Introduction
Klebsiella pneumoniae is a member of Enterobacterales order, and is commonly recognized as nosocomial pathogen that can cause various infectious syndromes. In humans, this bacterium is commonly found in the intestinal tract and fecal carriage rates may increase threefold during hospitalization. Selective pressure of antibiotics in the hospital setting gives rise to the evolvement of multiple genetic resistance mechanisms. Prolonged colonization with antibiotic-resistant
K. pneumoniae is common, especially in bedridden patients.[
1] Quarter-century after the first descriptions of extended-spectrum beta-lactamase (ESBL)-producing
K. pneumoniae in Croatian hospitals, more than 40 percent of clinical
Klebsiella isolates from our hospital are ESBL-producers.[
2,
3] Carbapenems are the treatment of choice for the infections caused by ESBL-producing organisms. In contrast to the Group 2 carbapenems (imipenem/ meropenem/ doripenem) frequently used for invasive infections, ertapenem is more frequently used in lower-risk patients without severe sepsis because it has no activity against non-fermenting bacteria.
However, the emergence of highly transmissible carbapenem-resistant (CR) isolates narrows the therapeutic choice and therefore poses a substantial threat to the hospital medicine. Different mechanisms are responsible for carbapenem resistance in
K. pneumoniae: (i) production of carbapenemases and (ii) hyperproduction of ESBL or AmpC enzymes combined with the major outer membrane proteins (OMPs) porin loss or upregulated efflux pump.[
4]
K. pneumoniae with reduced susceptibility to one or more carbapenems emerged sporadically in different geographic regions in Croatia in the last two decades. The first CR NDM-1 carbapenemase-producing
K. pneumoniae was isolated in 2008,[
5] followed by KPC[
6] and OXA-48[
7] carbapenemase-producing bacteria in 2012, the latter of which disseminated rapidly throughout the country. Although CR
K. pneumoniae has a high potential to cause outbreaks in healthcare settings, reports on the incidence of ertapenem resistance in
K. pneumoniae unrelated to the production of carbapenemases are limited.
Porin modification is an important bacterial strategy that confines the diffusion of the antibiotic into the cell. Mutation of non-selective porin channels OmpK35 and OmpK36, which are responsible for a remarkably rapid influx of β-lactams in the wild-type
K. pneumoniae isolate, have been implicated in reduced susceptibility to carbapenems.[
8] We documented an outbreak of an ESBL-producing ertapenem-resistant
K. pneumoniae strain in the Clinical Hospital Center Rijeka that occurred at the beginning of 2012 and lasted until the end of 2014. Hence, with growing prevalence of ESBL-producing
K. pneumoniae strains and the urgent need for optional antibiotic therapies, we aimed to analyze the epidemiological and microbiological characteristics of the outbreak. We explored the genetic relatedness of selected ertapenem-resistant clinical isolates and the underlying mechanisms that conferred the CR phenotype – the expression of genes encoding a component of the AcrAB-TolC efflux system and genes encoding OmpK35 and OmpK36 porins.
Methods
Study setting
A retrospective study was performed to identify ertapenem-resistant ESBL-producing Klebsiella pneumoniae isolates from various clinical samples collected from patients hospitalized in Clinical Hospital Centre Rijeka, Croatia. This institution is a tertiary-level teaching hospital situated in three geographically distinct locations. It is the major healthcare facility in its region, with 1069 acute care beds and outpatient clinic, and it serves a population of approximately 300,000 inhabitants mostly from Western (Costal) Croatia. It has 18 clinics (Anesthesiology and Intensive Care, Dermatology, Surgery, Neurosurgery, Internal Medicine, Infectious Diseases, Pediatrics, Gynecology, Oncology, Neurology, etc.) and 12 clinical institutes.
The Ethics Committee of the Clinical Hospital Centre Rijeka approved this study and waived the need for informed consent.
Routine susceptibility testing was carried out using the disk diffusion method as a part of routine laboratory practice. Minimum inhibitory concentration assays of selected antibiotics, molecular analyses and pulsed-field gel electrophoresis were specifically performed for this study.
