A Combination Extract of Gardeniae Fructus and Perillae Folium Exerts Anti-Inflammatory Effects on LPS-Activated RAW 264.7 Mouse Macrophages via an ER Stress-Induced CHOP Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GP
2.3. Total Flavonoid Content of GP
2.4. Cell Culture
2.5. NO Production Measurement
2.6. Assay for Intracellular Calcium Release
2.7. Multiplex Cytokine Assay
2.8. Assay for mRNA Expressions of Inflammatory Genes
2.9. Assay for Phosphorylation of P38 MAPK
2.10. Statistics
3. Results
3.1. Determination of Total Flavonoid Content of GP
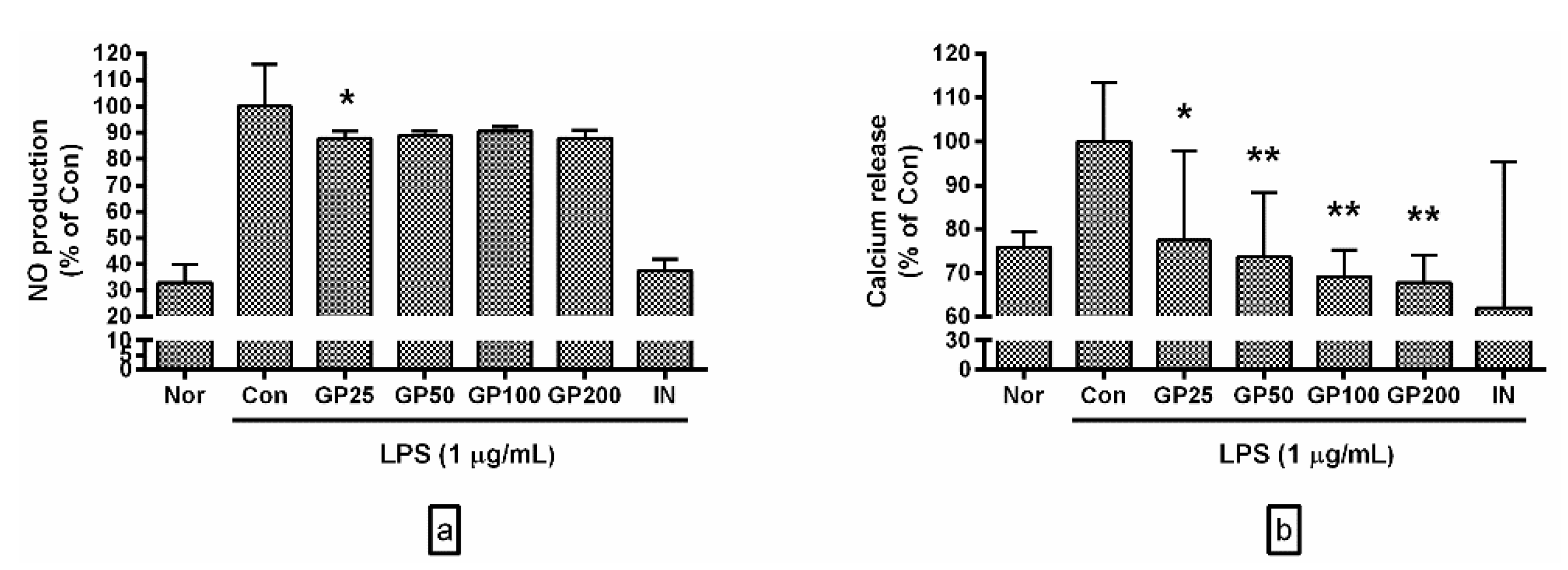
3.2. Effects of GP on Levels of NO and Ca2+
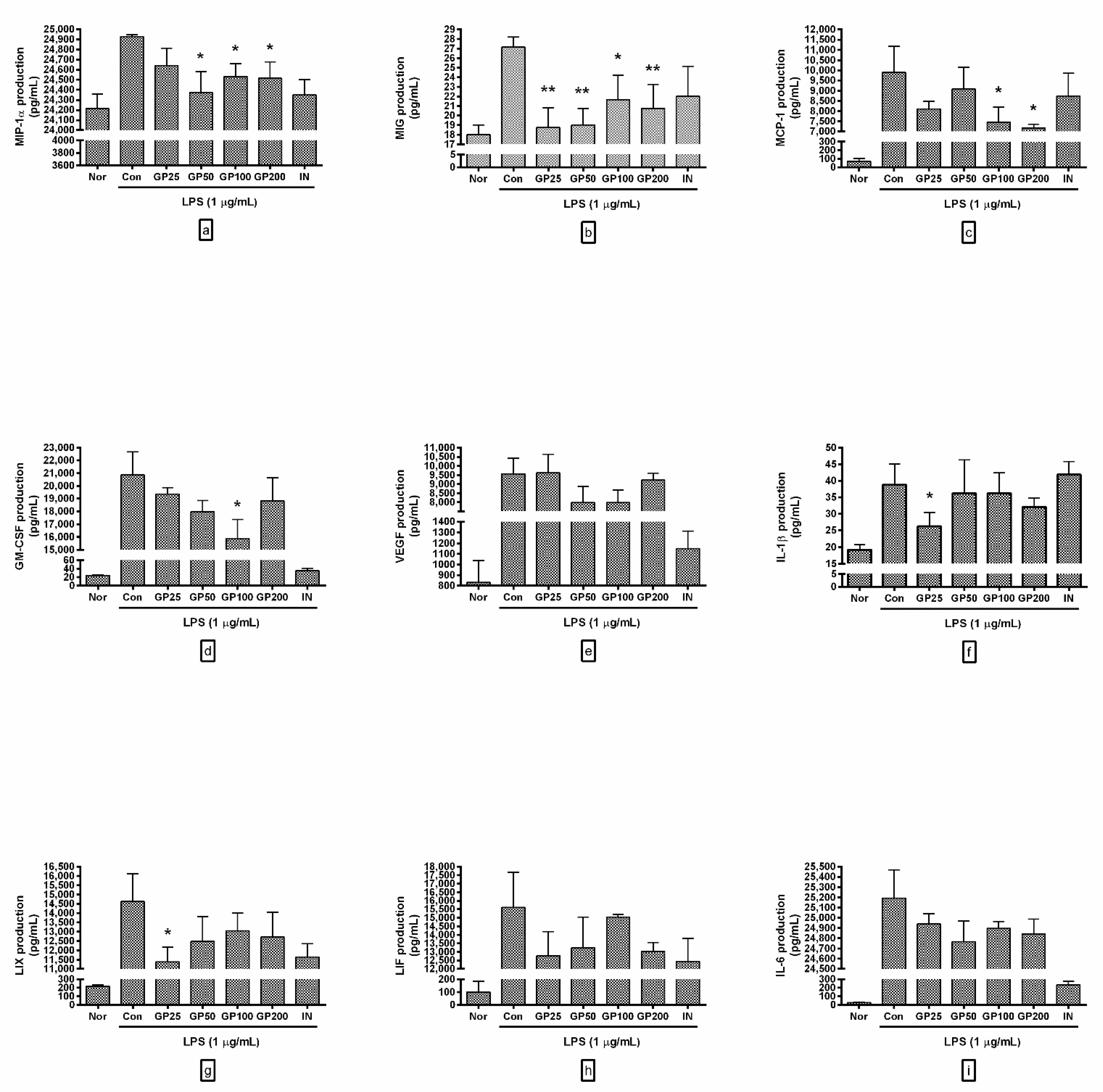
3.3. Effect of GP on Cytokine Production
- -
