Evaluation of a Combined MHE-NMPC Approach to Handle Plant-Model Mismatch in a Rotary Tablet Press
Abstract
1. Introduction
2. Material and Methods
2.1. State Estimation
2.2. Model Predictive Control (MPC)—Linear and Nonlinear
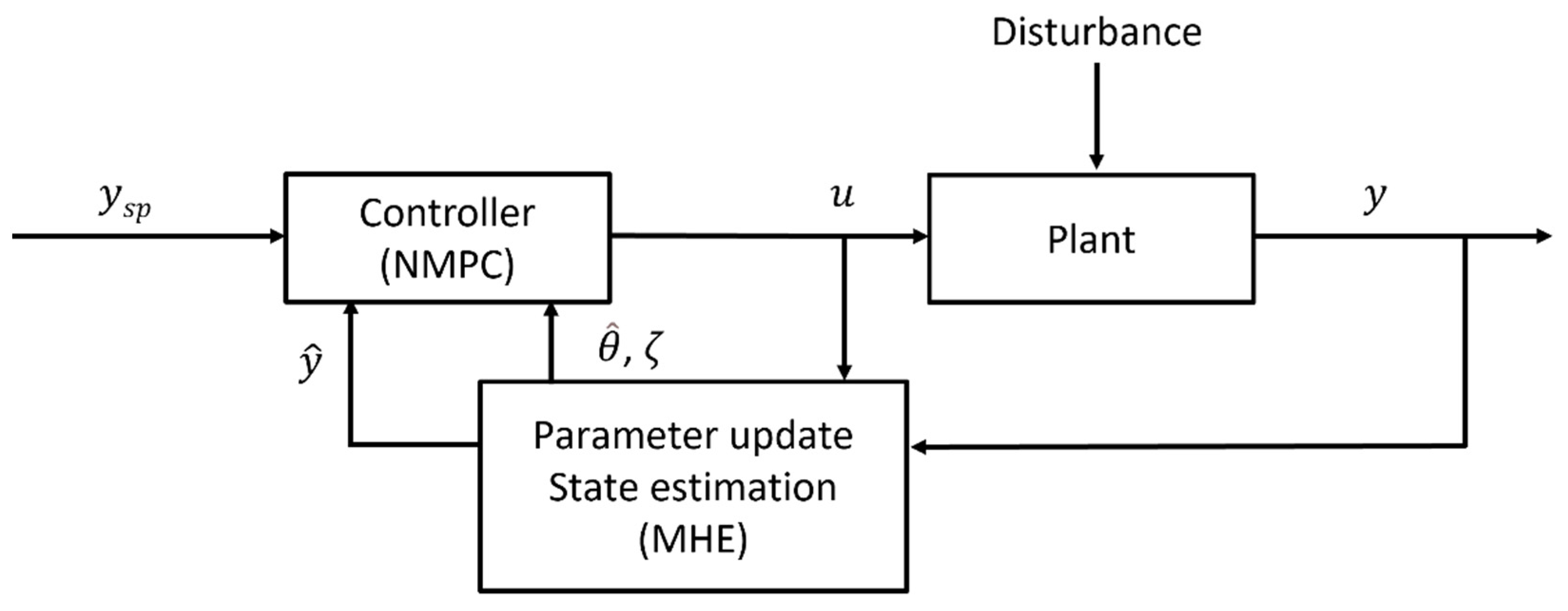
2.3. Moving Horizon Estimation-Based Nonlinear Model Predictive Control (MHE-NMPC) Framework
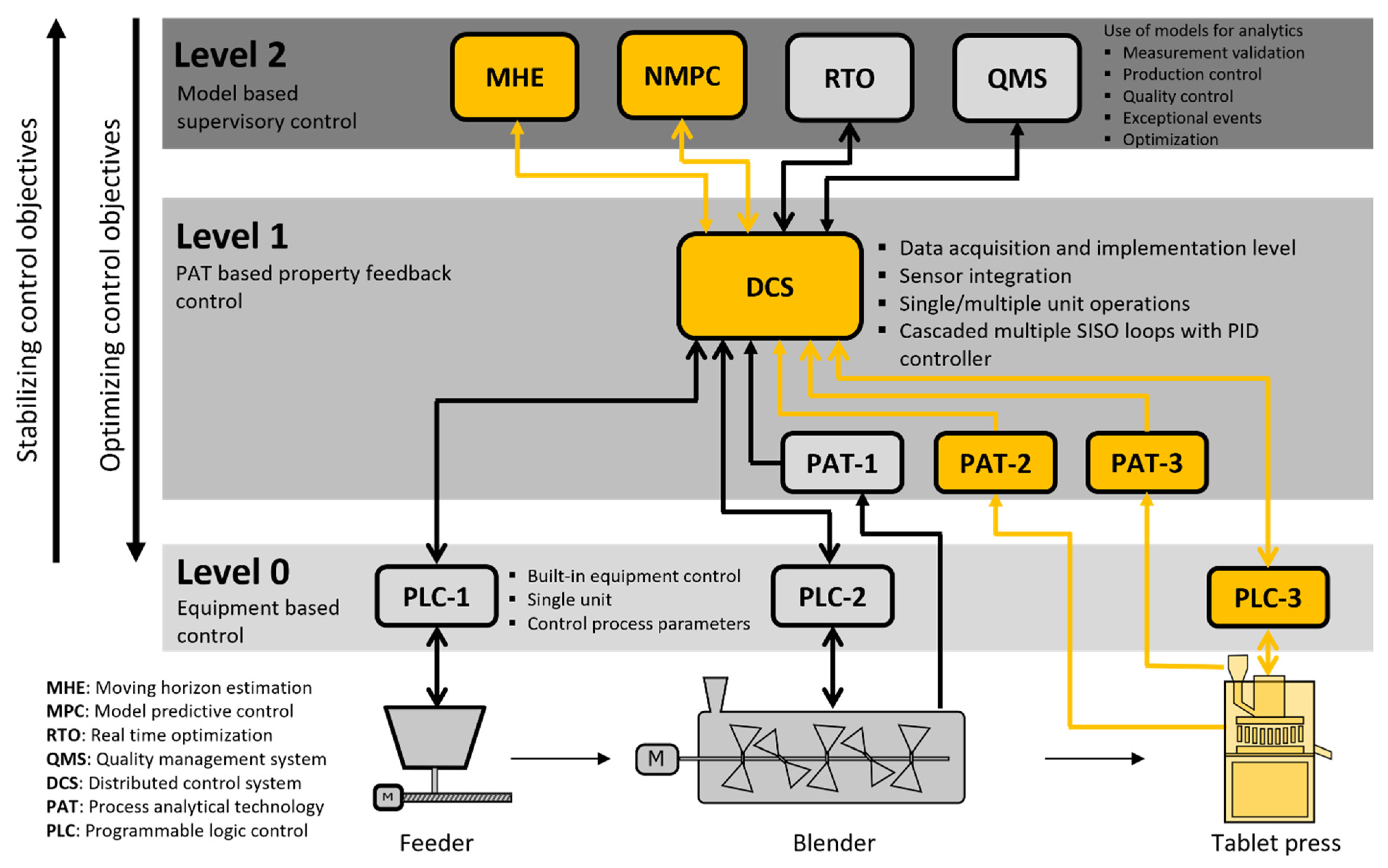
2.4. Implementation of a Real-Time Feasible MHE-NMPC Framework
3. Examples of Application to Continuous Direct Compression
3.1. Tablet Press Model
3.2. Case Study 1: Monitoring and Control of the Rotary Tablet Press in the Presence of Plant-Model Mismatch
3.3. Case Study 2: Monitoring and Control of the Rotary Tablet Press in the Presence of Uncertainty in the Glidant Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubert, C.; Lebrun, P.; Houari, S.; Ziemons, E.; Rozet, E.; Hubert, P. Improvement of a stability-indicating method by quality-by-design versus quality-by-testing: A case of a learning process. J. Pharm. Biomed. Anal. 2014, 88, 401–409. [Google Scholar] [CrossRef]
- Burcham, C.L.; Florence, A.J.; Johnson, M.D. Continuous manufacturing in pharmaceutical process development and manufacturing. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 253–281. [Google Scholar] [CrossRef] [PubMed]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic analysis of integrated continuous and batch pharmaceutical manufacturing: A case study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef]
- Piñeiro, D.P.; Nikolakopoulou, A.; Jäschke, J.; Braatz, R.D. Self-optimizing control of a continuous-flow pharmaceutical manufacturing plant. IFAC-PapersOnLine 2020, 53, 11601–11606. [Google Scholar] [CrossRef]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Karttunen, A.P.; Poms, J.; Sacher, S.; Sparén, A.; Ruiz Samblás, C.; Fransson, M.; Martin De Juan, L.; Remmelgas, J.; Wikström, H.; Hsiao, W.K.; et al. Robustness of a continuous direct compression line against disturbances in feeding. Int. J. Pharm. 2020, 574, 118882. [Google Scholar] [CrossRef]
- Gernaey, K.V.; Cervera-Padrell, A.E.; Woodley, J.M. Development of continuous pharmaceutical production processes supported by process systems engineering methods and tools. Future Med. Chem. 2012, 4, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Ierapetritou, M.; Muzzio, F.; Reklaitis, G. Perspectives on the continuous manufacturing of powder-based pharmaceutical processes. AIChE J. 2016, 62, 1846–1862. [Google Scholar] [CrossRef]