Bacterial isolates
A total of 74 ertapenem-non-susceptible K. pneumoniae isolates collected from January 2012 to December 2014 were the subject of this retrospective study. All isolates non-susceptible to ertapenem (MIC>0.5) with confirmed ESBL-producing phenotypes were collected as part of the routine hospital laboratory procedure and were not specially isolated for this study. Isolates were obtained from both clinical and active surveillance samples of patients hospitalized in different hospital wards. Duplicate isolates (the same species from the same patient) were excluded. Bacteria were stored at -70°C in MAST CRYOBANK™ cryogenic vials (MAST Diagnostica, Germany). The identification of K. pneumoniae complex was performed using the VITEK 2 system (bioMérieux, France). In this survey, we did not differentiate between colonizing and infecting isolates.
Antimicrobial susceptibility testing (AST)
Isolates were cultured at 37°C for 24 h on Mueller-Hinton agar (BD Difco, France), and inoculates were prepared in Mueller-Hinton broth (MHB) (BD Difco) at a density adjusted to a 0.5 MacFarland turbidity standard. AST was performed and interpreted according to EUCAST Standard.[
9] ESBL production was screened by phenotypic disc diffusion methods (double disk synergy assays) while AmpC beta-lactamases were tested using inhibitor-based method (cloxacillin with cefoxitin and phenylboronic acid with cefoxitin). The MIC of the antibiotics – ertapenem, doripenem, imipenem, meropenem, ciprofloxacin, cefotaxime, ceftazidime, gentamicin, amikacin, tigecycline and colistin was determined using E-test according to the manufacturer's instructions (AB Biodisk, Sweden). MIC
50 and MIC
90 levels were defined as the lowest concentration of the antibiotic at which 50% and 90% of the isolates were inhibited, respectively. ESBL production was confirmed by cefotaxime/cefotaxime-clavulanic acid and ceftazidime/ceftazidime-clavulanic acid ESBL strips (bioMérieux) on MH agar.
K. pneumoniae ATCC 700603 was used as quality control isolate for ESBL production. For detection of the major carbapenemases the Modified Hodge Test (MHT) and Carba NP Test were performed on isolates grown on MH agar plates as previously described.[
10]
K. pneumoniae ATCC BAA-1705 was used as positive control for MHT.
Molecular detection of carbapenemase resistance genes
A full 10-µL inoculation loop with a bacterial suspension equivalent to 0.5 McFarland standard was added into the sample reagent. After homogenization 1.7 mL were subsequently transferred into the cartridge and commercial Xpert® Carba-R multiplex real-time PCR assay (Cepheid, USA) designed for the qualitative detection of five carbapenemase genes (blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP-1) was carried on the GeneXpert System – the molecular testing platform (Cepheid).
mRNA expression level analysis
Relative expression levels of the acrA and tolC genes coding for the AcrAB-TolC efflux pumps proteins, and ompK35 and ompK36 genes coding for the outer membrane proteins was analyzed using reverse transcription-quantitative PCR (RT-qPCR) in: (i) isolates resistant to tigecycline, (ii) isolates resistant to ertapenem plus one of the other carbapenems, (iii) isolates resistant only to ertapenem and (iiii) susceptible control isolates. We have compared the gene expression levels of acrA, tolC, ompK35 and ompK36 genes between several groups of isolates. The variables that were compared are crossing point (CP) values for the qPCR amplification of cDNA of each gene between isolates from different groups. The CP values for the genes were normalized by the CP values for ATPs gene which is used as a reference gene in the qPCR. The p values are calculated for the comparison of CP values for each gene between different groups of isolates.
The isolates were cultured in brain heart infusion (BHI) medium until the suspension reached OD
600=0.6 (usually 2-3 hours). Total RNA was extracted using the High Pure RNA Isolation kit (Roche, Germany), and reverse transcribed into cDNA using the first-strand cDNA Synthesis Kit for RT-PCR (Roche). Resulting cDNA was analyzed by RT-qPCR using LightCycler FastStart DNA Master SYBR Green I (Roche) in the LightCycler 2.0 instrument (Roche) to determine if there is a difference in the relative expression of
acrA gene for efflux pump, and
tolC for outer membrane component of the efflux pump, and two porin channels genes –
ompK35 and
ompK36. The primers used for the expression analysis are listed in
Table 1.