- MIP-1α productions from RAW 264.7 with GP at concentrations of 25, 50, 100, and 200 µg/mL were 98.84 ± 0.7%, 97.78 ± 0.85%, 98.41 ± 0.52%, and 98.34 ± 0.66% of the control group treated with LPS only;
- -
- MIG were 69.02 ± 7.59%, 69.94 ± 6.38%, 79.75 ± 9.44%, and 76.38 ± 9.2%;
- -
- MCP-1 were 81.91 ± 3.86%, 91.69 ± 10.81%, 75.36 ± 7.39%, and 72.45 ± 1.89%;
- -
- GM-CSF were 92.83 ± 2.62%, 86.17 ± 4.82%, 76.12 ± 9.42%, and 90.23 ± 9.65%;
- -
- VEGF were 100.61 ± 10.56%, 83.55 ± 10.99%, 83.44 ± 8.75%, and 96.4 ± 4.14%;
- -
- IL-1β were 67.65 ± 10.6%, 93.43 ± 25.89%, 93.43 ± 15.8%, and 82.8 ± 6.69%;
- -
- LIX were 77.78 ± 5.39%, 85.26 ± 9.17%, 89.21 ± 6.45%, and 86.92 ± 9%;
- -
- LIF were 81.82 ± 9.14%, 84.81 ± 11.59%, 96.35 ± 1.02%, and 83.56 ± 3.29%;
- -
- IL-6 were 99 ± 0.4%, 98.31 ± 0.82%, 98.83 ± 0.27%, and 98.61 ± 0.59%.