- Kovács, B.; Péterfi, O.; Kovács-Deák, B.; Székely-Szentmiklósi, I.; Fülöp, I.; Bába, L.-I.; Boda, F. Quality-by-design in pharmaceutical development: From current perspectives to practical applications. Acta Pharm. 2021, 71, 497–526. [Google Scholar] [CrossRef]
- Beg, S.; Hasnain, M.S.; Rahman, M.; Swain, S. Introduction to Quality by Design (QbD): Fundamentals, principles, and applications. In Pharmaceutical Quality by Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]
- Adolfsson, Å.; Nyström, C. Tablet strength, porosity, elasticity and solid state structure of tablets compressed at high loads. Int. J. Pharm. 1996, 132, 95–106. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sun, Z.; Dong, Q.; Li, L.; Zang, H. A new perspective in understanding the dissolution behavior of nifedipine controlled release tablets by NIR spectroscopy with aquaphotomics. J. Mol. Struct. 2021, 1230, 129872. [Google Scholar] [CrossRef]
- de Meira, R.Z.C.; Maciel, A.B.; Murakami, F.S.; de Oliveira, P.R.; Bernardi, L.S. In vitro dissolution profile of dapagliflozin: Development, method validation, and analysis of commercial tablets. Int. J. Anal. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Tsunematsu, H.; Hifumi, H.; Kitamura, R.; Hirai, D.; Takeuchi, M.; Ohara, M.; Itai, S.; Iwao, Y. Analysis of available surface area can predict the long-term dissolution profile of tablets using short-term stability studies. Int. J. Pharm. 2020, 586, 119504. [Google Scholar] [CrossRef] [PubMed]
- Galata, D.L.; Farkas, A.; Könyves, Z.; Mészáros, L.A.; Szabó, E.; Csontos, I.; Pálos, A.; Marosi, G.; Nagy, Z.K.; Nagy, B. Fast, spectroscopy-based prediction of in vitro dissolution profile of extended release tablets using artificial neural networks. Pharmacy 2019, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Gonzalez, M.; Cuitiño, A.M. Quantification of lubrication and particle size distribution effects on tensile strength and stiffness of tablets. Powder Technol. 2018, 336, 360–374. [Google Scholar] [CrossRef]
- Olowosulu, A.K.; Apeji, Y.E. Quantifying the effect of glidant on the compaction and tableting properties of paracetamol granules. J. Res. Pharm. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Blackshields, C.A.; Crean, A.M. Continuous powder feeding for pharmaceutical solid dosage form manufacture: A short review. Pharm. Dev. Technol. 2018, 23, 554–560. [Google Scholar] [CrossRef]
- Engisch, W.E.; Muzzio, F.J. Feedrate deviations caused by hopper refill of loss-in-weight feeders. Powder Technol. 2015, 283, 389–400. [Google Scholar] [CrossRef]
- Hanson, J. Control of a system of loss-in-weight feeders for drug product continuous manufacturing. Powder Technol. 2018, 331, 236–243. [Google Scholar] [CrossRef]
