Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves
Abstract
:1. Introduction
2. Typical Preprocessing Means Applicable to Olive Leave Extraction
2.1. Drying
2.2. Size Reduction
2.3. Blanching
3. Future Perspectives
4. Conclusions
Funding
Conflicts of Interest
References
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Del Mar Contreras, M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 1–8. [Google Scholar]
- Gullon, P.; Gullon, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Lage, M.P.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 1–17. [Google Scholar] [CrossRef]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2017, 98, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.D.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Ekechukwu, O.V. Review of solar-energy drying systems I: An overview of drying principles and theory. Energy Convers. Manag. 1999, 40, 593–613. [Google Scholar] [CrossRef]
- Yang, H.; Sombatngamwilai, T.; Yu, W.Y.; Kuo, M.I. Drying applications during value-added sustainable processing for selected mass-produced food coproducts. Processes 2020, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Gowen, A.; Abu-Ghannam, N.; Frias, J.; Oliveira, J. Optimisation of dehydration and rehydration properties of cooked chickpeas (Cicer arietinum L.) undergoing microwave–hot air combination drying. Trends Food Sci. Technol. 2006, 17, 177–183. [Google Scholar] [CrossRef]
- Norton, B. Industrial and Agricultural Applications of Solar Heat. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Oxford, UK, 2012; Volume 3, pp. 567–594. [Google Scholar]
- Ndukwu, M.C.; Dirioha, C.; Abam, F.I.; Ihediwa, V.E. Heat and mass transfer parameters in the drying of cocoyam slice. Case Stud. Therm. Eng. 2017, 9, 62–71. [Google Scholar] [CrossRef]
- Iqbal, J.M.; Akbar, W.M.; Aftab, M.R. Heat and mass transfer modeling for fruit drying: A review. MOJ Food Process. Technol. 2019, 7, 69–73. [Google Scholar] [CrossRef]
- Ekechukwu, O.V.; Norton, B. Review of solar-energy drying systems II: An overview of solar drying technology. Energy Convers. Manag. 1999, 40, 615–655. [Google Scholar] [CrossRef]
- Tomar, V.; Tiwari, G.N.; Norton, B. Solar dryers for tropical food preservation: Thermophysics of crops, systems and components. Solar Energy 2017, 154, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Afaneh, I.; Yateem, H.; Al-Rimawi, F. Effect of olive leaves drying on the content of oleuropein. Am. J. Analyt. Chem. 2015, 6, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech. J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, V.S.; Matela, H.M.; Baptist, A. Total antioxidant capacity using ferric reducing antioxidant power and 2, 2-diphenyl-1 picryl hydrazyl methods and phenolic composition of fresh and dried drumstick (Moringa oleifera) leaves. Int. J. Green Pharm. 2013, 7, 66–72. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Goula, A.M.; Adamopoulos, K.G.; Gerasopoulos, D. Valorization of olive leaves: Spray drying of olive leaf extract. Waste Biomass Valorization 2018, 9, 619–633. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.T.; Blanco, B.; Beltrán, S.; Niknam, S.M. Freeze dried extract from olive leaves: Valorisation, extraction kinetics and extract characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Shukla, S. Freeze drying process: A review. Int. J. Pharm. Sci. Res. 2011, 2, 3061–3068. [Google Scholar]