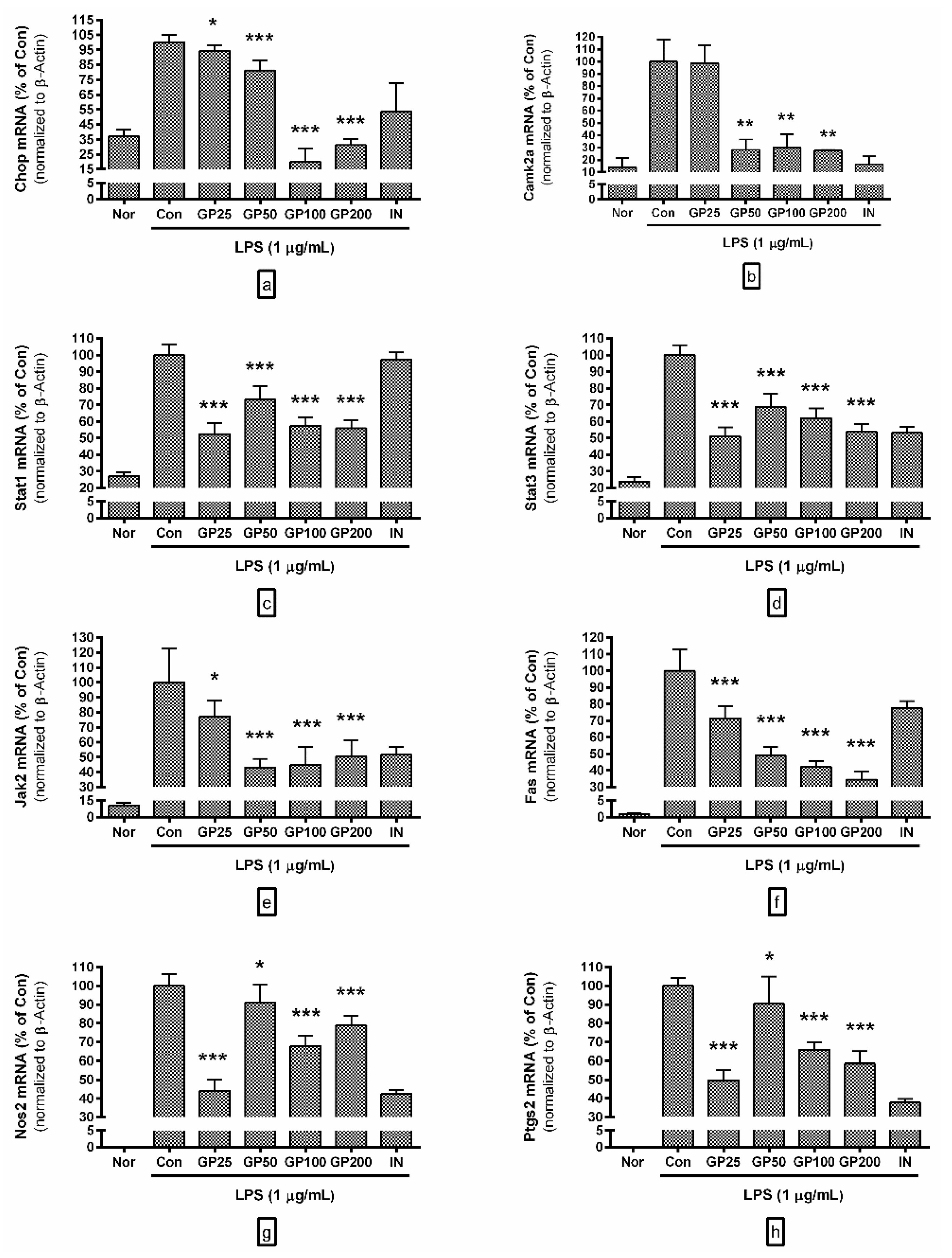
3.4. Effect of GP on mRNA Expressions of Inflammatory Genes
- -
- Chop mRNA levels in LPS-activated RAW 264.7 with GP at concentrations of 25, 50, 100, and 200 µg/mL were 94.24 ± 4%, 81.17 ± 6.88%, 19.87 ± 9.02%, and 31.12 ± 4.13% of the control group treated with LPS only;
- -
- Camk2a were 98.74 ± 14.5, 28.17 ± 8.37%, 30.13 ± 10.88%, and 27.62 ± 0.56%;
- -
- Stat1 were 52.52 ± 6.34, 73.17 ± 8.11%, 57.45 ± 5.04%, and 55.89 ± 4.94%;
- -
- Stat3 were 51.04 ± 5.49, 68.85 ± 7.68%, 62.08 ± 5.93%, and 53.83 ± 4.5%;
- -
- Jak2 were 77.22 ± 10.46, 43.18 ± 5.75%, 44.82 ± 12.07%, and 50.42 ± 10.86%;
- -
- Fas were 71.59 ± 7.23, 49.15 ± 4.99%, 42.11 ± 3.66%, and 34.57 ± 4.67%;
- -
- Nos2 were 44.11 ± 5.77, 91.15 ± 9.71%, 67.8 ± 5.61%, and 78.73 ± 5.26%;
- -
- Ptgs2 were 49.65 ± 5.36, 90.5 ± 14.47%, 65.88 ± 3.94%, and 58.52 ± 6.52%.
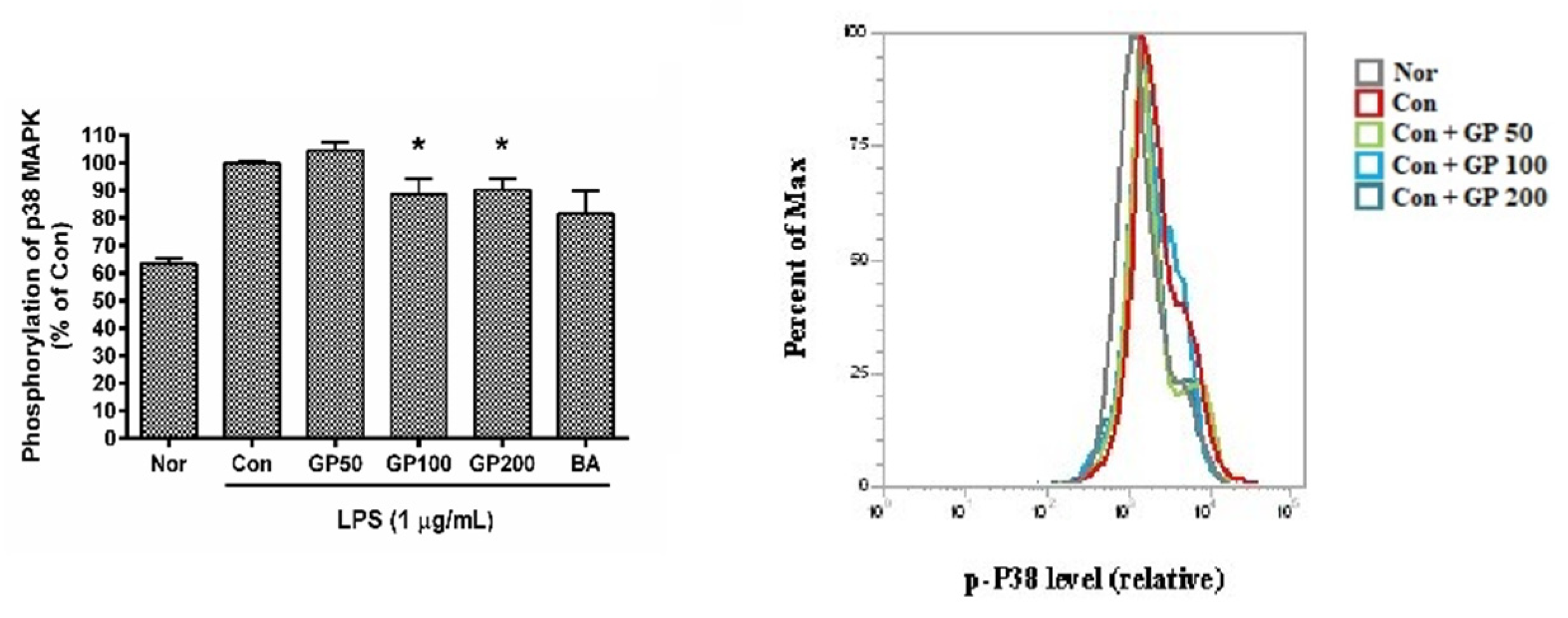
3.5. Effect of GP on Phosphorylation of P38 MAPK
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Si-Tahar, M.; Touqui, L.; Chignard, M. Innate immunity and inflammation—Two facets of the same anti-infectious reaction. Clin. Exp. Immunol. 2009, 156, 194–198. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [Green Version]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zahringer, U.; Seydel, U.; Di Padova, F. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol a inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-kappaB, Akt and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Y.M.; Yu, Y.; Dong, N.; Yin, H.N.; Sheng, Z.Y. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group Box protein 1 in rat peritoneal macrophages. Shock 2007, 27, 55–60. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.; Park, S.K.; Kim, H.; Bae, H.; Kim, H.M.; Ko, S.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-Inflammatory effects of scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.F.; Li, F.; Zhang, H.Y. Fructus Gardenia (Gardenia Jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013, 15, 94–110. [Google Scholar] [CrossRef]
