Convalescent Plasma as Potential Promising COVID-19 Treatment, Another Achievement in Administration’s Fight Against Pandemic. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment (accessed on 14 April 2021).
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P. Convalescent plasma in the management of moderate COVID-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020, 371, m3939. [Google Scholar] [CrossRef] [PubMed]
- Simonovich, V.A.; Pratx, L.D.B.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M. del L.; et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Marc, G.P.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Salazar, E.; Christensen, P.A.; Graviss, E.A.; Nguyen, D.T.; Castillo, B.; Chen, J.; Lopez, B.V.; Eagar, T.N.; Yi, X.; Zhao, P.; et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am. J. Pathol. 2021, 191, 90–107. [Google Scholar] [CrossRef]
- Shenoy, A.G.; Hettinger, A.Z.; Fernandez, S.J.; Blumenthal, J.; Baez, V. Early mortality benefit with COVID-19 convalescent plasma: A matched control study. Br. J. Haematol. 2021, 192, 706–713. [Google Scholar] [CrossRef]
- Alsharidah, S.; Ayed, M.; Ameen, R.M.; Alhuraish, F.; Rouheldeen, N.A.; Alshammari, F.R.; Embaireeg, A.; Almelahi, M.; Adel, M.; Dawoud, M.E.; et al. COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: A multicenter interventional study. Int. J. Infect. Dis. 2021, 103, 439–446. [Google Scholar] [CrossRef]
- Rogers, R.; Shehadeh, F.; Mylona, E.K.; Rich, J.; Neill, M.; Touzard-Romo, F.; Geffert, S.; Larkin, J.; Bailey, J.A.; Lu, S.; et al. Convalescent plasma for patients with severe COVID-19: A matched cohort study. Clin. Infect. Dis. 2020, 73, e208–e214. [Google Scholar] [CrossRef]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef]
- Clark, E.; Guilpain, P.; Filip, I.L.; Pansu, N.; Bihan, C.L.; Cartron, G.; Tchernonog, E.; Roubille, C.; Morquin, D.; Makinson, A.; et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: A report of rapid recovery. Br. J. Haematol. 2020, 190, E154–E156. [Google Scholar] [CrossRef]
- Ferrari, S.; Caprioli, C.; Weber, A.; Rambaldi, A.; Lussana, F. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk. Lymphoma. 2021, 62, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- The United States Food and Drug Administration. Clinical Memorandum—COVID-19 Convalescent Plasma. Available online: https://www.fda.gov/media/141480/download (accessed on 14 April 2021).
- Gasparyan, A.Y.; Misra, D.P.; Yessirkepov, M.; Zimba, O. Perspectives of immune therapy in coronavirus disease 2019. J Korean Med. Sci. 2020, 35, e176. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020, 21, 2272. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.I.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Jin, J.; Simmons, G. Antiviral functions of monoclonal antibodies against chikungunya virus. Viruses 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Zhao, J.; Pedotti, M.; Simonelli, L.; Agnihothram, S.; Fett, C.; Fernandez-Rodriguez, B.; Foglierini, M.; Agatic, G.; Vanzetta, F.; et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc. Natl. Acad. Sci. USA 2015, 112, 10473–10478. [Google Scholar] [CrossRef]
- De Wit, E.; Feldmann, F.; Horne, E.; Okumura, A.; Cameroni, E.; Haddock, E.; Saturday, G.; Scott, D.; Gopal, R.; Zambon, M.; et al. Prophylactic efficacy of a human monoclonal antibody against MERS-CoV in the common marmoset. Antivir. Res. 2019, 163, 70–74. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Issues Advice on Use of Regdanvimab for Treating COVID-19. 2021. Available online: https://www.ema.europa.eu/en/news/ema-issues-advice-use-regdanvimab-treating-covid-19 (accessed on 11 April 2021).