- Harnkarnsujarit, N.; Kawai, K.; Watanabe, M.; Suzuki, T. Effects of freezing on microstructure and rehydration properties of freeze-dried soybean curd. J. Food Eng. 2016, 184, 10–20. [Google Scholar] [CrossRef]
- Ghelichkhani, G.; Modaresi, M.H.; Rashidi, L.; Shariatifar, N.; Homapour, M.; Arabameri, M. Effect of the spray and freeze dryers on the bioactive compounds of olive leaf aqueous extract by chemometrics of HCA and PCA. J. Food Meas. Charact. 2019, 13, 2751–2763. [Google Scholar] [CrossRef]
- Martinho, D.; Karmali, A.; Rosa, E. Extraction of Phenolic Compounds from Olive Leaf Extracts and Their Effect on Proliferation of Human Carcinoma Cell Lines. J. Agric. Sci. 2019, 10, 1271–1285. [Google Scholar] [CrossRef] [Green Version]
- Erbay, Z.; Icier, F. Optimization of hot air drying of olive leaves using response surface methodology. J. Food Eng. 2009, 91, 533–541. [Google Scholar] [CrossRef]
- Bahloul, N.; Boudhrioua, N.; Kouhila, M.; Kechaou, N. Effect of convective solar drying on colour, total phenols and radical scavenging activity of olive leaves (Olea europaea L.). Int. J. Food Sci. Technol. 2009, 44, 2561–2567. [Google Scholar] [CrossRef]
- Helvaci, H.U.; Menon, A.; Aydemir, L.Y.; Korel, F.; Akkurt, G.G. Drying of olive leaves in a geothermal dryer and determination of quality parameters of dried product. Energy Procedia 2019, 161, 108–114. [Google Scholar] [CrossRef]
- Nourhène, B.; Mohammed, K.; Nabil, K. Experimental and mathematical investigations of convective solar drying of four varieties of olive leaves. Food Bioprod. Process. 2008, 86, 176–184. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Slimen, I.B.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Oyinloye, T.M.; Yoon, W.B. Effect of freeze-drying on quality and grinding process of food produce: A review. Processes 2020, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Bitra, V.S.P.; Womac, A.R.; Cannayen, I.; Miu, P.I.; Yang, Y.T.; Sokhansanj, S. Comminution energy consumption of biomass in knife mill and its particle size characterization. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting 2009, Reno, NV, USA, 21–24 June 2009; Paper No. 095898. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009. [Google Scholar]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Karam, M.C.; Petit, J.; Zimmer, D.; Djantou, E.B.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Gao, W.; Chen, F.; Wang, X.; Meng, Q. Recent advances in processing food powders by using superfine grinding techniques: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2222–2255. [Google Scholar] [CrossRef] [PubMed]
- Stamatopoulos, K.; Chatzilazarou, A.; Katsoyannos, E. Optimization of Multistage Extraction of Olive Leaves for Recovery of Phenolic Compounds at Moderated Temperatures and Short Extraction Times. Foods 2014, 3, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Stamatopoulos, K.; Katsoyannos, E.; Chatzilazarou, A.; Konteles, S.J. Improvement of oleuropein extractability by optimising steam blanching process as pre-treatment of olive leaf extraction via response surface methodology. Food Chem. 2012, 133, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Marazeeq, K.; Haddadin, M.S.Y.; Abdulla, B.; Haddadin, J.S. Biological Activities of Olive Leaves Extract from Nabali Baladi Variety against Lipid and Protein Oxidation. Int. J. Biol. Biotech. 