Gene expression data was analyzed by Relative Expression Software Tool (REST), comparing mRNA levels of the genes in the clinical isolates with ATPs as the reference gene (reaction efficiency 0.9524). Statistical analysis is performed in the REST using pair wise fixed reallocation randomization test.[
13] Results are presented as mean values ± standard errors. A p value of <0.05 was considered statistically significant.
Pulsed-field gel electrophoresis (PFGE)
K. pneumoniae isolates were genotyped by pulsed-field gel electrophoresis of XbaI restricted fragments. PFGE was performed as previously described with our modifications.[
14,
15] Bacterial plugs were sliced in 1-2 mm slices, that were digested with 20 U of XbaI restriction endonuclease (Fermentas, Lithuania) at 37°C overnight, and then transferred to 0.5 × TBE buffer, loaded on the comb, and run in 1% agarose gel in CHEF-DR III pulsed-field electrophoresis system (Bio-Rad, USA) at 6 V/cm, 120° field angle, 14°C, with initial and final switch times between 5 and 30 sec for 22 h. Gels were analyzed with GelCompar II software (Applied Maths NV, Belgium). PFGE patterns were analyzed by BioNumerics GelCompar II software. A dendrogram showing clonal relationship of the isolates was constructed using the unweighted-pair-group method of the arithmetic average clustering with the Dice similarity coefficient. An optimization of 1.2%, tolerance of 1.2%, and tolerance change of 1.2% were applied.
Results
Specimen sources
The majority of isolates were recovered from mid-stream urine (46%, n=34) or catheter specimen of urine (CSU) (17%, n=13), followed by tracheal aspirate/bronchoalveolar lavage (BAL) (17%, n=13), abscess/drainage (6%, n=5), blood (4%, n=3) and other (8%, n=6). The distribution analysis of K. pneumoniae complex isolates around clinical wards indicated that approximately 30% isolates were from the Intensive Care Unit (ICU) – mostly isolated from CSU or tracheal aspirate/BAL, and just a few K. pneumoniae were isolated from blood culture). Surgery (general surgery, abdominal surgery and neurosurgery) accounted for approximately 20%, while nephrology accounted for 10% of the total isolates (data not shown).
Antimicrobial susceptibility rates
According to the laboratory´s data, during a three-year study period a total of 1884
K. pneumoniae complex strains were isolated in our laboratory from different clinical samples. The overall proportion of ESBL producers among
K. pneumoniae complex strains was 43.10% (n=812). Reduced susceptibility to carbapenems in all
K. pneumoniae complex isolates in the three-year period was noted as stated: 0.10% isolates were resistant to imipenem, 0.26% isolates to doripenem, 0.32% isolates to meropenem, while 11.4% isolates were resistant to ertapenem (data not shown). The
K. pneumoniae complex isolates (n=74) with similar pattern of resistance selected for this study were subjected to additional MIC testing by E-tests. In general,
K. pneumoniae complex isolates showed multidrug-resistance (MDR) to at least one antimicrobial agent from three or more antibiotic classes. All isolates were resistant to amoxicillin, amoxicillin/clavulanate and trimethoprim/sulfamethoxazole (data not shown) as well as to broad-spectrum cephalosporins (cefotaxime and ceftazidime), ciprofloxacin and gentamicin (
Table 2).
In addition to 100% ertapenem resistance, 3%, 7% and 8% of selected 74 isolates showed reduced susceptibility to imipenem, doripenem and meropenem, respectively. Resistance to tigecycline was noted in 10% of these isolates while amikacin and colistin appeared to be the most effective drugs as 100% of these isolates were susceptible.
Phenotypic and molecular tests for carbapenemase detection
The phenotypic Modified Hodge test and Carba NP test were negative in all 74 isolates. No carbapenemases genes, blaKPC, blaNDM, blaVIM, blaOXA-48, and blaIMP-1, were detected by the Cepheid Xpert® Carba-R assay.
Efflux pump expression and porin mutation
The expression of the
acrA and
tolC genes coding for the AcrAB-TolC efflux pump proteins and
ompK35 and
ompK36 porin-encoding genes, was analyzed using RT-qPCR. The expression of genes in isolates resistant to tigecycline, isolates resistant or intermediate resistant to at least one other carbapenem (imipenem, meropenem or doripenem), and in the randomly selected six ertapenem-resistant isolates was compared to the control susceptible wild type
K. pneumoniae isolates. The results of the comparisons are listed in the
Table 3.