- Kim, C.; Ryu, D.-K.; Lee, J.; Kim, Y.-I.; Seo, J.-M.; Kim, Y.-G.; Jeong, J.-H.; Kim, M.; Kim, J.-I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Wang-Lin, S.X.; Balthasar, J.P. Pharmacokinetic and pharmacodynamic considerations for the use of monoclonal antibodies in the treatment of bacterial infections. Antibodies 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Beeler, J.A.; Coelingh, K. van W. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: Effect of mutation upon fusion function. J. Virol. 1989, 63, 2941–2950. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Matson, M.A.; Schenker, E.; Stein, M.; Zamfirova, V.; Nguyen, H.-B.; Bergman, G.E. Safety and efficacy results of simulated post-exposure prophylaxis with human immune globulin (HRIG; KEDRAB) co-administered with active vaccine in healthy subjects: A comparative phase 2/3 trial. Hum. Vaccines Immunother. 2019, 16, 452–459. [Google Scholar] [CrossRef]
- Cole, S.P. Monoclonal antibodies. Can. Fam. Physician. 1987, 33, 369–372. [Google Scholar]
- Gross, A.; Schoendube, J.; Zimmermann, S.; Steeb, M.; Zengerle, R.; Koltay, P. Technologies for single-cell isolation. Int. J. Mol. Sci. 2015, 16, 16897–16919. [Google Scholar] [CrossRef]
- Lushova, A.A.; Biazrova, M.G.; Prilipov, A.G.; Sadykova, G.K.; Kopylov, T.A.; Filatov, A.V. Next-generation techniques for discovering human monoclonal antibodies. Mol. Biol. 2017, 51, 782–787. [Google Scholar] [CrossRef]
- Throsby, M.; Van Den Brink, E.; Jongeneelen, M.; Poon, L.L.M.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; Van Deventer, E.; Guan, Y.I.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef]
- Harvey, B.R.; Georgiou, G.; Hayhurst, A.; Jeong, K.J.; Iverson, B.L.; Rogers, G.K. Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries. Proc. Natl. Acad. Sci. USA 2004, 101, 9193–9198. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Zwick, M.B.; Burton, D.R. Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage. Protein Eng. Des. Sel. 2007, 20, 81–90. [Google Scholar] [CrossRef]
- Zhou, C.; Shen, W.D. Mammalian cell surface display of full length IgG. Methods Mol. Biol. 2012, 907, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.H.; Marissen, W.E.; Kramer, R.A.; Rice, A.B.; Weldon, W.C.; Niezgoda, M.; Hanlon, C.A.; Thijsse, S.; Backus, H.H.J.; De Kruif, J.; et al. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 2005, 79, 9062–9068. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.M.; Hill, E.M.; AlDeghaither, D.; Weiner, L.M. Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 2015, 67, 28–45. [Google Scholar] [CrossRef]
- Phage, M. Display identification of immunodominant epitopes and autoantibodies in autoimmune diseases. Curr. Biosci. 2021, 1, e02. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Makdasi, E.; Alcalay, R.; Mechaly, A.; Levy, Y.; Bercovich-Kinori, A.; Zauberman, A.; Tamir, H.; Yahalom-Ronen, Y.; Israeli, M. ’a.; et al. A panel of human neutralizing mAbs targeting SARS-CoV-2 spike at multiple epitopes. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.U.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of human single-domain antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e5. [Google Scholar] [CrossRef]
- Amersdorfer, P.; Wong, C.; Smith, T.; Chen, S.; Deshpande, S.; Sheridan, R.; Marks, J.D. Genetic and immunological comparison of anti-botulinum type A antibodies from immune and non-immune human phage libraries. Vaccine 2002, 20, 1640–1648. [Google Scholar] [CrossRef]
- Trott, M.; Weiß, S.; Antoni, S.; Koch, J.; Von Briesen, H.; Hust, M.; Dietrich, U. Functional characterization of two scFv-Fc antibodies from an HIV controller selected on soluble HIV-1 Env complexes: A neutralizing V3- and a trimer-specific gp41 antibody. PLoS ONE 2014, 9, e107089. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O’neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012, 489, 526–532. [Google Scholar] [CrossRef]
- Rahumatullah, A.; Karim, I.Z.A.; Noordin, R.; Lim, T.S. Antibody-based protective immunity against helminth infections: Antibody phage display derived antibodies against BmR1 antigen. Int. J. Mol. Sci. 2017, 18, 2376. [Google Scholar] [CrossRef]
- Grubbs, H.; Kahwaji, C.I. Physiology, active immunity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- World Health Organization. Draft Landscape and Tracker of COVID-19 Candidate Vaccines. 2021. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 23 April 2021).
- OurWorldinData. Coronavirus (COVID-19) Caccinations. 2021. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 23 April 2021).