- Harris, T.J. Assessment of control loop performance. Can. J. Chem. Eng. 1989, 67, 856–861. [Google Scholar] [CrossRef]
- Chen, M.; Xie, L.; Su, H. Impact of model-plant mismatch to minimum variance benchmark in control performance assessment. In Proceedings of the 2020 39th Chinese Control Conference (CCC)—IEEE, Shenyang, China, 27–30 July 2020; pp. 2252–2257. [Google Scholar]
- Wang, S.; Simkoff, J.M.; Baldea, M.; Chiang, L.H.; Castillo, I.; Bindlish, R.; Stanley, D.B. Autocovariance-based plant-model mismatch estimation for linear model predictive control. Syst. Control Lett. 2017, 104, 5–14. [Google Scholar] [CrossRef]
- Wang, S.; Simkoff, J.M.; Baldea, M.; Chiang, L.H.; Castillo, I.; Bindlish, R.; Stanley, D.B. Autocovariance-based MPC model mismatch estimation for systems with measurable disturbances. J. Process Control 2017, 55, 42–54. [Google Scholar] [CrossRef]
- Xu, X.; Simkoff, J.M.; Baldea, M.; Chiang, L.H.; Castillo, I.; Bindlish, R.; Ashcraft, B. Data-driven plant-model mismatch quantification for MIMO MPC systems with feedforward control path. In Proceedings of the 2020 American Control Conference (ACC), Denver, CO, USA, 1–3 July 2020; pp. 2760–2765. [Google Scholar]
- Badwe, A.S.; Gudi, R.D.; Patwardhan, R.S.; Shah, S.L.; Patwardhan, S.C. Detection of model-plant mismatch in MPC applications. J. Process. Control. 2009, 19, 1305–1313. [Google Scholar] [CrossRef]
- Chen, Y.; Ierapetritou, M. A framework of hybrid model development with identification of plant-model mismatch. AIChE J. 2020, 66. [Google Scholar] [CrossRef]
- Destro, F.; García Muñoz, S.; Bezzo, F.; Barolo, M. Powder composition monitoring in continuous pharmaceutical solid-dosage form manufacturing using state estimation—proof of concept. Int. J. Pharm. 2021, 605, 120808. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, Q.; Moreno, M.; Laird, C.; Nagy, Z.; Reklaitis, G. Robust state estimation of Feeding—blending systems in continuous pharmaceutical manufacturing. Chem. Eng. Res. Des. 2018, 134, 140–153. [Google Scholar] [CrossRef]
- Singh, R.; Ierapetritou, M.; Ramachandran, R. System-wide hybrid MPC–PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur. J. Pharm. Biopharm. 2013, 85, 1164–1182. [Google Scholar] [CrossRef]
- Mesbah, A.; Paulson, J.A.; Lakerveld, R.; Braatz, R.D. Model predictive control of an integrated continuous pharmaceutical manufacturing pilot plant. Org. Process Res. Dev. 2017, 21, 844–854. [Google Scholar] [CrossRef]