2016, 13, 283–291. [Google Scholar]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Gharekhani, M.; Ghorbani, M.; Rasoulnejad, N. Microwave-assisted extraction of phenolic and flavonoid compounds from Eucalyptus camaldulensis Dehn leaves as compared with ultrasound-assisted extraction. Lat. Am. Appl. Res. 2012, 42, 305–310. [Google Scholar]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Optimization of the aqueous extraction of phenolic compounds from olive leaves. Antioxidants 2014, 3, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; You, F.; Zhang, Y.L. Reduced pressure extraction of oleuropein from olive leaves (Olea europaea L.) with ultrasound assistance. Food Bioprod. Process. 2015, 93, 29–38. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 1–8. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Integrated process for sequential extraction of bioactive phenolic compounds and proteins from mill and field olive leaves and effects on the lignocellulosic profile. Foods 2019, 8, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şahin, S.; İlbay, Z.; Kırbaşlar, Ş.İ. Study on optimum extraction conditions for olive leaf extracts rich in polyphenol and flavonoid. Sep. Sci. Technol. 2015, 50, 1181–1189. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-assisted extraction of phenolic compounds from olive leaves; A comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Lins, P.G.; Pugine, S.M.P.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Canabarro, N.I.; Mazutti, M.A.; do Carmo Ferreira, M. Drying of olive (Olea europaea L.) leaves on a conveyor belt for supercritical extraction of bioactive compounds: Mathematical modeling of drying/extraction operations and analysis of extracts. Ind. Crops Prod. 2019, 136, 140–151. [Google Scholar] [CrossRef]
- Urzúa, C.; González, E.; Dueik, V.; Bouchon, P.; Giménez, B.; Robert, P. Olive leaves extract encapsulated by spray-drying in vacuum fried starch–gluten doughs. Food Bioprod. Process. 2017, 106, 171–180. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, J.Y.; Park, C.S.; Shetty, K.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Poklar Ulrih, N. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Khemakhem, I.; Ahmad-Qasem, M.H.; Catalán, E.B.; Micol, V.; García-Pérez, J.V.; Ayadi, M.A.; Bouaziz, M. Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason. Sonochem. 2017, 34, 466–473. [Google Scholar] [CrossRef]
- Wissam, Z.; Ali, A.; Rama, H. Optimization of extraction conditions for the recovery of phenolic compounds and antioxidants from Syrian olive leaves. J. Pharmacogn. Phytochem. 2016, 5, 390–394. [Google Scholar]
- Gao, X.; Zhu, D.; Liu, Y.; Zha, L.; Chen, D.; Guo, H. Physicochemical properties and anthocyanin bioaccessibility of downy rose-myrtle powder prepared by superfine grinding. Int. J. Food Prop. 2019, 22, 2022–2032. [Google Scholar] [CrossRef] [Green Version]
- Speroni, C.S.; Stiebe, J.; Guerra, D.R.; Bender, A.B.B.; Ballus, C.A.; dos Santos, D.R.; Morisso, F.D.P.; da Silva, L.P.; Emanuelli, T. Micronization and granulometric fractionation improve polyphenol content and antioxidant capacity of olive pomace. Ind. Crops Prod. 2019, 137, 347–355. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Zhang, G.; Tang, W. Effect of superfine grinding on the physicochemical properties and antioxidant activity of red grape pomace powders. Powder Technol. 2015, 286, 838–844. [Google Scholar] [CrossRef]