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Golob, J.L.; Lugogo, N.; Lauring, A.S.; Lok, A.S. SARS-CoV-2 vaccines: A triumph of science and collaboration. J. Clin. Investig. 2021, 6, 149187. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2015, 7, 842–854. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, J.; Chen, C.; Ye, X.; Xu, L.; Wang, W.; Zhao, Q.; Zhu, H.; Cheng, T.; Xia, N. A highly conserved epitope-vaccine candidate against varicella-zoster virus induces neutralizing antibodies in mice. Vaccine. 2016, 34, 1589–1596. [Google Scholar] [CrossRef]
- Guo, L.E.; Yin, R.; Liu, K.; Lv, X.; Li, Y.; Duan, X.; Chu, Y.; Xi, T.; Xing, Y. Immunological features and efficacy of a multi-epitope vaccine CTB-UE against H. pylori in BALB/c mice model. Appl. Microbiol. Biotechnol. 2013, 98, 3495–3507. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Ding, N.; Li, Y.; Shao, J.; Zhu, M.; Xie, Z.; Sun, K. Vaccine based on antibody-dependent cell-mediated cytotoxicity epitope on the H1N1 influenza virus increases mortality in vaccinated mice. Biochem. Biophys. Res. Commun. 2018, 503, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Sasidharan, S.; Saudagar, P. Computational design of a potential multi-epitope subunit vaccine using immunoinformatics to fight Ebola virus. Infect. Genet. Evol. 2020, 85, 104464. [Google Scholar] [CrossRef]
- Bemani, P.; Amirghofran, Z.; Mohammadi, M. Designing a multi-epitope vaccine against blood-stage of Plasmodium falciparum by in silico approaches. J. Mol. Graph. Model. 2020, 99, 107645. [Google Scholar] [CrossRef] [PubMed]
- Majee, P.; Jain, N.; Kumar, A. Designing of a multi-epitope vaccine candidate against Nipah virus by in silico approach: A putative prophylactic solution for the deadly virus. J. Biomol. Struct. Dyn. 2020, 39, 1461–1480. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kaushik, V. In-Silico Proteomic Exploratory Quest: Crafting T-Cell Epitope Vaccine Against Whipples Disease. Int. J. Pept. Res. Ther. 2020, 27, 169–179. [Google Scholar] [CrossRef]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2020, 22, 74–85. [Google Scholar] [CrossRef]
- Kar, T.; Narsaria, U.; Basak, S.; Deb, D.; Castiglione, F.; Mueller, D.M.; Srivastava, A.P. A candidate multi-epitope vaccine against SARS-CoV-2. Sci. Rep. 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Toth, I.; Skwarczynski, M. Peptide-based vaccines. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. [Google Scholar] [CrossRef]
- Slingluff, C.L., Jr.; Petroni, G.R.; Chianese-Bullock, K.A.; Wages, N.A.; Olson, W.C.; Smith, K.T.; Haden, K.; Dengel, L.T.; Dickinson, A.; Reed, C.; et al. Trial to evaluate the immunogenicity and safety of a melanoma helper peptide vaccine plus incomplete Freunds adjuvant, cyclophosphamide, and polyICLC (Mel63). J. Immunother. Cancer 2021, 9, e000934. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide vaccine: Progress and challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Kaumaya, P.T. B-cell epitope peptide cancer vaccines: A new paradigm for combination immunotherapies with novel checkpoint peptide vaccine. Future Oncol. 2020, 16, 1767–1791. [Google Scholar] [CrossRef]
- Di Natale, C.; La Manna, S.; De Benedictis, I.; Brandi, P.; Marasco, D. Perspectives in peptide-based vaccination strategies for syndrome coronavirus 2 pandemic. Front. Pharmacol. 2020, 11, 578382. [Google Scholar] [CrossRef]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.-Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef]
- Rini, B.I.; Stenzl, A.; Zdrojowy, R.; Kogan, M.; Shkolnik, M.; Oudard, S.; Weikert, S.; Bracarda, S.; Crabb, S.J.; Bedke, J.; et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 1599–1611. [Google Scholar] [CrossRef]
