Introducing Oxygen Vacancies in Li4Ti5O12 via Hydrogen Reduction for High-Power Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Modification of LTO
2.2. Material Characterization
2.3. Electrochemical Measurement
2.4. Density Functional Theory (DFT) Calculations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gangaja, B.; Nair, S.; Santhanagopalan, D. Surface-Engineered Li4Ti5O12 Nanostructures for High-Power Li-Ion Batteries. Nano-Micro Lett. 2020, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Muhetaer, A.; Li, J.; Wang, F.; Liu, C.; Li, Q.; Xu, D. Ultrathin Li4Ti5O12 Nanosheet Based Hierarchical Microspheres for High-Rate and Long-Cycle Life Li-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700950. [Google Scholar] [CrossRef]
- Lee, S.H.; Huang, C.; Grant, P.S. Multi-layered composite electrodes of high power Li4Ti5O12 and high capacity SnO2 for smart lithium ion storage. Energy Storage Mater. 2021, 38, 70–79. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, S.; Liu, J.; Niu, X.; Xiang, Y.; Li, T.; Chen, J.S. Highly Efficient Na+ Storage in Uniform Thorn Ball-Like α-MnSe/C Nanospheres. Acta Metall. Sin. Engl. Lett. 2021, 34, 373–382. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.; Gao, M.; Sun, X.; Zhang, J.; Liang, L.; Liu, Y.; Hou, L.; Yuan, C. Solid-state template-free fabrication of uniform Mo2C microflowers with lithium storage towards Li-ion batteries. Chin. Chem. Lett. 2020, 31, 1670–1673. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Tao, Y.; Li, Y.; Feng, J. Scalable construction of SiO/wrinkled MXene composite by a simple electrostatic self-assembly strategy as anode for high-energy lithium-ion batteries. Chin. Chem. Lett. 2020, 31, 980–983. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, H.; Fan, Y.; Ju, G.; Zhao, H.; Fang, J.; Zhang, J.; Xu, J. A novel Mo-based oxide β-SnMoO4 as anode for lithium ion battery. Chin. Chem. Lett. 2020, 31, 210–216. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Hou, M.; Fan, L.; Wang, Y.; Xia, Y. Carbon-coated Li4Ti5O12 nanoparticles with high electrochemical performance as anode material in sodium-ion batteries. J. Mater. Chem. A 2017, 5, 10902–10908. [Google Scholar] [CrossRef]