- Cheng, S.; Zhou, F.; Xu, Y.; Liu, X.; Zhang, Y.; Gu, M.; Su, Z.; Zhao, D.; Zhang, L.; Jia, Y. Geniposide regulates the miR-101/MKP-1/p38 pathway and alleviates atherosclerosis inflammatory injury in ApoE(-/-) mice. Immunobiology 2019, 224, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Park, H.C.; So, Y.; Nam, B.; Han, S.N.; Kim, J.B. Comparison of the anti-inflammatory activities of supercritical carbon dioxide versus ethanol extracts from leaves of perilla frutescens britt. radiation mutant. Molecules 2017, 22, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.H.; Yoo, J.M.; Lee, E.; Lee, B.; Cho, W.K.; Park, K.I.; Yeul Ma, J. Anti-Inflammatory effects of perillae herba ethanolic extract against TNF-alpha/IFN-Gamma-Stimulated human keratinocyte HaCaT cells. J. Ethnopharmacol. 2018, 211, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Park, W. Rubi fructus water extract alleviates LPS-Stimulated macrophage activation via an ER stress-induced calcium/CHOP signaling pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory effects of angelica sinensis (Oliv.) diels water extract on RAW 264.7 induced with lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Hartley, M.A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell. Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef] [Green Version]
- Barsness, K.A.; Bensard, D.D.; Partrick, D.A.; Calkins, C.M.; Hendrickson, R.J.; McIntyre, R.C., Jr. Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J. Pediatr. Surg. 2004, 39, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Bradford, W.D.; Croker, B.P.; Tisher, C.C. Kidney lesions in rocky mountain spotted fever: A light-, immunofluorescence-, and electron-microscopic study. Am. J. Pathol. 1979, 97, 381–392. [Google Scholar] [PubMed]
- Jalbert, E.; Crawford, T.Q.; D’Antoni, M.L.; Keating, S.M.; Norris, P.J.; Nakamoto, B.K.; Seto, T.; Parikh, N.I.; Shikuma, C.M.; Ndhlovu, L.C.; et al. IL-1Beta enriched monocytes mount massive il-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS ONE 2013, 8, e75500. [Google Scholar] [CrossRef]
- Yuk, S.; Lim, E.; Lee, J.Y.; Lee, Y.; Kim, Y.; Lee, T.H.; Park, S.K.; Bae, H.; Kim, H.M.; Ko, S. Antiinflammatory effects of epimedium brevicornum water extract on lipopolysaccharide-activated RAW264.7 macrophages. Phytother. Res. 2010, 24, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, J.Y.; Han, H.S.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Kim, H.M.; Ko, S.G.; An, H.J.; Lee, Y.J.; et al. Immunomodulatory effects of liriope platyphylla water extract on lipopolysaccharide-activated mouse macrophage. Nutrients 2012, 4, 1887–1897. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, Y.; Li, M.; Wang, J.; Zhang, Y.; Du, Y.; Yang, Y. Study on mechanism of iridoid glycosides derivatives from fructus gardeniae in jiangxi province by network pharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 4062813. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Shen, L.; Yao, L.; Ma, Y.; Yu, D.; Chen, J.; Li, P.; Chen, Y.; Zhang, C. Isolation and purification of six iridoid glycosides from gardenia jasminoides fruit by medium-pressure liquid chromatography combined with macroporous resin chromatography. J. Sep. Sci. 2015, 38, 4119–4126. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, Y.; Li, L.; Li, W.; Yin, X. Contents of diterpenoid pigments in didifferent parts of fruit from gardenia jasminoides. Zhongguo Zhong Yao Za Zhi 2009, 34, 1395–1397. [Google Scholar] [PubMed]
- Zuo, Y.M.; Xu, Y.L.; Zhang, Z.L.; Liu, D.H.; Cai, M.T. Study on phenylpropanoids of gardenia jasminoides. Zhong Yao Cai 2015, 38, 2311–2313. [Google Scholar] [PubMed]
- Lu, D.; Zhang, W.; Jiang, Y.; Zhang, Y.; Pan, D.; Zhang, D.; Yao, X.; Yu, Y. Two new triterpenoids from gardenia jasminoides fruits. Nat. Prod. Res. 2019, 33, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.J.; Shu, Q.; Xu, B.Q.; Peng, L.M.; He, Y. Orthogonal test design for optimization of the extraction of flavonid from the fructus gardeniae. Biomed. Environ. Sci. 2011, 24, 688–693. [Google Scholar]
- Ruan, J.; Liu, L.; Shan, X.; Xia, B.; Fu, Q. Anti-Depressant effects of oil from fructus gardeniae via PKA-CREB-BDNF signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Shin, M.R.; Lee, A.R.; Seo, B.I.; Park, H.J.; Roh, S.S. Improvement of inflammation through antioxidant pathway of gardeniae fructus 50% EtOH Extract (GE) from acute reflux esophagitis rats. Biomed. Res. Int. 2020, 2020, 4826176. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yao, N.; Wang, S.; An, D.; Cao, K.; Wei, J.; Li, N.; Zhao, D.; Wang, L.; Chen, X.; et al. Fructus gardeniae-induced gastrointestinal injury was associated with the inflammatory response mediated by the disturbance of vitamin b6, phenylalanine, arachidonic acid, taurine and hypotaurine metabolism. J. Ethnopharmacol. 2019, 235, 47–55. [Google Scholar] [CrossRef]