- Zhong, C.; Zu, Y.; Zhao, X.; Li, Y.; Ge, Y.; Wu, W.; Zhang, Y.; Li, Y.; Guo, D. Effect of superfine grinding on physicochemical and antioxidant properties of pomegranate peel. Int. J. Food Sci. Technol. 2016, 51, 212–221. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Tang, X.; Wei, Z.; Zhang, M. Particle size of insoluble dietary fiber from rice bran affects its phenolic profile, bioaccessibility and functional properties. LWT-Food Sci. Technol. 2018, 87, 450–456. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Chin, K.B. Evaluation of ball-milling time on the physicochemical and antioxidant properties of persimmon by-products powder. Innov. Food Sci. Emerg. Technol. 2016, 37, 115–124. [Google Scholar] [CrossRef]
- Hong, S.J.; Das, P.R.; Eun, J.B. Effects of superfine grinding using ball-milling on the physical properties, chemical composition, and antioxidant properties of Quercus salicina (Blume) leaf powders. J. Sci. Food Agric. 2021, 101, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wu, Z.; Ameer, K.; Li, S.; Ramachandraiah, K. Particle size of ginseng (Panax ginseng Meyer) insoluble dietary fiber and its effect on physicochemical properties and antioxidant activities. Appl. Biol. Chem. 2020, 63, 1–10. [Google Scholar] [CrossRef]
- Alsaud, N.; Farid, M. Insight into the Influence of Grinding on the Extraction Efficiency of Selected Bioactive Compounds from Various Plant Leaves. Appl. Sci. 2020, 10, 6362. [Google Scholar] [CrossRef]
- Chen, G.; Wang, L.; Zhang, F.; Li, C.; Kan, J. Effect of superfine grinding on physicochemical properties of mulberry leaf powder. TCSAE 2015, 31, 307–314. [Google Scholar]
- Hu, J.; Chen, Y.; Ni, D. Effect of superfine grinding on quality and antioxidant property of fine green tea powders. LWT-Food Sci. Technol. 2012, 45, 8–12. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Junghare, H.; Hamjade, M.; Patil, C.; Girase, S.B.; Lele, M.M. A Review on cryogenic grinding. Int. J. Curr. Eng. Technol. 2017, 7, 420–423. [Google Scholar]
- Saxena, S.N.; Sharma, Y.K.; Rathore, S.S.; Singh, K.K.; Barnwal, P.; Saxena, R.; Upadhyaya, P.; Anwer, M.M. Effect of cryogenic grinding on volatile oil, oleoresin content and anti-oxidant properties of coriander (Coriandrum sativum L.) genotypes. J. Food Sci. Technol. 2013, 52, 568–573. [Google Scholar] [CrossRef]
- Saxena, S.N.; Saxena, P.; Rathore, S.S.; Sharma, L.K.; Saxena, R.; Barnwal, P. Effect of cryogenic grinding on phenolic compounds and antioxidant properties of fenugreek (Trigonella foenum-graecum L.) seed extract. J. Spices Aromat. Crops 2016, 25, 73–78. [Google Scholar]
- Bellik, F.Z.; Benkaci-Ali, F.; Alsafra, Z.; Eppe, G.; Tata, S.; Sabaou, N.; Zidani, R. Chemical composition, kinetic study and antimicrobial activity of essential oils from Cymbopogon schoenanthus L. Spreng extracted by conventional and microwave-assisted techniques using cryogenic grinding. Ind. Crops Prod. 2019, 139, 1–12. [Google Scholar] [CrossRef]
- Sharma, L.K.; Agarwal, D.; Meena, S.K.; Rathore, S.S.; Saxena, S.N. Effect of cryogenic grinding on oil yield, phenolics and antioxidant properties of ajwain (Trachyspermum ammi L.). Int. J. Seed Spices 2015, 5, 82–85. [Google Scholar]
- Herrero, M.; Castro-Puyana, M.; Rocamora-Reverte, L.; Ferragut, J.A.; Cifuentes, A.; Ibáñez, E. Formation and relevance of 5-hydroxymethylfurfural in bioactive subcritical water extracts from olive leaves. Food Res. Int. 2012, 47, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching–A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Otero, D.M.; Oliveira, F.M.; Lorini, A.; Antunes, B.D.F.; Oliveira, R.M.; Zambiazi, R.C. Oleuropein: Methods for extraction, purifying and applying. Rev. Ceres 2020, 67, 315–329. [Google Scholar] [CrossRef]