The first comparison is between isolates resistant to tigecycline and control K. pneumoniae isolates susceptible to all antibiotics. We did not find any statistically significant difference in the expression of acrA and tolC genes between tigecycline-resistant isolates and susceptible control group. However, we found significantly reduced expression of OmpK35 porin in isolates resistant to tigecycline compared to the control isolates by a mean factor of 0.012 (p=0.003), which implies that the expression of OmpK35 porin in the tigecycline-resistant isolates is around 80 times lower than in the susceptible control samples.
The second comparison is between isolates resistant to more than one carbapenem and control K. pneumoniae isolates susceptible to all antibiotics. A statistically significant upregulation of acrA gene was found in the group of isolates resistant or intermediate resistant to at least one other carbapenem, apart from ertapenem, however the expression was upregulated by a mean factor of 1.195 (p=0.023), which implies only slight upregulation. We did not find any difference in tolC expression. The isolates resistant or intermediate resistant to at least one other carbapenem, showed significantly (around 9 times) lower expression of ompK36 porin-encoding gene, by a mean factor of 0.114 (p=0.032).
The third comparison is between isolates resistant only to ertapenem and control K. pneumoniae isolates susceptible to all antibiotics. The comparison of the expression of the aforementioned genes was done for the six randomly selected isolates resistant only to ertapenem. The only difference found was strongly reduced expression of the OmpK35 porin (by a mean factor of 0.015), however it was not statistically significant (p=0.075).
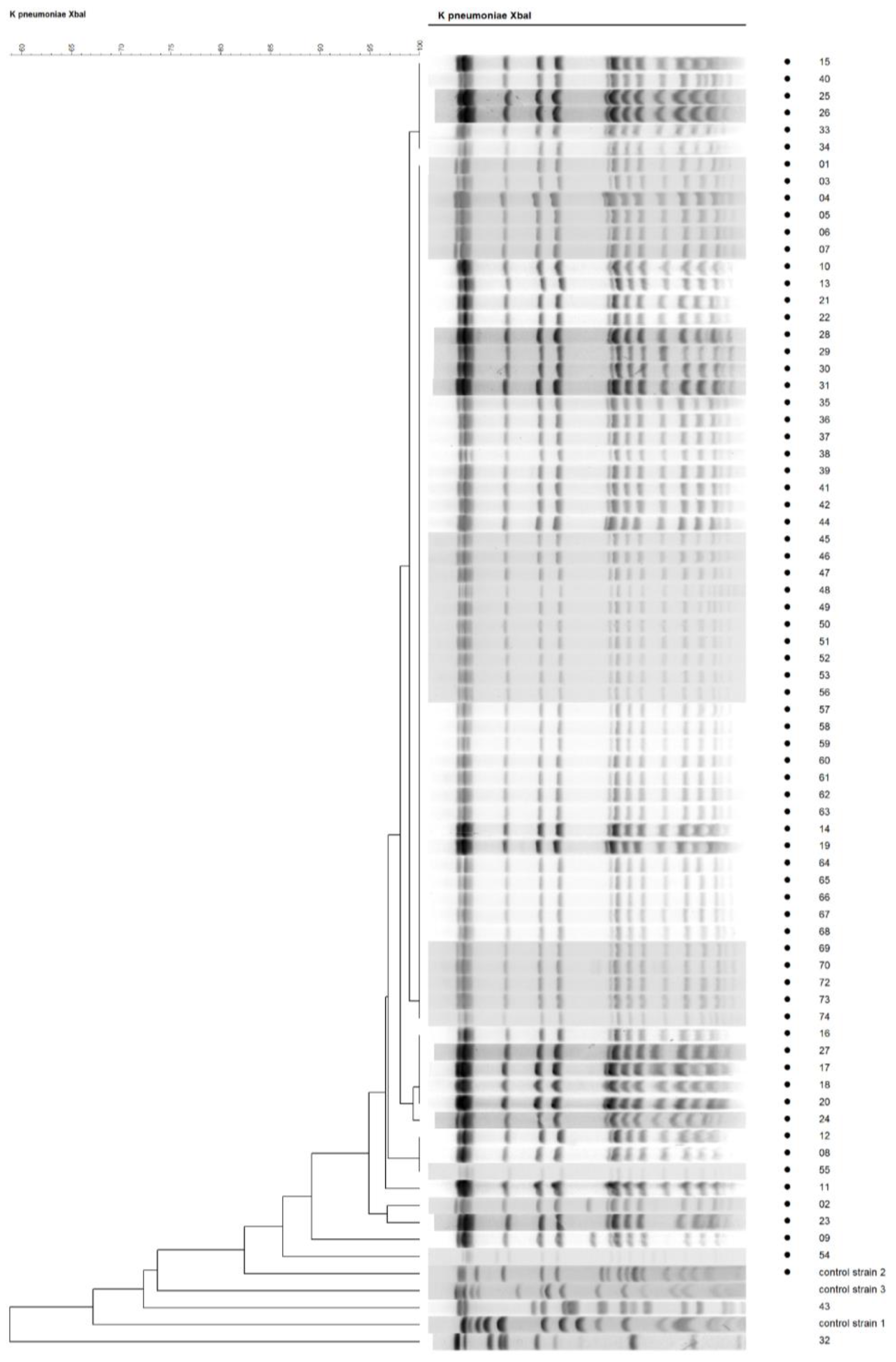
The genetic relationships among isolates – results of PFGE analysis
The selected 74 ertapenem-resistant ESBL-producing
K. pneumoniae strains isolated from different wards and specimen types were further analyzed by PFGE to determine the clonal relationship between them. Typeability by PFGE was 100%. The phylogenetic tree constructed upon PFGE analysis showed that almost all clinical samples were clonally related, as 72 out of 74 (97 %) belonged to the same pulsogroup based on >80% similarity criterion, while 67 out of 74 (91%) showed >95% genetic similarity implying the clonal spread of the single isolate throughout the hospital (
Figure 1).
Discussion
K. pneumoniae has a high degree of environmental stability – the emergence of high-risk clones that are very efficient at colonizing the human host and responsible for outbreaks in hospitals across Europe is worrying. According to the European Centre for Disease Prevention and Control (ECDC) surveillance report the percentage of MDR
K. pneumoniae invasive isolates with combined resistance to fluoroquinolones, third-generation cephalosporins and aminoglycosides in Croatian hospitals in last few years varied from 23% to 32%.[
16] Carbapenems are considered as one of the last resort antibiotics used for the treatment of nosocomial infections caused by Gram-negative bacteria resistant to third-generation of cephalosporins.[
