1. Introduction
The Anaerobic Digestion (AD) of biodegradable wastes is a bio-process in which the organic matter in effluents and residues is ultimately converted to methane (CH
4) and carbon dioxide (CO
2). Biogas from AD typically contains 50% to 70% CH
4, 30% to 50% CO
2, ≤1% N
2, and 10ppmv–2000ppmv H
2S depending on the substrate and processing conditions [
1,
2,
3].
AD is commonly described by four major steps performed by microorganisms and driven by thermodynamic principles: (i) hydrolysis, (ii) acidogenesis, (iii) acetogenesis and (iv) methanogenesis. Since microorganisms are not able to assimilate particulate organic matter, the first stage in AD is the hydrolysis of complex particulate materials into simpler dissolved materials, which can cross the cell walls of fermentative bacteria. This conversion of particulate materials into dissolved materials is achieved through the action of exoenzymes excreted by hydrolytic bacteria. Soluble products from the hydrolysis phase are metabolized inside the cells of fermentative bacteria, being converted into several simpler compounds, which are then excreted by the cells. The compounds produced include: volatile fatty acids, alcohols, lactic acid, carbon dioxide, hydrogen (H2), ammonia (NH3) and hydrogen sulfide (H2S), in addition to new bacterial cells. As volatile fatty acids are the main product of fermentative organisms, they are usually called acidogenic fermentative bacteria. Acetogenic bacteria are responsible for oxidizing the products generated in the acidogenic phase into suitable substrates for methanogenic microorganisms. The products generated in this process are H2, CO2, and acetate. The final step in the global process of anaerobic degradation, methanogenesis, is the conversion of acetate into CH4 and CO2 by aceticlastic methanogens and of H2 and CO2 into CH4 by hydrogenotrophic methanogens.
Biogas can be used for a number of purposes, including electricity production (most common), heat generation and as raw material for industries [
1,
2,
3]. Biogas production has been a reality since the 1930s for the stabilization of sewage sludge. What has changed over the years is that its production has been optimized and achieved at industrial scale with a higher efficiency, degree of complexity and specification, particularly in developed countries. Biogas production can reduce emission of Greenhouse Gases (GHG), provide a renewable source of energy and reduce impacts of pollution by waste disposal [
4]. Furthermore, the activation of methane and/or carbon dioxide present in biogas enables its chemical conversion into higher value biochemicals and biofuels [
5].
One possible process for methane conversion into a valuable chemical is the Oxidative Coupling of Methane (OCM), which is its heterogeneous catalytic oxidation into ethylene: a major feedstock for chemical and polymer production. It is of scientific consensus that OCM occurs via three steps (simplified): (i) activation of methane to methyl radical through a C–H bond breaking and a hydrogen abstraction, (ii) homogeneous coupling of two methyl radicals to ethane in the gas phase, and (iii) oxidative dehydrogenation of ethane to ethylene [
6]. These steps are summarized in Equations (2) and (5). However, many parallel side reactions also occur, which adds complexity to the downstream separations. The full reaction network considered in this study, as proposed by [
7], is given in Equations (1)–(10). A trade-off between methane conversion and selectivity towards C
products (ethane and ethylene) is observed, which is typical of selective oxidation reactions.
OCM is not yet commercially applied, but the surge in shale gas exploration has made it a potential route for producing valuable ethylene from cheap methane sources and without wildly fluctuating prices of crude oil [
6]. A demonstration plant has been built and put into operation by Siluria Technologies in Texas, U.S. [
8].
In order for the ethylene production from biogas via OCM to be economically competitive with the consolidated production from oil [
9], a significant amount of biogas (process feed) is required to merely approach the economies of scale typical of this industry. Consequentially, a great amount of a biodegradable effluent (substrate) must be available for AD. Agroindustry wastes such as whey (dairy industry effluent), glycerin (biodiesel production effluent), and sugarcane stillage (also called vinasse, which is the sugar and ethanol production effluent) are all produced in large quantities worldwide and, due to their high organic load, pose an environmental threat if not properly treated. Several studies have shown the feasibility of treating these effluents by anaerobiosis with the associated production of bio-energy, i.e., methane and/or hydrogen [
10,
11,
12,
13,
14,
15,
16,
17].
Among these effluents, vinasse stands out as a particularly good candidate for large-scale biogas generation. It is the main liquid stream from first-generation ethanol production process from sugarcane, beet, sweet sorghum, grape, corn, wheat, rice, cassava, potato, and others [
18]. Together, the U.S. and Brazil produced 85% of the world’s ethanol in 2017. The vast majority of U.S. ethanol is produced from corn, while Brazil primarily uses sugarcane [
19].