- Piffet, C.; Vertruyen, B.; Caes, S.; Thomassin, J.-M.; Broze, G.; Malherbe, C.; Boschini, F.; Cloots, R.; Mahmoud, A. Aqueous processing of flexible, free-standing Li4Ti5O12 electrodes for Li-ion batteries. Chem. Eng. J. 2020, 397, 125508. [Google Scholar] [CrossRef]
- Qi, S.; He, J.; Liu, J.; Wang, H.; Wu, M.; Li, F.; Wu, D.; Li, X.; Ma, J. Phosphonium Bromides Regulating Solid Electrolyte Interphase Components and Optimizing Solvation Sheath Structure for Suppressing Lithium Dendrite Growth. Adv. Funct. Mater. 2020, 31, 2009013. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Shan, Z.; Xia, D.; Na, R.; Liu, H.; Wang, B.; Tian, J. Nitrogen, sulfur Co-doped porous graphene boosting Li4Ti5O12 anode performance for high-rate and long-life lithium ion batteries. Energy Storage Mater. 2020, 27, 387–395. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Lin, J.; Yang, J.; Wu, F.; Li, L.; Chen, R. Structural and electrochemical characteristics of hierarchical Li4Ti5O12 as high-rate anode material for lithium-ion batteries. Electrochim. Acta 2021, 368, 137470. [Google Scholar] [CrossRef]
- Wang, R.; Cao, X.; Zhao, D.; Zhu, L.; Xie, L.; Li, J.; Miao, Y. Enhancing Lithium Storage Performances of the Li4Ti5O12 Anode by Introducing the CuV2O6 Phase. ACS Appl. Mater. Interfaces 2020, 12, 39170–39180. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tan, X.; Chen, T.; Chen, S.; Zhang, S. Fast Li-ion transport and uniform Li-ion flux enabled by a double–layered polymer electrolyte for high performance Li metal battery. Energy Storage Mater. 2020, 32, 55–64. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Tian, G.; Zhang, Q.; Knapp, M.; Ehrenberg, H.; Chen, G.; Shen, Z.; Yang, G.; Gu, L.; et al. Lithium lanthanum titanate perovskite as an anode for lithium ion batteries. Nat. Commun. 2020, 11, 3490. [Google Scholar] [CrossRef]