17] Thus, the emergence of carbapenem resistance in
K. pneumoniae requires careful monitoring because of the potential for rapid spread of resistance genes through plasmid transfer and limited treatment options. Patients infected or colonized with CR
K. pneumoniae may introduce bacteria into the healthcare environment leading to dissemination of organisms to healthcare personnel and other patients. Carbapenemase-positive isolates of
K. pneumoniae have the highest transmissibility to spread in hospital environments both within and between countries, which have been shown in a recent European survey.[
18] However, a few years ago, outbreaks in southern Europe caused by an ertapenem-resistant, porin-deficient ESBL-producing
K. pneumoniae raised awareness of emergence and spread of carbapenem resistance derived from other mechanisms.[
19,
20]
This study presents a retrospective analysis of the distribution and antibiotic resistance patterns of ertapenem-resistant ESBL-producing
K. pneumoniae strains causing clonal outbreak in University Hospital Rijeka. According to laboratory´s data, resistance to ertapenem in our hospital started from 2012 to increase remarkably. During the three years period reduced susceptibility to ertapenem was noted in as many as 11.41% of all
K. pneumoniae isolates. In order to analyze the mechanisms of resistance and genetic relations, retrospective screening was performed on a collection of 74 ertapenem-non-susceptible
K. pneumoniae isolates selected during three years. The highest number of these isolates were from urine (63%), mostly obtained from patients hospitalized in the ICU (30%) and surgical wards (20%), which is not surprising since prolonged hospital stay with indwelling urethral catheter is one of the risk factors for acquiring nosocomial infection. Phenotypic and molecular analyzes ruled out carbapenemases production in all isolates. Apart from carbapenemase production,
K. pneumoniae isolates possess several mechanisms to evade the activity of carbapenems. Recently, a global survey of major porins from ertapenem non-susceptible
K. pneumoniae isolates lacking carbapenemases revealed that porin disruption is a widespread phenomenon, and in combination with ESBLs and/or AmpC enzymes, likely accounts for the elevated ertapenem MICs.[
21] All studied ertapenem-resistant
K. pneumoniae strains were ESBL producers. According to the laboratory´s data, the ESBL-producing strains isolated in our laboratory represented 43.10% of the total
K. pneumoniae isolates. Ertapenem is the most sensitive indicator of carbapenem resistance affected by carbapenemase production. Our data support the finding that ertapenem is also the most sensitive carbapenem to permeability defects, as the majority (97%, 93% and 92%) of the isolates remained susceptible to imipenem, doripenem or meropenem, respectively. Therefore, ertapenem MICs often increase when an organism loses one or more of its porins. The presence of ESBL enzymes combined with alterations in OmpK35 and OmpK36 porins expression can result in ertapenem MICs in the intermediate or resistant range, while other carbapenems can remain susceptible or have raised MICs.[
21] However, mutations in