- Singh, R.; Sahay, A.; Karry, K.M.; Muzzio, F.; Ierapetritou, M.; Ramachandran, R. Implementation of an advanced hybrid MPC–PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. Int. J. Pharm. 2014, 473, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Jelsch, M.; Roggo, Y.; Kleinebudde, P.; Krumme, M. Model predictive control in pharmaceutical continuous manufacturing: A review from a user’s perspective. Eur. J. Pharm. Biopharm. 2021, 159, 137–142. [Google Scholar] [CrossRef]
- Jacob, N.C.; Dhib, R. Unscented kalman filter based nonlinear model predictive control of a LDPE autoclave reactor. J. Process. Control 2011, 21, 1332–1344. [Google Scholar] [CrossRef]
- Magni, L.; Opizzi, S.; Scattolini, R. Tracking of non-square nonlinear systems via model predictive control. In Proceedings of the 2001 European Control Conference (ECC), Porto, Portugal, 4–7 September 2001; pp. 951–956. [Google Scholar]
- Magni, L.; Scattolini, R. Tracking of non-Square nonlinear continuous time systems with piecewise constant model predictive control. J. Process Control 2007, 17, 631–640. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, Y.; Han, H.; Tan, T. PID Control of Non-Square Systems and Its Application in the Fuel Cell Voltage. In Proceedings of the 2012 24th Chinese Control and Decision Conference (CCDC), Taiyuan, China, 23–25 May 2012; pp. 4091–4094. [Google Scholar]
- Billups, M.; Singh, R. Systematic framework for implementation of material traceability into continuous pharmaceutical tablet manufacturing process. J. Pharm. Innov. 2020, 15, 51–65. [Google Scholar] [CrossRef]
- Bhaskar, A.; Singh, R. Residence Time Distribution (RTD)-based control system for continuous pharmaceutical manufacturing process. J. Pharm. Innov. 2019, 14, 316–331. [Google Scholar] [CrossRef]
- De Assis, A.J.; Maciel Filho, R. Soft sensors development for on-line bioreactor state estimation. Comput. Chem. Eng. 2000, 24, 1099–1103. [Google Scholar] [CrossRef]
- Papachristos, C.; Dang, T.; Khattak, S.; Mascarich, F.; Khedekar, N.; Alexis, K. Modeling, control, state estimation and path planning methods for autonomous multirotor aerial robots. Found. Trends Robot. 2018, 7, 180–250. [Google Scholar] [CrossRef]
- Mansouri, M.M.; Nounou, H.N.; Nounou, M.N. Nonlinear control and estimation in induction machine using state estimation techniques. Syst. Sci. Control Eng. 2014, 2, 642–654. [Google Scholar] [CrossRef][Green Version]
- Shenoy, A.V.; Prakash, J.; Prasad, V.; Shah, S.L.; McAuley, K.B. Practical issues in state estimation using particle filters: Case studies with polymer reactors. J. Process Control 2013, 23, 120–131. [Google Scholar] [CrossRef]