- Gong, S.H.; Lee, J.H.; Chun, D.W.; Bae, J.-H.; Kim, S.-C.; Yu, S.; Nahm, S.; Kim, H.-S. Effects of Cr doping on structural and electrochemical properties of Li4Ti5O12 nanostructure for sodium-ion battery anode. J. Energy. Chem. 2021, 59, 465–472. [Google Scholar] [CrossRef]
- Zhang, Q.; Verde, M.G.; Seo, J.K.; Li, X.; Meng, Y.S. Structural and electrochemical properties of Gd-doped Li4Ti5O12 as anode material with improved rate capability for lithium-ion batteries. J. Power Sources 2015, 280, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, C.; Li, B.; Jiang, D.; Kang, S.; Li, X.; Wang, Y. Preparation and characterization of W-doped Li4Ti5O12 anode material for enhancing the high rate performance. Electrochim. Acta 2013, 107, 139–146. [Google Scholar] [CrossRef]
- Zhao, F.; Xue, P.; Ge, H.; Li, L.; Wang, B. Na-Doped Li4Ti5O12 as an Anode Material for Sodium-Ion Battery with Superior Rate and Cycling Performance. J. Electrochem. Soc. 2016, 163, A690–A695. [Google Scholar] [CrossRef]
- Liang, K.; He, H.; Ren, Y.; Luan, J.; Wang, H.; Ren, Y.; Huang, X. Ti3+ self-doped Li4Ti5O12 with rich oxygen vacancies for advanced lithium-ion batteries. Ionics 2020, 26, 1739–1747. [Google Scholar] [CrossRef]
- Lyu, P.; Zhu, J.; Han, C.; Qiang, L.; Zhang, L.; Mei, B.; He, J.; Liu, X.; Bian, Z.; Li, H. Self-Driven Reactive Oxygen Species Generation via Interfacial Oxygen Vacancies on Carbon-Coated TiO2-x with Versatile Applications. ACS Appl. Mater. Interfaces 2021, 13, 2033–2043. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Joo, J.H.; Samuelis, D.; Maier, J. Oxygen-Deficient TiO2−δ Nanoparticles via Hydrogen Reduction for High Rate Capability Lithium Batteries. Chem. Mater. 2012, 24, 543–551. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect Engineering of Oxygen-Deficient Manganese Oxide to Achieve High-Performing Aqueous Zinc Ion Battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Xu, H.; Sun, S.; Xu, Y.; Zhou, M.; Gao, X.; Sun, Z. Plasma-Introduced Oxygen Defects Confined in Li4Ti5O12 Nanosheets for Boosting Lithium-Ion Diffusion. ACS Appl. Mater. Interfaces 2019, 11, 17384–17392. [Google Scholar] [CrossRef]