- Moynihan, J.S.; Blair, J.; Coombes, A.; D’mEllo, F.; Howard, C.R. Enhanced immunogenicity of a hepatitis B virus peptide vaccine using oligosaccharide ester derivative microparticles. Vaccine 2002, 20, 1870–1876. [Google Scholar] [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef]
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Böhm, T.; Flaig, M.; Bourquin, C.; Oduncu, F.S. Phage idiotype vaccination: First phase I/II clinical trial in patients with multiple myeloma. J. Transl. Med. 2014, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Willis, A.E.; Perham, R.N. Multiple display of foreign peptides on a filamentous bacteriophage. J. Mol. Biol. 1991, 220, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, K.S.; Yang, M.; Mao, C. Phage-enabled nanomedicine: From probes to therapeutics in precision medicine. Angew. Chem. Int. Ed. Engl. 2016, 56, 1964–1992. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Denisov, D.; Groisman, B.; Smelyanski, L.; Meyuhas, R.; Gross, G.; Denisova, G.; Gershoni, J.M. The mapping and reconstitution of a conformational discontinuous B-cell epitope of HIV-1. J. Mol. Biol. 2003, 334, 87–101. [Google Scholar] [CrossRef]
- Oleksiewicz, M.B.; Bøtner, A.; Toft, P.; Normann, P.; Storgaard, T. Epitope mapping porcine reproductive and respiratory syndrome virus by phage display: The nsp2 fragment of the replicase polyprotein contains a cluster of B-cell epitopes. J. Virol. 2001, 75, 3277–3290. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.-F.; Fang, D.-Y.; Yan, H.-J.; Zhou, J.-J.; Zhou, J.-M.; Liang, Y.U.; Gao, Y.; Zhao, W.; Long, B.-G. Selection of SARS-coronavirus-specific B cell epitopes by phage peptide library screening and evaluation of the immunological effect of epitope-based peptides on mice. Virology. 2007, 359, 264–274. [Google Scholar] [CrossRef]
- Zamecnik, C.R.; Rajan, J.V.; Yamauchi, K.A.; Mann, S.A.; Sowa, G.M.; Zorn, K.C.; Alvarenga, B.D.; Stone, M.; Norris, P.J.; Gu, W.; et al. ReScan, a multiplex diagnostic pipeline, pans human sera for SARS-CoV-2 antigens. Cell Rep. Med. 2020, 1, 100123. [Google Scholar] [CrossRef]
- DeFrancesco, L. COVID-19 antibodies on trial. Nat. Biotechnol. 2020, 39, 1242–1252. [Google Scholar] [CrossRef]
- Both, L.; Banyard, A.C.; Van Dolleweerd, C.; Wright, E.; Ma, J.K.-C.; Fooks, A.R. Monoclonal antibodies for prophylactic and therapeutic use against viral infections. Vaccine 2013, 31, 1553–1559. [Google Scholar] [CrossRef]
- Lebozec, K.; Jandrot-Perrus, M.; Avenard, G.; Favre-Bulle, O.; Billiald, P. Quality and cost assessment of a recombinant antibody fragment produced from mammalian, yeast and prokaryotic host cells: A case study prior to pharmaceutical development. New Biotechnol. 2018, 44, 31–40. [Google Scholar] [CrossRef]
- Deeks, E.D. Certolizumab pegol: A review in inflammatory autoimmune diseases. BioDrugs 2016, 30, 607–617. [Google Scholar] [CrossRef]
- Lien, S.; Lowman, H.B. Therapeutic anti-VEGF antibodies. In Therapeutic antibodies. Handbook of experimental pharmacology; Chernajovsky, Y., Nissim, A., Eds.; Springer Nature: Berlin, 2008. [Google Scholar] [CrossRef]
- Bournazos, S.; Ravetch, J.V. Anti-retroviral antibody FcγR-mediated effector functions. Immunol. Rev. 2017, 275, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.A.C.; Teow, S.-Y. Review of current cell-penetrating antibody developments for HIV-1 therapy. Molecules 2018, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert. Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. MRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.A.; Arbabi-Ghahroudi, M.; Scott, J.K. Beyond phage display: Non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold. Front. Microbiol. 2015, 6, 755. [Google Scholar] [CrossRef] [PubMed]
- Antibody Society. Antibody Therapeutics Approved or in Regulatory Review in the EU or US. 2021. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 22 April 2021).