- Fang, H.; Tian, N.; Wang, Y.; Zhou, M.; Haile, M.A. Nonlinear bayesian estimation: From kalman filtering to a broader horizon. IEEE/CAA J. Autom. Sin. 2018, 5, 401–417. [Google Scholar] [CrossRef]
- Rawlings, J.B.; Bakshi, B.R. Particle filtering and moving horizon estimation. Comput. Chem. Eng. 2006, 30, 1529–1541. [Google Scholar] [CrossRef]
- Mohd Ali, J.; Ha Hoang, N.; Hussain, M.A.; Dochain, D. Review and classification of recent observers applied in chemical process systems. Comput. Chem. Eng. 2015, 76, 27–41. [Google Scholar] [CrossRef]
- Xia, C.; Pan, Z.; Zhang, S.; Polden, J.; Wang, L.; Li, H.; Xu, Y.; Chen, S. Model predictive control of layer width in wire arc additive manufacturing. J. Manuf. Process. 2020, 58, 179–186. [Google Scholar] [CrossRef]
- Cavanini, L.; Ippoliti, G.; Camacho, E.F. Model predictive control for a linear parameter varying model of an UAV. J. Intell. Robot. Syst. 2021, 101, 1–18. [Google Scholar] [CrossRef]
- Hewing, L.; Wabersich, K.P.; Menner, M.; Zeilinger, M.N. Learning-based model predictive control: Toward safe learning in control. Annu. Rev. Control Robot. Auton. Syst. 2020, 3, 269–296. [Google Scholar] [CrossRef]
- Drgoňa, J.; Arroyo, J.; Cupeiro Figueroa, I.; Blum, D.; Arendt, K.; Kim, D.; Ollé, E.P.; Oravec, J.; Wetter, M.; Vrabie, D.L.; et al. All you need to know about model predictive control for buildings. Annu. Rev. Control 2020, 50, 190–232. [Google Scholar] [CrossRef]
- Yang, S.; Wan, M.P.; Ng, B.F.; Dubey, S.; Henze, G.P.; Chen, W.; Baskaran, K. Experimental study of model predictive control for an air-conditioning system with dedicated outdoor air system. Appl. Energy 2020, 257, 113920. [Google Scholar] [CrossRef]
- Zavala, V.M.; Biegler, L.T. The advanced-step NMPC controller: Optimality, stability and robustness. Automatica 2009, 45, 86–93. [Google Scholar] [CrossRef]
- Salem, F.; Mosaad, M.I. A comparison between MPC and optimal PID controllers: Case studies. In Proceedings of the Michael Faraday IET International Summit 2015, Kolkata, India, 12–14 September 2015. [Google Scholar]
- Martínez, L.; Peinado, A.; Liesum, L.; Betz, G. Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: Influence of mass flow and rotation speed variations. Eur. J. Pharm. Biopharm. 2013, 84, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Vanarase, A.U.; Alcalà, M.; Jerez Rozo, J.I.; Muzzio, F.J.; Romañach, R.J. Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy. Chem. Eng. Sci. 2010, 65, 5728–5733. [Google Scholar] [CrossRef]
- Kirchengast, M.; Celikovic, S.; Rehrl, J.; Sacher, S.; Kruisz, J.; Khinast, J.; Horn, M. Ensuring tablet quality via model-based control of a continuous direct compaction process. Int. J. Pharm. 2019, 567, 118457. [Google Scholar] [CrossRef]