- Dong, C.; Dong, W.; Lin, X.; Zhao, Y.; Li, R.; Huang, F. Recent progress and perspectives of defective oxide anode materials for advanced lithium ion battery. EnergyChem 2020, 2, 100045. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, R.; Fang, Y.; Zhang, P. Three-Dimensional Oxygen-Deficient Li4Ti5O12 Nanospheres as High-Performance Anode for Lithium Ion Batteries. Electrochim. Acta 2016, 211, 1041–1047. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, J.; Hang, X.; Zhang, X.; Xie, J.; Pan, B.; Xie, Y. Half-metallicity in single-layered manganese dioxide nanosheets by defect engineering. Angew. Chem. Int. Edit. 2015, 54, 1195–1199. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Jiang, J.; Liu, J.; Deng, J.; Ping, X.; Wei, Z. Manipulating the surface composition of Pt-Ru bimetallic nanoparticles to control the methanol oxidation reaction pathway. Chem. Commun. 2020, 56, 2419–2422. [Google Scholar] [CrossRef]
- Wang, A.; Cao, Z.; Wang, J.; Wang, S.; Li, C.; Li, N.; Xie, L.; Xiang, Y.; Li, T.; Niu, X.; et al. Vacancy defect modulation in hot-casted NiO film for efficient inverted planar perovskite solar cells. J. Energy. Chem. 2020, 48, 426–434. [Google Scholar] [CrossRef]
- Ye, J.; Zhai, X.; Chen, L.; Guo, W.; Gu, T.; Shi, Y.; Hou, J.; Han, F.; Liu, Y.; Fan, C.; et al. Oxygen vacancies enriched nickel cobalt based nanoflower cathodes: Mechanism and application of the enhanced energy storage. J. Energy. Chem. 2021, 62, 252–261. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, R.; Shao, Q.; Zhu, T.; Huang, X. Oxygen Vacancies in Amorphous InOx Nanoribbons Enhance CO2 Adsorption and Activation for CO2 Electroreduction. Angew. Chem. Int. Edit. 2019, 58, 5609–5613. [Google Scholar] [CrossRef]
- Li, J.; Shu, C.; Liu, C.; Chen, X.; Hu, A.; Long, J. Rationalizing the Effect of Oxygen Vacancy on Oxygen Electrocatalysis in Li-O2 Battery. Small 2020, 16, 2001812. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Liu, Z.; Zhang, Y.; Ma, C.; Deng, H.; Zhang, S.; Wang, S. Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single Fe-N4 Site. Adv. Mater. 2021, 33, 2003238. [Google Scholar] [CrossRef] [PubMed]