ompK35 and
ompK36 genes or decrease/loss of the OmpK35 or OmpK36 porins expression were previously reported as the mechanism of resistance to other carbapenems in the carbapenemase-negative strains.[
22,
23] In the present study, comparing the expression of OmpK35 and OmpK36 porins in the isolates resistant or intermediate resistant to imipenem, doripenem or meropenem, we confirmed that the reduced expression of OmpK36 porin channel might be involved in the development of resistance to those carbapenems. Further, our results show that OmpK35 downregulation is probably involved in the development of the ertapenem resistance since the expression of OmpK35 was strongly reduced in the ertapenem-resistant isolates compared to the control, even though it was not statistically significant.
We next aimed to investigate the possible contribution of AcrAB-TolC efflux pump in carbapenem resistance. Interestingly, we found slight upregulation of the acrA gene of a mere 20% in isolates resistant to one or more carbapenems, which has not been reported previously. The overexpression of an efflux pump system in CR isolates might have contributed towards carbapenem resistance. Further investigation is warranted to clarify the role of AcrAB-TolC pump in developing resistance against the carbapenem group of antibiotics.
MDR
K. pneumoniae clonal isolate studied here remained susceptible to amikacin - outstanding among antimicrobials because of its high and stable rates of
K. pneumoniae susceptibility. Although aminoglycosides may be used in monotherapy to treat urinary tract infections caused by susceptible ESBL-producers, the aminoglycoside use in systemic infections must be supported by other active therapy.[
24] Monotherapy with amikacin is associated with increase in multidrug resistance originating from production of aminoglycoside-modifying enzymes.
Beside amikacin, colistin appeared to be the most effective drug as all tested isolates were susceptible. Since colistin is one of the last-resort antibiotics for CR
K. pneumoniae infections, its resistance, which is continually increasing worldwide, represents a treatment challenge. Thus, in a recent surveillance of CR
K. pneumoniae complex from Croatian hospitals emerging colistin resistance has been reported.[
25]
Tigecycline is one of the last resort treatments for multidrug-resistant Gram-negative bacterial infections. We observed the worrying occurrence of tigecycline resistance in isolates characterized in the current study. Recent data indicate that
K. pneumoniae tigecycline resistance could be associated with overexpression of AcrAB and OqxAB efflux pumps.[
26,
27] In the present study we did not detect the overexpression of AcrAB-TolC efflux pump in tigecycline-resistant
K. pneumoniae isolates. Tigecycline resistance, which evolved in 10% of ertapenem resistant isolates during the course of the monoclonal outbreak presented here, could be the result of strong reduction of the expression of OmpK35 porin. However, we cannot rule out other mechanisms of the resistance that were not tested in our study, since the reduction of OmpK35 was also detected in the isolates resistant only to ertapenem.