- Deng, L.Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.H.; Wang, J.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef]
- Zeitoun, M.A.M.; Mansour, H.M.; Ezzat, S.; El Sohaimy, S.A. Effect of pretreatment of olive leaves on phenolic content and antioxidant activity. Am. J. Food Technol. 2017, 12, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Sucharitha, P.; Satyanarayana, S.V.; Reddy, K.B. Pretreatment and optimization of processing conditions for extraction of oleuropein from olive leaves using central composite design. Pharmacogn. Res. 2019, 11, 178–187. [Google Scholar]
- Nobosse, P.; Fombang, E.N.; Mbofung, C.M.F. The effect of steam blanching and drying method on nutrients, phytochemicals and antioxidant activity of Moringa (Moringa oleifera L.) leaves. Am. J. Food Sci. Technol. 2017, 5, 53–60. [Google Scholar]
- Musco, J.; McHugh, T.H.; Pan, Z.; Avena-Bustillos, R. Olive Leaf Powder. U.S. Patent Number US 9,724,376 B2, 8 August 2017. [Google Scholar]
- Majetic Germek, V.; Bencek, M.; Lukic, B.; Broznic, D.; Koprivnjak, O. Natural enrichment of refined rapeseed oil with phenols and chlorophylls from olive leaves of Oblica cultivar. Croat. J. Food Sci. Technol. 2019, 11, 202–209. [Google Scholar] [CrossRef] [Green Version]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Evolution of the phenolic compounds profile of olive leaf extract encapsulated by spray-drying during in vitro gastrointestinal digestion. Food Chem. 2019, 279, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, R.G. Subcritical water extraction of mannitol from olive leaves. J. Food Eng. 2009, 93, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Paladino, S.; Zuritz, C. Antioxidant grape seed (Vitis vinifera L.) extracts: Efficiency of different solvents on the extraction process. Rev. Fac. Cienc. Agrar. 2011, 43, 187–199. [Google Scholar]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
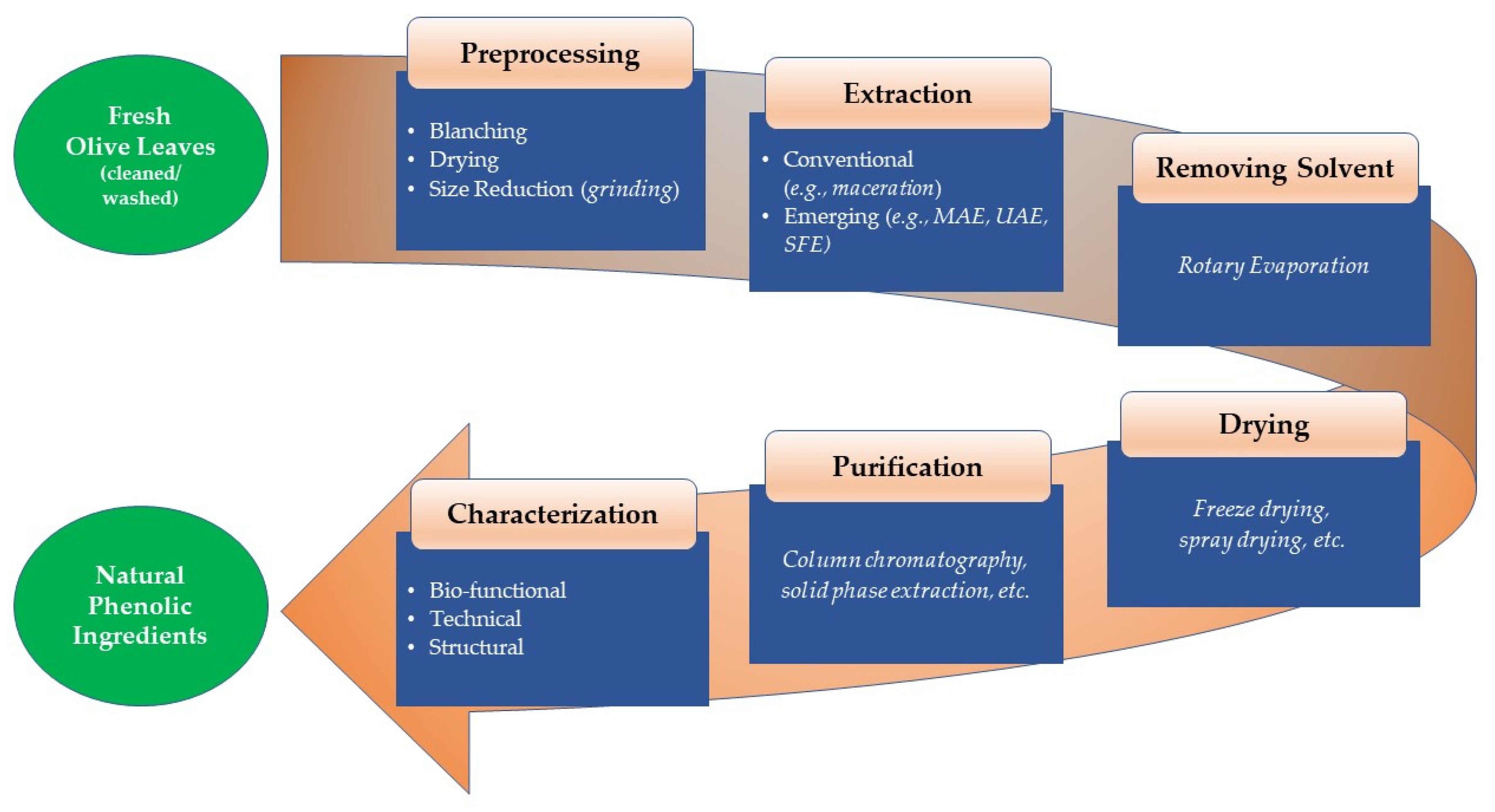
| Main Processing Factors for Phenolic Extraction | Drying & Size Reduction Prior to Extraction | Key Finding(s) | Reference |
|---|---|---|---|
| Leaves (pre-blanched) assessed as follows: - Optimization via a single-stage extraction (particle size was among the key independent variables) - Further optimized via multistage extraction system (compared to conventional method 40 °C, 48 h) | Drying: oven-dried with an air tray oven (60 °C, 4 h). Size reduction: dry ground and sieved through 0.05, 0.1, 0.2, 0.315, and 1.0 mm. | - Single-stage extraction: Optimized conditions include 0.315 mm particle size, 70% ethanolic extraction, solid-liquid ratio of 1:7 - Multi-step extraction: Optimization with three stages (30 min, 85 °C) improved TPC (166.6 mg/g); Oleuropein (103.1 mg/g); luteolin-7-O-glucoside (33.7 mg/g); verbascoside (16.0 mg/g); apigenin-7-O-glucoside (13.8 mg/g). - Multistage extraction enabled a 10-fold higher antioxidant activity compared to conventional extraction. | [37] |
| Steam blanching and hot water blanching (blanching time and particle size of fresh leaves accounted for the key parameters through optimization of blanching) | - For blanching optimization: particle size of fresh leaves ranged: above 20 mm, 20–11 mm, and 3–1 mm. - For extraction: leaves (optimally blanched), air dried (60 °C for 4 h), and ground to 1 mm. | Optimized steam blanching (10 min, 20–11 mm particle size) improved oleuropein extraction (8.28 g/kg leaves d.w.), and antioxidant effects (4 to 13-fold increase, compared to those obtained from non-blanched ones). | [38] |