- Sadighi, Z.; Huang, J.; Qin, L.; Yao, S.; Cui, J.; Kim, J.-K. Positive role of oxygen vacancy in electrochemical performance of CoMn2O4 cathodes for Li-O2 batteries. J. Power Sources 2017, 365, 134–147. [Google Scholar] [CrossRef]
- Guo, M.; Chen, H.; Wang, S.; Dai, S.; Ding, L.-X.; Wang, H. TiN-coated micron-sized tantalum-doped Li4Ti5O12 with enhanced anodic performance for lithium-ion batteries. J. Alloy. Compd. 2016, 687, 746–753. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Chabot, V.; Gu, M.; Wang, C.; Xiao, X.; Chen, Z. Dual phase Li4Ti5O12–TiO2 nanowire arrays as integrated anodes for high-rate lithium-ion batteries. Nano Energy 2014, 9, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Huang, F.; Zhao, W.; Liu, Z.; Wan, D. Synthesis of graphene-supported Li4Ti5O12 nanosheets for high rate battery application. J. Mater. Chem. 2012, 22, 11257. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, P.; Yuan, T.; Ruan, J.; Peng, C.; Pang, Y.; Sun, H.; Yang, J.; Zheng, S. Molecular self-assembly of a nanorod N-Li4Ti5O12/TiO2/C anode for superior lithium ion storage. J. Mater. Chem. A 2018, 6, 15755–15761. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, X.; Wang, D.; Deng, T.; Wang, H.; Liu, X.; Shi, X.; Li, Z.; Chen, H.; Meng, X.; et al. Oxygen Vacancies Boost delta-Bi2O3 as a High-Performance Electrode for Rechargeable Aqueous Batteries. ACS Appl. Mater. Interfaces 2019, 11, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Hou, Y.; Fan, Y.; Zhai, Y.; Wang, Y.; Sun, Z.; Fan, R.; Dang, F.; Wang, J. Oxygen vacancy derived local build-in electric field in mesoporous hollow Co3O4microspheres promotes high-performance Li-ion batteries. J. Mater. Chem. A 2018, 6, 6967–6976. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Quan, W.; Hong, Y.; Zhang, Z.; Tang, Z.; Li, J. Ti3+-free three-phase Li4Ti5O12/TiO2 for high-rate lithium ion batteries: Capacity and conductivity enhancement by phase boundaries. Nano Energy 2017, 32, 294–301. [Google Scholar] [CrossRef]