Resistance to at least one carbapenem, frequently to ertapenem, defines the CR phenotype. Ertapenem is the most vulnerable carbapenem to hydrolysis by β-lactamases and the emergence of ertapenem resistance needs to be monitored closely. A randomized controlled trial found that ertapenem could be efficiently used as de-escalation therapy for susceptible ESBL-producing Enterobacterales, especially for urinary tract infections (UTIs).[
28] Meropenem and imipenem often remain moderately active against strains with low-level ertapenem resistance. If susceptibility of carbapenemase-negative Enterobacterales to Group 2 carbapenem is retained, treatment with a susceptible carbapenem agent as monotherapy is generally sufficient. Our study has shown approximately the equal susceptibility of
K. pneumoniae isolates to all Group 2 carbapenem agents. Although meropenem showed higher MIC
50s (1 μg/mL) in comparison to imipenem (0.19 μg/mL), it is probably not likely to be clinically significant for most susceptible isolates. As there are is clear difference in efficacy between meropenem and imipenem, the choice to use one over the other carbapenem is principally based on toxicity profiles in specific patients. Meropenem would be preferred option in patients with bacterial meningitis, underlying central nervous system disease or impaired renal function.[
29]
Doripenem is a relatively new carbapenem and the clinical studies for its use are rare. High clinical efficacy and safety of doripenem in the treatment of acute bacterial infections is reported recently in a systemic review and meta-analysis of randomized controlled trials.[
30] We demonstrated here similar
in vitro activity of doripenem against ertapenem-resistant, ESBL-producing
K. pneumoniae complex as other Group 2 carbapenem agents. Accordingly, doripenem could be a therapeutic option for the treatment of patients with complicated UTI, intra-abdominal infections and ventilator-associated pneumonia caused by ESBL-producing
K. pneumoniae isolates.
Group 2 carbapenems are an effective treatment against the ertapenem-resistant isolates. However, in case of susceptible isolates with higher MIC to Group 2 carbapenems a high-dose prolonged-infusion regimen is generally recommended.[
31,
32] Pharmacokinetic optimization of antibiotic therapy increases the likelihood of appropriate time above the MIC and the effective elimination of pathogens. Several ertapenem-resistant clinical isolates of
K. pneumoniae showed Group 2 carbapenem MICs of 2 μg/mL, in which case the combination therapy with another active drug should be considered. In general, aminoglycosides are preferred as a second agent added to the extended-infusion carbapenem. The use of carbapenems combination therapy is likely ineffective for CR isolates with carbapenem MICs 4 μg/mL or greater.[
33] In that case the use of a new β-lactam/β-lactamase inhibitor combinations (ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam) or cefiderocol, a novel siderophore cephalosporin, should be considered.
The limitation of this study is a lack of differentiation between colonizing and infecting K. pneumoniae isolates. It is important to differentiate them and compare their antibiotic resistance and molecular typing patterns in future studies, as hospitalized patients can become infected with the same strain that they carried. Characterization of carriage isolates could help make treatment decisions in patients that are at risk.
We next analyzed clonal relationships of isolates using PFGE method. Molecular typing results obtained from the PFGE patterns showed indistinguishable patterns, suggesting the presence of an epidemic clone circulating within the 13 different hospital wards situated in two separate hospital buildingslocations. Therefore, in the current study multifactorial events resulted in carbapenem resistance phenotype, whereupon the positive selection imposed by the extensive use of antimicrobials (third generation cephalosporins and fluoroquinolones) favored the expansion of the resistant clone, promoting its diffusion within the hospital. Although porin-deficient mutants may have reduced fitness and be less likely to spread in healthcare settings, this ertapenem-resistant ESBL-producing clone emerged rapidly in our hospital. To prevent further hospital-wide dissemination of this MDR isolate, infection control measures were taken quickly. The outbreak control measures were: good clean-up of the hospital environment and medical devices, cohorting and screening of patients (rectal surveillance cultures performed in high-risk wards), prevention of patient transfer to other units, and creating awareness in staff about hand-washing. Restrictive antimicrobial policy consisted of limitation of third generation cephalosporins and fluoroquinolones use during a nine-month period, as well as education of staff about restriction policies and criteria. The management of the outbreak by the healthcare workers, in collaboration with microbiologists and the hospital Infection Control Committee, allowed a successful interruption of the “silent” dissemination of this clone.