| Extraction solvents (methanol, ethanol, water, and acetone) | Drying: dried at room temperature in the dark. Size reduction: dry ground to pass through a 20-mesh screen. | - Leaves extracted with 80% methanol exhibited higher TPC (392 mg GAE/g extract); total flavonoids (71 mg rutin equivalent/g); total tannins (18 mg GAE/g). - Leaves extracted with ethanol (80%) exhibited DPPH antiradical activity (IC50 = 1082.35 µg/mL). Total antioxidant activity (via linoleic acid system) was 76.36% with 2400 µg/mL extract. | [39] |
| Combining supercritical fluid extraction (with CO2) and pressurized liquid extraction (PLE) | Drying: dried in the shade (ventilated). Size reduction: dry ground to 3 mm particle size. | Oleuropein reached 10.44%, 9.5%, and 9.9%, with DPPH scavenging effects of 127.3, 145.3, and 138.6 µg/mL in defatted residues, using water (150 °C), ethanol (60%, 50 °C), and water (50 °C), respectively. | [40] |
| Freezing (conventional and liquid nitrogen) and drying (hot air drying and freeze drying) techniques | Drying: hot air-dried (70 °C for 50 min, 120 °C for 12 min) and freeze-dried. Size reduction: dry ground to 0.05 mm particle size. | Using hot air drying (120 °C): - Increased phenolics particularly oleuropein (108.6 mg/g d.w.). - Antioxidant capacity via ferric reducing antioxidant power (FRAP) reached 109 mg Trolox equivalents (TE)/g d.w. | [16] |
| Optimization of MAE compared to conventional and UAE | Drying: dried at ventilated room temperature. Size reduction: dry ground to pass through a 60-mesh. | Competitive effectiveness of MAE (5 min, 50% ethanol) in increasing TPC (76.6 mg GAE/g), and flavonoids (5.8 mg quercetin equivalent/g extract). | [41] |
| Hybrid extraction protocol (conventional ethanol extraction subsequent with supercritical fluid antisolvent extraction) | Olive leaves with 8% moisture content ground at room temperature to 1 mm particle size. | Concentrated yield of oleuropein powder reached up to 36% (35 °C, 150 bar). | [42] |
| Optimization of aqueous extraction using water | Drying: dried at 120 °C for 90 min. Size reduction: dry ground to 0.1 mm. | Maximum TPC (32.4 mg GAE/g) yielded through extraction at 90 °C for 70 min, solid/solvent ratio of 1:60 g/mL Antioxidant capacity, using DPPH and FRAP, reached 85.26 and 91.03 mg TE/g, respectively. | [43] |
| Optimization of UAE | Drying: air-dried at 40 °C Size reduction: dry ground to a 0.5 mm | Increased yield of oleuropein (10.65%) using 50% acetone, 60 °C, 10 min. | [44] |
| Extraction methods (solvent extraction, UAE, and reduced pressure extraction) | Drying: dried at ambient temperature (no exposure to solar radiation). Size reduction: ground with a high-speed crusher to pass through a 40–60 mesh. | Increased oleuropein via combined UAE and reduced pressure extraction (92.3% extraction efficiency in a single run). | [45] |
| Olive leaves (dried and fresh) from different cultivars | Drying: freeze-dried Size reduction: ground to 0.1 mm | - TPC ranged 7.72–24.65 and 2.09–8.44 mg GAE/g in dried and fresh leaves, respectively. - Effective in inhibiting proliferation of human carcinoma cell line (e.g., freeze dried leaves ranged from 0.07 to 2.40 µg phenolic constituents/well). | [26] |
| - Extraction methods (MAE, Soxhlet) - Extraction solvents | Drying: open air-dried in the dark. Size reduction: ground and sieved (<2 mm) | Higher TPC (76.1 mg GAE/g), and antioxidant activity (78.0 mg TE/g) in Soxhlet extracted leaves (50% ethanol). Oleuropein was the key component. MAE was comparably effective. | [46] |