Considering the Brazilian scenario, vinasse is derived from the ethanol distillation step, leaving the columns at ≈ 360
. The presence of melanoidins and the high organic acid content gives it a dark-brownish color and low pH, respectively. Sugarcane processing plants usually generate from 10L to 15L of vinasse per
of produced ethanol and, in 2019, the Brazilian Ministry of Agriculture, Livestock and Food Supply estimated a production of
L of ethanol [
20,
21]. This leads to an estimated
L to
L of vinasse in this country alone in a single year. The vinasse is currently applied for fertilization, but if not properly conditioned, vinasse may lead to pollution of soil and water bodies. AD is an effective way to reduce its high organic load, i.e., COD, which may reach values up to 65000 mg
O2 L
-1, and make it suitable for use in fertilization while simultaneously producing biogas [
22].
The Southeast region of Brazil and, notably the state of São Paulo, concentrates a great portion of the bioethanol production in the country. An interactive map has recently been created by researchers at University of São Paulo containing the availability of organic wastes (substrates), the biogas and biomethane production potential, as well as the available infrastructure such as pipelines and compression stations [
23]. The total estimated biogas production potential lies at around
Nm
3 year
-1 (
Nm
3CH4 year
-1) with around 88% of the total stemming from vinasse AD [
23]. Based on the yields obtained in the present study (
kg
C2H4 kg
CH4-1), this could be used to produce around 900 kt of ethylene per year and potentially replace some 9 Mt year
-1 of oil (assuming
t
C2H4 t
Naphtha-1 and
t
Naphtha t
Oil-1). This region is also very industrialized and hosts 42% of the total Brazilian population [
24], therefore also containing the infrastructure and market demand that are required for a project such as this.
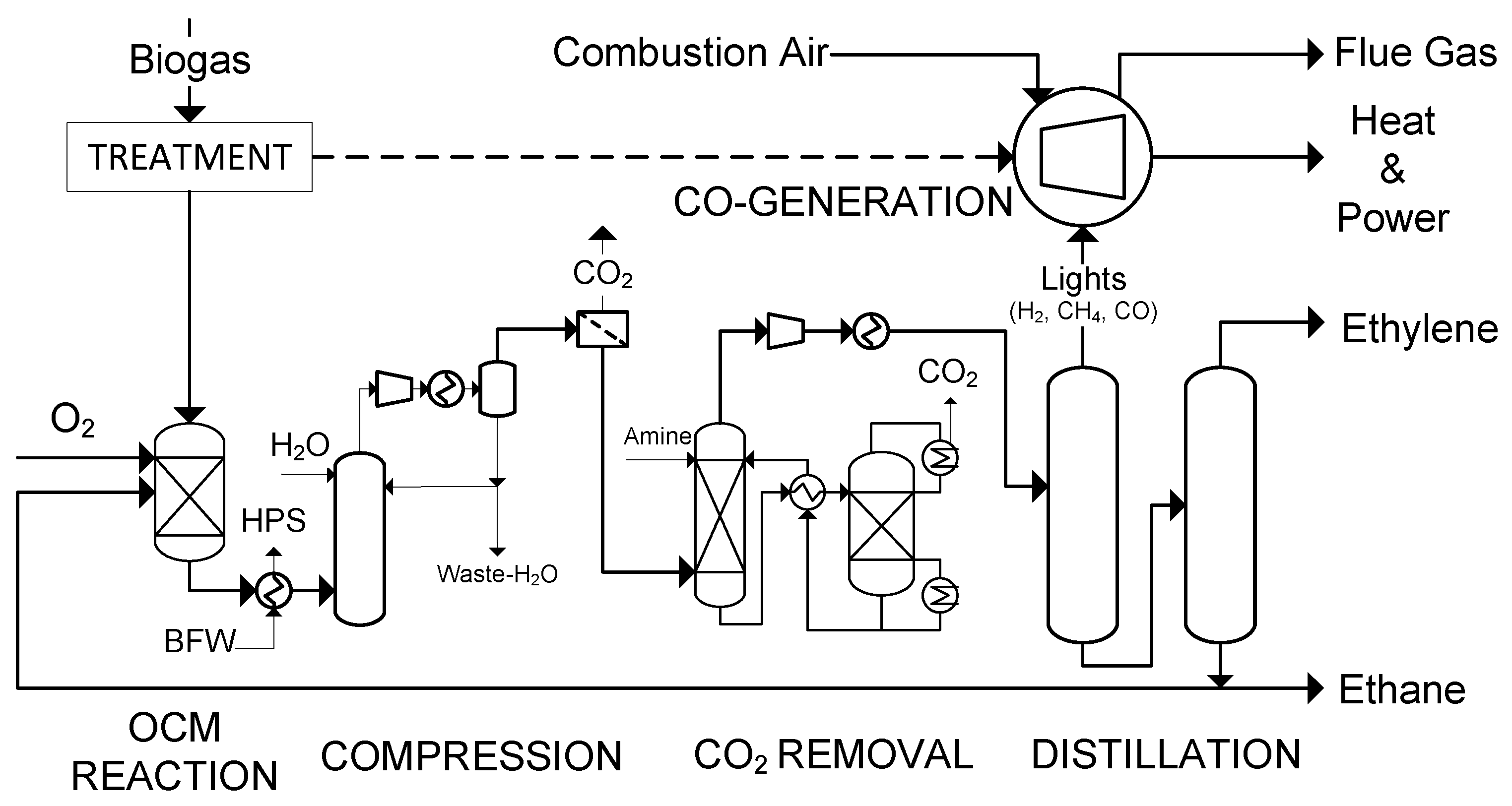
This contribution investigates the potential and competitiveness of industrial bio-ethylene production via OCM using biogas produced by biological anaerobiosis of sugarcane vinasse as a feedstock. The main goals are to apply process simulation models to conceptually design an economic Biogas-based Oxidative Coupling of Methane (Bio-OCM) process using the structure depicted in
Figure 1 and to assess its techno-economic feasibility. A model of the OCM reaction section applying adiabatic Packed Bed Reactors (PBRs) is used to maximize C
product yield by manipulating the operating conditions. For the CO
2 removal section, a superstructure optimization is applied to determine whether to use a standalone amine-absorption configuration or a hybrid configuration employing both membrane and absorption. Two different process configurations are compared for the distillation section. The first distillation configuration only applies external low-temperature refrigeration, while the second distillation configuration adiabatically expands fractions of the process streams to reduce external refrigeration consumption. Once the optimal process design is defined, the bio-ethylene production cost is estimated and compared to typical market values for fossil ethylene. Finally, a Monte Carlo simulation is performed to encompass uncertainties in the cost estimations.
2. Materials and Methods