- Li, C.; Lan, M.; Lv, J.; Zhang, Y.; Gao, X.; Gao, X.; Dong, L.; Luo, G.; Zhang, H.; Sun, J. Screening of the hepatotoxic components in fructus gardeniae and their effects on rat liver BRL-3A cells. Molecules 2019, 24, 3920. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Gao, F.; Chang, R.; Zhang, X.; Zhong, J.; Wen, J.; Wu, J.; Zhou, T. Metabolomics based comprehensive investigation of gardeniae fructus induced hepatotoxicity. Food Chem. Toxicol. 2021, 153, 112250. [Google Scholar] [CrossRef]
- Zhao, B.T.; Lee, K.R.; Lee, J.H.; Min, B.S.; Son, J.K.; Woo, M.H. Quality evaluation of perillae folium by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1521–1529. [Google Scholar] [CrossRef]
- Jiang, T.; Guo, K.; Liu, L.; Tian, W.; Xie, X.; Wen, S.; Wen, C. Integrated transcriptomic and metabolomic data reveal the flavonoid biosynthesis metabolic pathway in perilla frutescens (L.) leaves. Sci. Rep. 2020, 10, 16207. [Google Scholar] [CrossRef]
- Xu, L.; Ma, T.; Wang, X.; Liu, W.; Li, J.; Wang, X. Preparative separation of seven polyphenols from perillae folium via pH-zone-refining counter-current chromatography combined with high-speed counter-current chromatography. Anal. Methods 2021, 13, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Si, Y.; Wang, W.; Zhang, S.; Sun, S.; Liu, X.; Wang, R.; Wang, W. Isolation, characterization and xanthine oxidase inhibitory activities of flavonoids from the leaves of perilla frutescens. Nat. Prod. Res. 2020, 34, 2566–2572. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Higashihara, H.; Ukiya, M.; Watanabe, K.; Kimura, Y.; Hasegawa, J.; Nishino, H. Triterpene acids from the leaves of perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Sun, W.; Ma, P.; Yao, C.; Fan, Y.; Li, S.; Yuan, J.; Zhang, Z.; Li, X.; Lin, M.; et al. Multiple components rapidly screened from perilla leaves attenuate asthma airway inflammation by synergistic targeting on Syk. J. Inflamm. Res. 2020, 13, 897–911. [Google Scholar] [CrossRef]
- Bae, J.S.; Han, M.; Shin, H.S.; Kim, M.K.; Shin, C.Y.; Lee, D.H.; Chung, J.H. Perilla frutescens leaves extract ameliorates ultraviolet radiation-induced extracellular matrix damage in human dermal fibroblasts and hairless mice skin. J. Ethnopharmacol. 2017, 195, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Huo, G.; Liu, S.; Li, F.; Chen, W.; Jiang, D. Luteolin suppresses inflammation through inhibiting cAMP-phosphodiesterases activity and expression of adhesion molecules in microvascular endothelial cells. Inflammopharmacology 2019, 27, 773–780. [Google Scholar] [CrossRef]