| Extraction methods (MAE, UAE, maceration) | Drying: oven-dried (24 h, 40 °C). Size reduction: ground to pass through a 60-mesh. | MAE extracts (86 °C, 3 min) exhibited higher TPC (104.22 mg GAE/g), with 90.03% antioxidant activity. | [47] |
| - Preprocessing leaves: drying, non-drying (fresh leaves) - Solvent variations | Drying: freeze-dried (−50 °C, 36 h, 0.08 mbar); hot air oven dried (120 °C, 8 min). Moisture content < 1%. Grinding: milled using a blender | - Hot air-dried leaves extracted by 30% ethanol exhibited highest TPC (151 mg/g d.w.), with DPPH-scavenging activity of 922 µmol TE/g. - The use of water (100%) comparably effects on increased TPC (144 mg/g) of hot air-dried leaves. | [48] |
| - Successive extraction techniques - Samples: Olive mill leaves and collected leaves from olive trees | Drying: air-dried Size reduction: ground to 1 mm particle size | - TPC in extracts from olive mill leaves: 4476–6167 mg GAE/100 g. - Extracts from Olive tree leaves (UAE prior to alkaline extraction) contained TPC around 13,108 mg GAE/100 g; oleuropein (12,694 mg/100 g); luteolin 7-O-glucoside 903 mg/100 g; with antioxidant efficiency of 59,651 µmol TE/100 g - Highest concentration of oleuropein in olive mill leaves was 1790 mg/100 g extract. | [49] |
| Optimization of UAE extraction | Dried leaves were ground to 0.9−2.0 mm prior to extraction | - Extraction with 43.61% ethanol, 34.18 °C, 59 min exhibited increased TPC (43.825 mg GAE/g dried leaves). - Total flavonoids (31.992 mg catechin equivalents/g dried leaves) through 70% ethanol, 34.44 °C, 60 min. - DPPH inhibiting capacity ranged 89.3%–90.5% | [50] |
| - Extraction solvents (ethanol, methanol, acetone, and water) -Extraction methods (MAE and maceration) | Drying: dried in the shade Size reduction: ground to pass through a 60-mesh size screen | TPC using ethanol (50%) represented 88.298 and 69.027 mg GAE/g extract d.w. via MAE and maceration, respectively. | [51] |
| Pressurized liquid extraction using water and ethanol | Drying: dried at ambient condition (not exposed to solar radiation) for about 50 days (depending on relative humidity). Size reduction: cryogenically ground using liquid nitrogen. | - TPC yielded 58.7 and 45.8 mg GAE/g, using water (200 °C) and ethanol (150 °C), respectively. - Through water extraction, hydroxytyrosol was the principal phenolic component (up to 8.542 mg/g extract). Through ethanol extraction, oleuropein was the principal component (up to 6.156 mg/g extract). - Extraction with water (200 °C), and ethanol (150 °C) showed effective DPPH scavenging activities (EC50 = 18.6 and 27.4 µg/mL, respectively). | [52] |
| Solvent extraction (80% methanol) | Dried/micronized olive leaves (commercial powders) | - Extraction enabled TPC up to 131.7 mg GAE/g leaves d.w.), total flavonoids with 19.4 mg quercetin equivalents/g, and oleuropein 25.5 mg/g d.w. - Antioxidant effects: 281.8 mg TE/g, and EC50 13.8 µg/mL using FRAP and DPPH, respectively. | [53] |
| Effect of drying on supercritical extracts | Drying: conveyer belt dryer (air temperatures range: 50, 60 and 70 °C; residence time: 180, 120 and 60 min). Size reduction: ground with a knife mill for 5 min, and sieved (274 µm particle mean diameter). | Drying at 60 °C for 120 min presented higher TPC (36.1 mg GAE/g d.w.) in supercritical extracts, with 73% DPPH inhibiting activity, EC50 = 1.1 µg/mL | [54] |