- Xu, G.; Tian, Y.; Wei, X.; Yang, L.; Chu, P.K. Free-standing electrodes composed of carbon-coated Li4Ti5O12 nanosheets and reduced graphene oxide for advanced sodium ion batteries. J. Power Sources 2017, 337, 180–188. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Q.; Li, S.; Mao, J. High-temperature effect on electrochemical performance of Li4Ti5O12 based anode material for Li-ion batteries. J. Alloy. Compd. 2018, 753, 192–202. [Google Scholar] [CrossRef]
- Ma, J.; Wei, Y.; Gan, L.; Wang, C.; Xia, H.; Lv, W.; Li, J.; Li, B.; Yang, Q.-H.; Kang, F.; et al. Abundant grain boundaries activate highly efficient lithium ion transportation in high rate Li4Ti5O12 compact microspheres. J. Mater. Chem. A 2019, 7, 1168–1176. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.J.; Wu, M. Ultrathin Anatase TiO2 Nanosheets Embedded with TiO2-B Nanodomains for Lithium-Ion Storage: Capacity Enhancement by Phase Boundaries. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Chen, C.; Xu, H.; Zhou, T.; Guo, Z.; Chen, L.; Yan, M.; Mai, L.; Hu, P.; Cheng, S.; Huang, Y.; et al. Integrated Intercalation-Based and Interfacial Sodium Storage in Graphene-Wrapped Porous Li4Ti5O12Nanofibers Composite Aerogel. Adv. Energy Mater. 2016, 6, 1600322. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Li, X.; Tang, M.; Gan, A.; Hu, Y.-Y. Enhanced rate performance of Li4Ti5O12 anodes with bridged grain boundaries. J. Power Sources 2017, 354, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhao, S.-X.; Peng, H.; Lin, Y.-H.; Nan, C.-W.; Cao, G.-Z. Hierarchical porous Li4Ti5O12–TiO2 composite anode materials with pseudocapacitive effect for high-rate and low-temperature applications. J. Mater. Chem. A 2018, 6, 14339–14351. [Google Scholar] [CrossRef]
- Li, G.; Blake, G.R.; Palstra, T.T. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Li, Y.; Guo, X.; Shen, Y.; Wang, D.; Zhang, Y.; Wang, Z. Fabrication of Li4Ti5O12-TiO2 Nanosheets with Structural Defects as High-Rate and Long-Life Anodes for Lithium-Ion Batteries. Sci. Rep. 2017, 7, 2960. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhou, K.; Huang, Z.; Wei, M. Iso-Oriented Anatase TiO2 Mesocages as a High Performance Anode Material for Sodium-Ion Storage. Sci. Rep. 2015, 5, 11960. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Z.; Liu, J.; Song, Y.; Li, T.; Xiang, Y.; Chen, J.S.; Yan, Q. SeC Bonding Promoting Fast and Durable Na+ Storage in Yolk-Shell SnSe2 @SeC. Small 2020, 16, 2002486. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, M.; Zhang, C.; Wang, C.; Liang, L.; Fang, Y.; Wu, M.; Cheng, L.; Lei, Y. Oxygen vacancies: Effective strategy to boost sodium storage of amorphous electrode materials. Nano Energy 2017, 38, 304–312. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with Expanded Interlayer Distance as High-Rate Lithium/Sodium-Ion Battery Anode Materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef]
- Deng, X.; Wei, Z.; Cui, C.; Liu, Q.; Wang, C.; Ma, J. Oxygen-deficient anatase TiO2@C nanospindles with pseudocapacitive contribution for enhancing lithium storage. J. Mater. Chem. A 2018, 6, 4013–4022. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Yi, T.-F.; Fang, Z.-K.; Deng, L.; Wang, L.; Xie, Y.; Zhu, Y.-R.; Yao, J.-H.; Dai, C. Enhanced electrochemical performance of a novel Li4Ti5O12 composite as anode material for lithium-ion battery in a broad voltage window. Ceram. Int. 2015, 41, 2336–2341. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Tang, L.; He, Y.-B.; Gan, L.; Li, J.; Du, H.; Li, B.; Lin, Z.; Kang, F. A robust strategy for crafting monodisperse Li4Ti5O12 nanospheres as superior rate anode for lithium ion batteries. Nano Energy 2016, 21, 133–144. [Google Scholar] [CrossRef]
- Ge, H.; Cui, L.; Zhang, B.; Ma, T.-Y.; Song, X.-M. Ag quantum dots promoted Li4Ti5O12/TiO2 nanosheets with ultrahigh reversible capacity and super rate performance for power lithium-ion batteries. J. Mater. Chem. A 2016, 4, 16886–16895. [Google Scholar] [CrossRef]
- Yuan, T.; Yu, X.; Cai, R.; Zhou, Y.; Shao, Z. Synthesis of pristine and carbon-coated Li4Ti5O12 and their low-temperature electrochemical performance. J. Power Sources 2010, 195, 4997–5004. [Google Scholar] [CrossRef]