- López-Negrete, R.; Biegler, L.T. A moving horizon estimator for processes with multi-rate measurements: A nonlinear programming sensitivity approach. J. Process. Control. 2012, 22, 677–688. [Google Scholar] [CrossRef]
- Rao, C.V.; Rawlings, J.B.; Mayne, D.Q. Constrained state estimation for nonlinear discrete-time systems: Stability and moving horizon approximations. IEEE Trans. Autom. Control 2003, 48, 246–258. [Google Scholar] [CrossRef]
- Huang, R.; Biegler, L.T.; Patwardhan, S.C. Fast offset-free nonlinear model predictive control based on moving horizon estimation. Ind. Eng. Chem. Res. 2010, 49, 7882–7890. [Google Scholar] [CrossRef]
- Su, Q.; Bommireddy, Y.; Shah, Y.; Ganesh, S.; Moreno, M.; Liu, J.; Gonzalez, M.; Yazdanpanah, N.; O’Connor, T.; Reklaitis, G.V.; et al. Data reconciliation in the Quality-by-Design (QbD) implementation of pharmaceutical continuous tablet manufacturing. Int. J. Pharm. 2019, 563, 259–272. [Google Scholar] [CrossRef]
- Medina-González, S.; Huang, Y.-S.; Bachawala, S.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A NMPC strategy applied to a continuous direct compaction tablet manufacturing. In Proceedings of the AIChE 2020 Annual Meeting, Virtual, 16–20 November 2020; p. 716b. [Google Scholar]
- Kawakita, K.; Lüdde, K.-H. Some considerations on powder compression equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Gonzalez, M. Generalized loading-unloading contact laws for elasto-plastic spheres with bonding strength. J. Mech. Phys. Solids 2019, 122, 633–656. [Google Scholar] [CrossRef]
- Su, Q.; Moreno, M.; Giridhar, A.; Reklaitis, G.V.; Nagy, Z.K. A systematic framework for process control design and risk analysis in continuous pharmaceutical solid-dosage manufacturing. J. Pharm. Innov. 2017, 12, 327–346. [Google Scholar] [CrossRef]








| Case Study 1 | Case Study 2 | |||
|---|---|---|---|---|
| Purpose | Assess Control Performance in the Presence of Different Levels of PMM | Assess Control Performance When Uncertainty in Glidant Concentration Is Present | ||
| Assumption | Glidant Concentration Can Be Manipulated | Glidant Concentration Needs to Be Estimated | ||
| Model Parameters | No PMM | Mild PMM | High PMM | Nominal Operation |
| 0.036 | 0.036 | 0.036 | 0.036 | |
| 0.030 | 0.030 | 0.050 | 0.030 | |
| (g/cm3) | 0.365 | 0.390 | 0.410 | 0.365 |
| 0.265 | 0.290 | 0.230 | 0.265 | |
| Kawakita: a | 0.80 | 0.77 | 0.84 | 0.80 |
| Kawakita: 1/b (MPa) | 10.26 | 10.26 | 8.55 | 10.26 |
| (g/cm3) | 1.53 | 1.53 | 1.51 | 1.53 |
| 0.08 | 0.08 | 0.08 | 0.08 | |