| - Microencapsulation of olive leaves - Frying methods: starch gluten fried dough added with microencapsulated leaves | Drying: pre-blanched leaves dried in force air oven (at 45 °C for 18 h). Grinding device: windmilled. | - Olive leave extract: TPC was 25.7 mg GAE/mL extract; oleuropein was 28.4 mg/mL extract: EC50 = 0.15 mg GAE/mL extract (DPPH) and 109 µmol TE/mL extract (FRAP). - Highest TPC in atmospheric fried dough containing microencapsulated leaves. | [55] |
| Olive leaf extract (80% ethanol) and fractions | Drying: dried at 40 ± 5 °C for 6 h Size reduction: ground to pass through a 20–30 mesh | - Ethanolic extract (80%) contained TPC (148 mg/g); total flavonoids (58 mg naringin equivalents/g); oleuropein (the main phenol, 102.11 mg/100 g). Rutin, vanillin, and caffeic acid (minor phenols) represented 1.38, 0.66, and 0.31 mg/100 g, respectively. - Among the fractions: butanol fraction showed greatest antioxidant activity with highest TPC (175 mg/g), and flavonoids (75 mg/g). | [56] |
| Optimization via UAE extraction | Drying: air-dried at 25 °C for 7 days. Size reduction: coarsely ground using mortar and pestle. | Compared to maceration, oleuropein increased (30%) with UAE (70% ethanol, 25 °C, 2 h, solid: solvent ratio of 1:5). | [57] |
| Extraction kinetics and temperature with UAE and conventional | Dried in a tunnel microwave dryer (70 °C, 1200 W, 10 min) and ground prior to extraction. | - Oleuropein, TPC, and antioxidant capacity increased with the rise of temperature (through both UAE and conventional). - Oleuropein ranged from 6.48 to 6.65 g/100 g d.w.) through UAE that enabled 88% oleuropein extraction in the 1st min. - Using UAE at low temperature (10 °C) competitively exhibited higher oleuropein (5.71 g/100 g d.w.) in 10 min, compared to the conventional (5.15 g/100 g d.w.). | [58] |
| Drying of aqueous extracts (freeze-drying and spray-drying) | Leaves (after being washed) kept in the shade (48 h), and ground (80-mesh screen). | - Freeze-dried extracts: TPC (446.63 mg GAE/g d.w.), total flavonoids (298.16 mg quercetin/g), tannins (117.32 mg GAE/g), with 96.57% antioxidant activity. - Spray-dried extracts: TPC (442.84 mg GAE/g d.w.), flavonoids (396.4 mg quercetin/g), tannins (128.71 mg GAE/g), with 96.05% antioxidant activity. | [25] |
| Optimization of extraction conditions including drying methods and solvent types/ratio | Drying methods: shade-drying; microwave (2450 MHZ, 80 sec); and vacuum (– 0.5 bar, 55 °C, 24 h). Size reduced by grinding. | - Microwave drying of fresh leaves provided the highest TPC (6.45 g GAE/100 g dried leaves). - Favorable extraction conditions (40% ethanol 60 °C, 120 min) enabled high antioxidant activity (IC50 = 18.92 µg/mL), with a TPC around 6.63 g/100 g. | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safarzadeh Markhali, F. Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves. Processes 2021, 9, 1662. https://doi.org/10.3390/pr9091662
Safarzadeh Markhali F. Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves. Processes. 2021; 9(9):1662. https://doi.org/10.3390/pr9091662
Chicago/Turabian StyleSafarzadeh Markhali, Fereshteh. 2021. "Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves" Processes 9, no. 9: 1662. https://doi.org/10.3390/pr9091662
APA StyleSafarzadeh Markhali, F. (2021). Roles of Drying, Size Reduction, and Blanching in Sustainable Extraction of Phenolics from Olive Leaves. Processes, 9(9), 1662. https://doi.org/10.3390/pr9091662