- Wang, D.; Shan, Z.; Na, R.; Huang, W.; Tian, J. Solvothermal synthesis of hedgehog-like mesoporous rutile TiO2 with improved lithium storage properties. J. Power Sources 2017, 337, 11–17. [Google Scholar] [CrossRef]
- Wang, X.; Hao, H.; Liu, J.; Huang, T.; Yu, A. A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries. Electrochim. Acta 2011, 56, 4065–4069. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Tan, S.; Feng, H.; Cheng, Z.; Zhao, J.; Zhao, A.; Wang, B.; Luo, Y.; Yang, J.; et al. Role of point defects on the reactivity of reconstructed anatase titanium dioxide (001) surface. Nat. Commun. 2013, 4, 2214. [Google Scholar] [CrossRef] [PubMed]
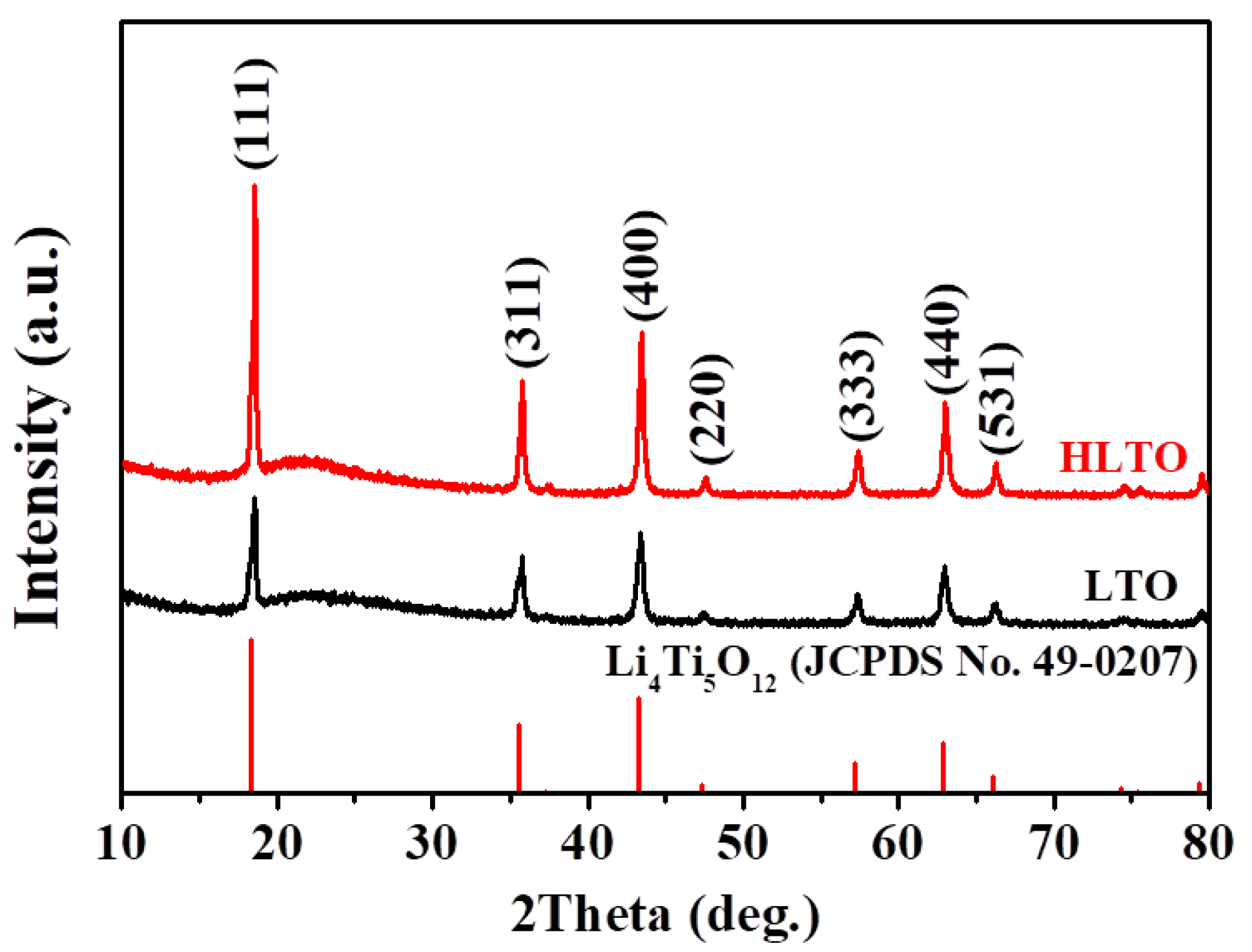

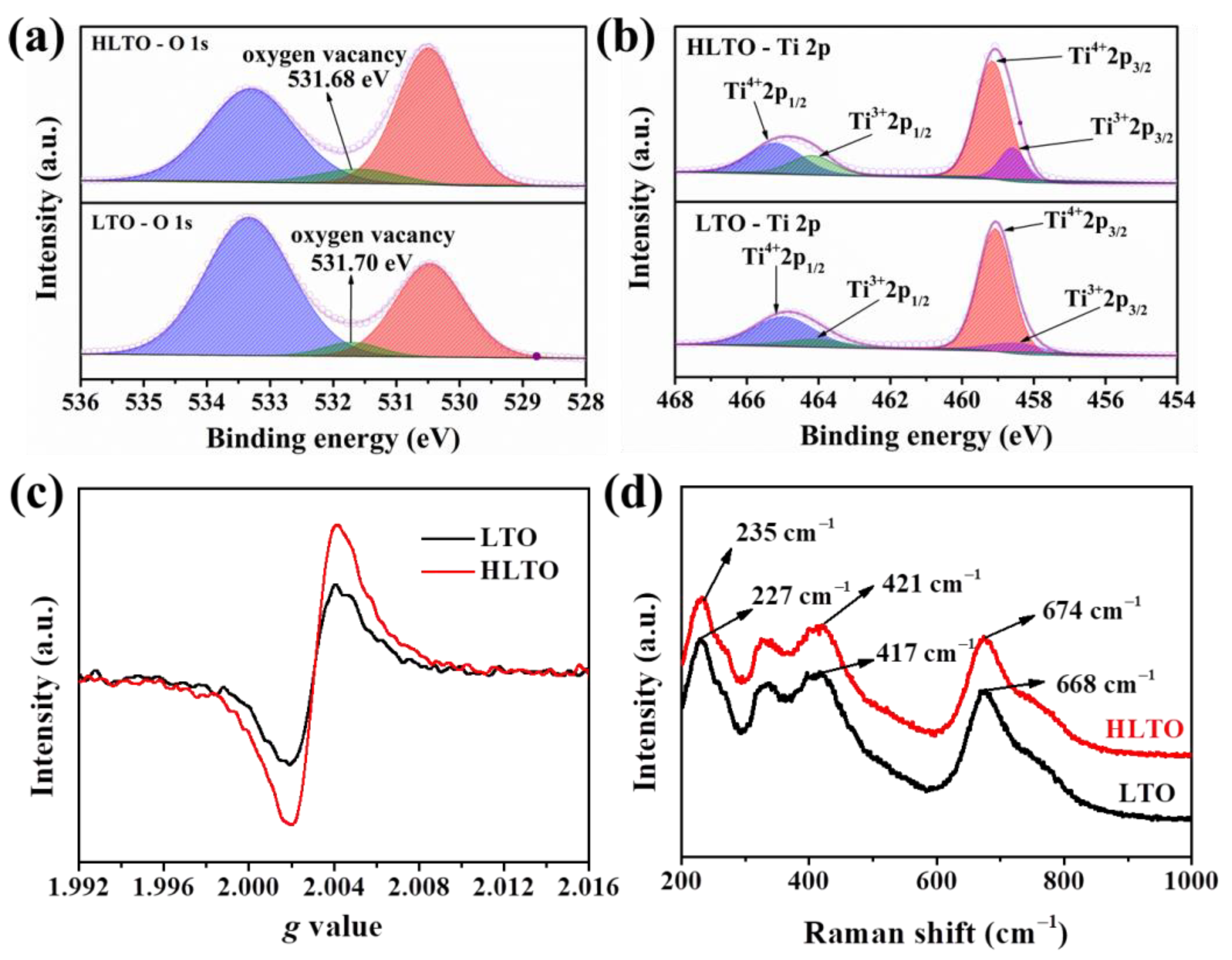
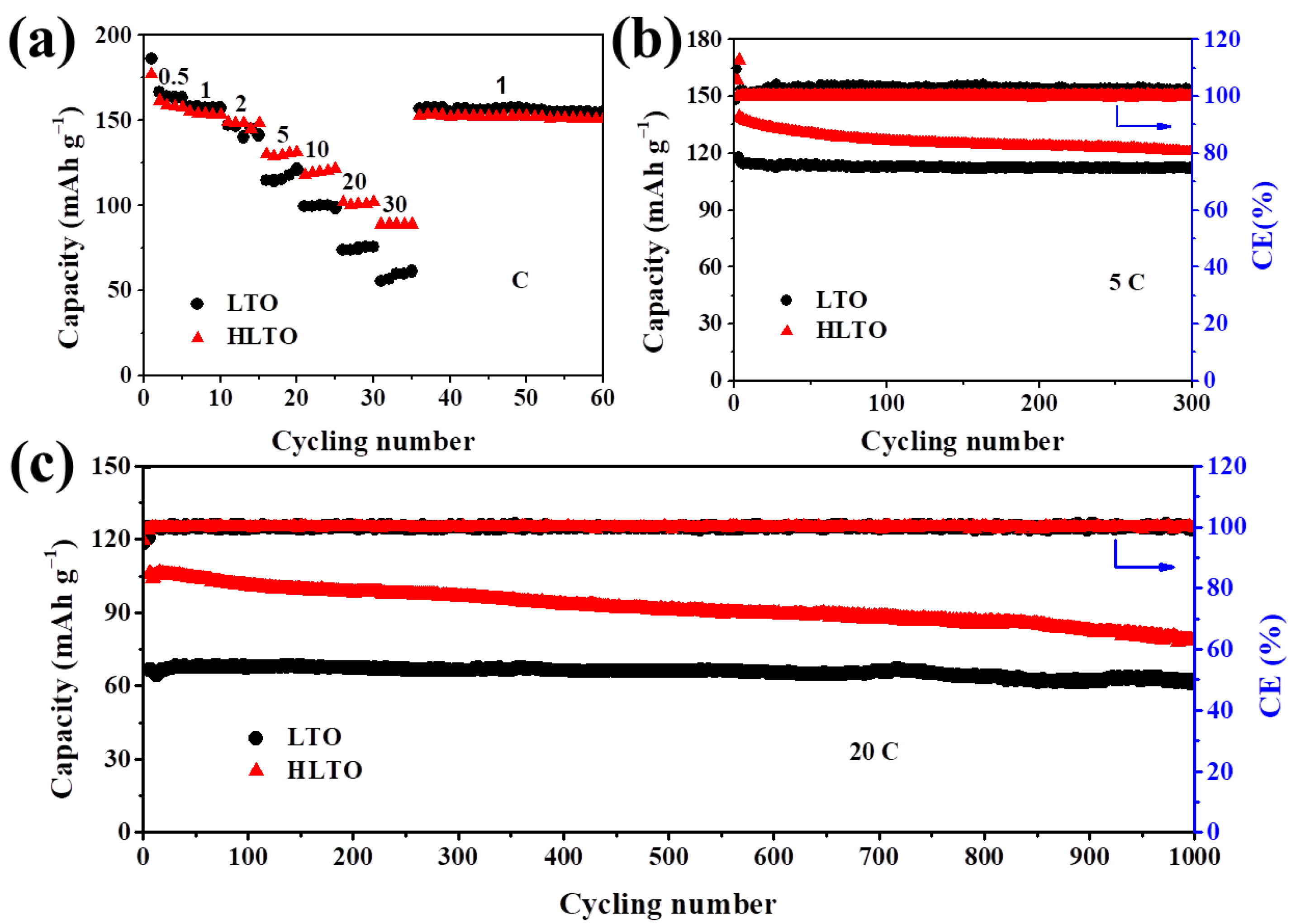


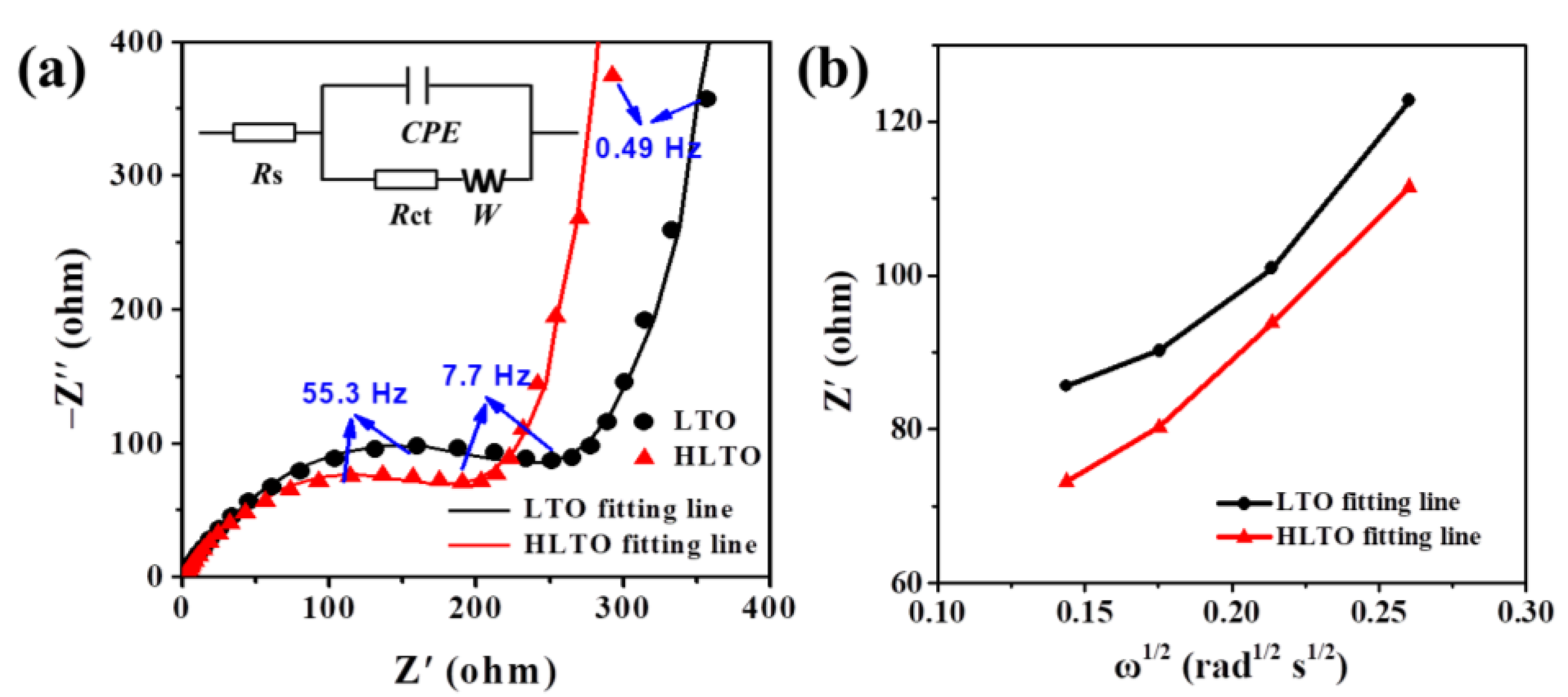

| Electrodes | Rs (Ω cm−2) | Rct (Ω cm−2) | σ | DLi+ (cm2 s−1) |
|---|---|---|---|---|
| LTO | 1.48 | 314.9 | 373.73 | 3.29 × 10−17 |
| HLTO | 0.87 | 254.3 | 297.58 | 5.18 × 10−17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Xiao, S.; Li, Z.; Li, X.; Liu, J.; Wu, R.; Chen, J. Introducing Oxygen Vacancies in Li4Ti5O12 via Hydrogen Reduction for High-Power Lithium-Ion Batteries. Processes 2021, 9, 1655. https://doi.org/10.3390/pr9091655
Zhou Y, Xiao S, Li Z, Li X, Liu J, Wu R, Chen J. Introducing Oxygen Vacancies in Li4Ti5O12 via Hydrogen Reduction for High-Power Lithium-Ion Batteries. Processes. 2021; 9(9):1655. https://doi.org/10.3390/pr9091655
Chicago/Turabian StyleZhou, Yiguang, Shuhao Xiao, Zhenzhe Li, Xinyan Li, Jintao Liu, Rui Wu, and Junsong Chen. 2021. "Introducing Oxygen Vacancies in Li4Ti5O12 via Hydrogen Reduction for High-Power Lithium-Ion Batteries" Processes 9, no. 9: 1655. https://doi.org/10.3390/pr9091655
APA StyleZhou, Y., Xiao, S., Li, Z., Li, X., Liu, J., Wu, R., & Chen, J. (2021). Introducing Oxygen Vacancies in Li4Ti5O12 via Hydrogen Reduction for High-Power Lithium-Ion Batteries. Processes, 9(9), 1655. https://doi.org/10.3390/pr9091655