| 0.57 | 0.57 | 0.57 | 0.57 | |
| (MPa) | 11.67 | 11.67 | 11.67 | 11.67 |
| 0.57 | 0.57 | 0.57 | 0.57 | |
| 0.61 | 0.61 | 0.61 | 0.61 | |
| 0.31 | 0.31 | 0.31 | 0.31 | |
| 0.38 | 0.38 | 0.38 | 0.38 | |
| 8.40 | 8.40 | 8.40 | 8.40 | |
| (g/cm3) | N/A | 0.450 | ||
| (g/cm3) | 0.330 | |||
| 0.361 | ||||
| 1.394 | ||||
| 23.326 | ||||
| Controlled variables | Tablet weight, pre-compression force, production rate, tensile strength |
| Manipulated variables | Dosing position, pre-compression thickness, main compression thickness, turret speed, silica concentration |
| Measured variables | Tablet weight, pre-compression force, main compression force, production rate |
| Uncertain model parameters | Bulk density, critical density, a: maximum degree of compression |
| Controlled Variables | Performance Metrics | No PMM | Mild PMM | High PMM |
|---|---|---|---|---|
| Tablet Weight | IAE | 6.83 | 7.00 | 7.05 |
| M2P (%) | 3.31 | 3.19 | 3.61 | |
| D2R (s) | 76 | 78 | 74 | |
| Tensile Strength | IAE | 9.95 | 10.18 | 39.07 |
| M2P (%) | 5.25 | 5.23 | 10.46 | |
| D2R (s) | 82 | 81 | 90 | |
| Production Rate | IAE | 8.84 | 8.26 | 8.41 |
| Controlled variables | Tablet weight, pre-compression force, production rate, tensile strength |
| Manipulated variables | Dosing position, pre-compression thickness, main compression thickness, turret speed |
| Measured variables | Tablet weight, pre-compression force, main compression force, production rate |
| Uncertain model parameters | Silica concentration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-S.; Sheriff, M.Z.; Bachawala, S.; Gonzalez, M.; Nagy, Z.K.; Reklaitis, G.V. Evaluation of a Combined MHE-NMPC Approach to Handle Plant-Model Mismatch in a Rotary Tablet Press. Processes 2021, 9, 1612. https://doi.org/10.3390/pr9091612
Huang Y-S, Sheriff MZ, Bachawala S, Gonzalez M, Nagy ZK, Reklaitis GV. Evaluation of a Combined MHE-NMPC Approach to Handle Plant-Model Mismatch in a Rotary Tablet Press. Processes. 2021; 9(9):1612. https://doi.org/10.3390/pr9091612
Chicago/Turabian StyleHuang, Yan-Shu, M. Ziyan Sheriff, Sunidhi Bachawala, Marcial Gonzalez, Zoltan K. Nagy, and Gintaras V. Reklaitis. 2021. "Evaluation of a Combined MHE-NMPC Approach to Handle Plant-Model Mismatch in a Rotary Tablet Press" Processes 9, no. 9: 1612. https://doi.org/10.3390/pr9091612
APA StyleHuang, Y.-S., Sheriff, M. Z., Bachawala, S., Gonzalez, M., Nagy, Z. K., & Reklaitis, G. V. (2021). Evaluation of a Combined MHE-NMPC Approach to Handle Plant-Model Mismatch in a Rotary Tablet Press. Processes, 9(9), 1612. https://doi.org/10.3390/pr9091612