- Huang, B.P.; Lin, C.H.; Chen, Y.C.; Kao, S.H. Anti-Inflammatory effects of perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264.7 cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Z.; Ron, D. Stress-Induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef] [Green Version]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 activation causes translocation of bax to the endoplasmic reticulum during the resolution of airway mucous cell hyperplasia by IFN-gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef] [Green Version]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent protein kinase II links Er stress with Fas and mitochondrial apoptosis pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage apoptosis in advanced atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. 1), E40–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.K.; Cheong, K.J.; Kim, H.Y.; Cheong, J. Endoplasmic reticulum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4. Biochem. J. 2011, 435, 431–439. [Google Scholar] [CrossRef] [PubMed]

| Name 1 | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Chop | CCACCACACCTGAAAGCAG | TCCTCATACCAGGCTTCCA |
| Camk2a | AGCCATCCTCACCACTAT | ATTCCTTCACGCCATCATT |
| Stat1 | TGAGATGTCCCGGATAGTGG | CGCCAGAGAGAAATTCGTGT |
| Stat3 | GTCTGCAGAGT TCAAGCACCT | TCCTCAGTCACGATCAAGGAG |
| Jak2 | TTGGTTTTGAATTATGGTGTCTGT | TCCAAATTTTACAAATTCTTGAACC |
| Fas | CGCTGTTTTCCCTTGCTG | CCTTGAGTATGAACTCTTAACTGTGAG |
| Nos2 | TGGAGGTTCTGGATGAGAGC | AATGTCCAGGAAGTAGGTGAGG |
| Ptgs2 | TCAAACAGTTTCTCTACAACAACTCC | ACATTTCTTCCCCCAGCAA |
| β-Actin | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W. A Combination Extract of Gardeniae Fructus and Perillae Folium Exerts Anti-Inflammatory Effects on LPS-Activated RAW 264.7 Mouse Macrophages via an ER Stress-Induced CHOP Pathway. Processes 2021, 9, 1632. https://doi.org/10.3390/pr9091632
Park W. A Combination Extract of Gardeniae Fructus and Perillae Folium Exerts Anti-Inflammatory Effects on LPS-Activated RAW 264.7 Mouse Macrophages via an ER Stress-Induced CHOP Pathway. Processes. 2021; 9(9):1632. https://doi.org/10.3390/pr9091632
Chicago/Turabian StylePark, Wansu. 2021. "A Combination Extract of Gardeniae Fructus and Perillae Folium Exerts Anti-Inflammatory Effects on LPS-Activated RAW 264.7 Mouse Macrophages via an ER Stress-Induced CHOP Pathway" Processes 9, no. 9: 1632. https://doi.org/10.3390/pr9091632