| Antibody | Target | Pathogen | Phases | NCT Number |
|---|---|---|---|---|
| 3BNC117-LS | CD4 binding site on the HIV-1 envelope | HIV | I, II | NCT04250636, NCT04319367 |
| 10-1074-LS | V3 glycan supersite on the HIV-1 envelope protein | HIV | NCT04250636, NCT04319367 | |
| PGT121.414.LS, VRC07-523LS | CD4 binding site of the HIV-1 envelope | HIV | I | NCT04212091 |
| Herpevizumab (HDIT101) | Epitope on HSV-1/2 glycoprotein B (gB) | HSV-1 | II | NCT04539483 |
| SAR440894 | E2 envelope protein of chikungunya virus | Chikungunya virus | I | NCT04441905 |
| MAU868 | Viral capsid protein, VP1 | BK virus | II | NCT04294472 |
| SYN023 (CTB011 + CTB012) | Non-overlapping epitopes on the rabies virus (RABV) glycoprotein (G) | Rabies virus | III | NCT04644484 |
| AV-1 | West Nile virus E protein | Dengue | I | NCT04273217 |
| Antibodies against Pseudomonas from patients’ B lymphocytes | Pseudomonas | Pseudomonas aeruginosa | NCT04335383 | |
| Bezlotoxumab | Toxin B | Clostridioides difficile | II | NCT03829475 |
| SCTA01 | S protein | SARS-CoV-2 | I | NCT04483375 |
| REGN10933+REG N10987 | S protein | SARS-CoV-2 | I, II, III | NCT04519437, NCT04426695, NCT04425629, NCT04452318 |
| TY027 | S protein | SARS-CoV-2 | I, III | NCT04429529, NCT04649515 |
| JS016 | S protein | SARS-CoV-2 | I | NCT04441918 |
| DZIF-10c (BI767551) | From recovered COVID-19 patients | SARS-CoV-2 | I, II | NCT04631705 |
| DZIF-10c | From recovered COVID-19 patients | SARS-CoV-2 | I, II | NCT04631666 |
| VIR-7831 | From patient recovered from SARS in 2003 | SARS-CoV-2 | II, III | NCT04545060 |
| CIS43LS | Circumsporozoite protein (PfCSP) | Malaria | I | NCT04206332 |
| TB31F | Pfs48/45 | Malaria | I | NCT04238689 |
| Antibody | Target | Pathogen | Phases | NCT Number |
|---|---|---|---|---|
| UB-421 | CD4 | HIV | II | NCT03164447, NCT04404049, NCT03743376 |
| Garadacimab (CSL312) | Factor XII/XIIa | SARS-CoV-2 | II | NCT04409509 |
| Mavrilimumab (KPL-301, CAM3001) | GM-CSF α | SARS-CoV-2 | II, III | NCT04447469 |
| Mavrilimumab | GM-CSF α | SARS-CoV-2 | II | NCT04397497 |
| Leronlimab | CCR5 | SARS-CoV-2 | II | NCT04343651, NCT04347239 |
| TJ003234 | Granulocyte-monocyte stimulating factor (GM-CSF) | SARS-CoV-2 | II, III | NCT04341116 |
| Lenzilumab | GM-CSF | SARS-CoV-2 | III | NCT04351152 |
| Ravulizumab | Complement component C5 | SARS-CoV-2 | III | NCT04369469 |
| CPI-006 | CD73 cell-surface ectonucleotidase | SARS-CoV-2 | I | NCT04464395 |
| Crizanlizumab | P-selectin | SARS-CoV-2 | II | NCT04435184 |
| REGN-COV2, Tocilizumab | IL-6 | SARS-CoV-2 | II, III | NCT04381936 |
| Gimsilumab | Granulocyte-monocyte stimulating factor (GM-CSF) | SARS-CoV-2 | II | NCT04351243 |
| Pamrevlumab | Connective tissue growth factor (CTGF) | SARS-CoV-2 | II | NCT04432298 |
| Canakinumab | IL-1β | SARS-CoV-2 | NCT04348448 | |
| Tocilizumab | IL6 | SARS-CoV-2 | IV | NCT04377750 |
© GERMS 2021.
Share and Cite
Palma, M. Perspectives on Passive Antibody Therapy and Peptide-Based Vaccines Against Emerging Pathogens Like SARS-CoV-2. GERMS 2021, 11, 287-305. https://doi.org/10.18683/germs.2021.1264
Palma M. Perspectives on Passive Antibody Therapy and Peptide-Based Vaccines Against Emerging Pathogens Like SARS-CoV-2. GERMS. 2021; 11(2):287-305. https://doi.org/10.18683/germs.2021.1264
Chicago/Turabian StylePalma, Marco. 2021. "Perspectives on Passive Antibody Therapy and Peptide-Based Vaccines Against Emerging Pathogens Like SARS-CoV-2" GERMS 11, no. 2: 287-305. https://doi.org/10.18683/germs.2021.1264
APA StylePalma, M. (2021). Perspectives on Passive Antibody Therapy and Peptide-Based Vaccines Against Emerging Pathogens Like SARS-CoV-2. GERMS, 11(2), 287-305. https://doi.org/10.18683/germs.2021.1264