The process flow diagram for the Bio-OCM process considered herein is depicted in
Figure 1. The process plant consumes biogas and oxygen as educts, while producing ethylene as the main product and ethane and light off-gases as side-products. Biogas is first subjected to a regular treatment step for the removal of impurities such as H
2S and NH
3. The CO
2 removal step, i.e., biogas upgrade, is avoided and the CO
2 present in biogas serves to dilute the educts in the feed stream to the OCM reactor. This helps to contain the intense reactions’ heat release. The treated biogas stream is fed to the OCM reactor together with an oxygen stream. The hot reaction gases are cooled by generating High Pressure Steam (HPS) and compressed prior to the CO
2 removal section. CO
2 removal is achieved either by a hybrid permeation-absorption process or by a standalone amine-absorption process. The final hydrocarbon separation is achieved by distillation. The off-gas (lights) stream containing mostly the unreacted methane is exploited energetically in a Combined Heat and Power (CHP) unit. The process model focuses on the three main sections of the proposed Bio-OCM process, i.e., reaction section, compression and CO
2 removal section, and distillation section, while the vinasse treatment by AD, the CHP, and the air separation plants are not considered here.
In this study, the feed to the plant is assumed to be treated biogas and an oxygen-rich stream ( 95 mol O
2 + 5 mol N
2) both at 313
and
. The total inlet methane flowrate is specified at 15000 Nm
3CH4 h
-1, which can represent 50 mol% to 70 mol% of the biogas composition with the rest being solely CO
2. Such biogas production volumes can only be achieved at large bioethanol plants, i.e., approximately 1000000 m
3Ethanolyear
-1 or by combining the output of two or more plants. A previous study on a medium-sized bioethanol plant producing 1320 Nm
3 h
-1CH4 revealed a rather insufficient production volume in order to achieve decent economies of scale and dilute investment in expensive process equipment [
5].
The main product is polymer-grade ethylene (
mol
C2H4 mol
-1) and the resulting plant output is 4632 t
C2H4 year
-1. The side products are a refinery-grade ethane stream and a methane-rich light off-gases stream. Both side streams are sold for additional revenue as detailed in
Section 2.4.6.
The models described in the following sections are implemented in the process simulation software Aspen Plus and Aspen Custom Modeler v10. Further details on the models are provided in [
25] and the Aspen Plus model files are made available in [
26]. All process optimizations have been performed via a self-programmed Python interface to Aspen Plus [
27] and by applying Differential Evolution as optimization algorithm [
28].
2.1. Reaction Section Model
The OCM reaction is carried out in adiabatic Packed Bed Reactors (PBRs) with oxygen as the limiting reactant. Due to the high temperatures involved and high exothermicity of the reaction network, supplying heat and controlling temperature are difficult tasks. Therefore, industrial operation with PBR in isothermal regime is unrealistic and adiabatic regime is likely the only current option available with this reactor type [
29]. To cope with low per-pass product yields and high temperature rises, two reactors in series with intermediate cooling and oxygen feed are adopted in this study.
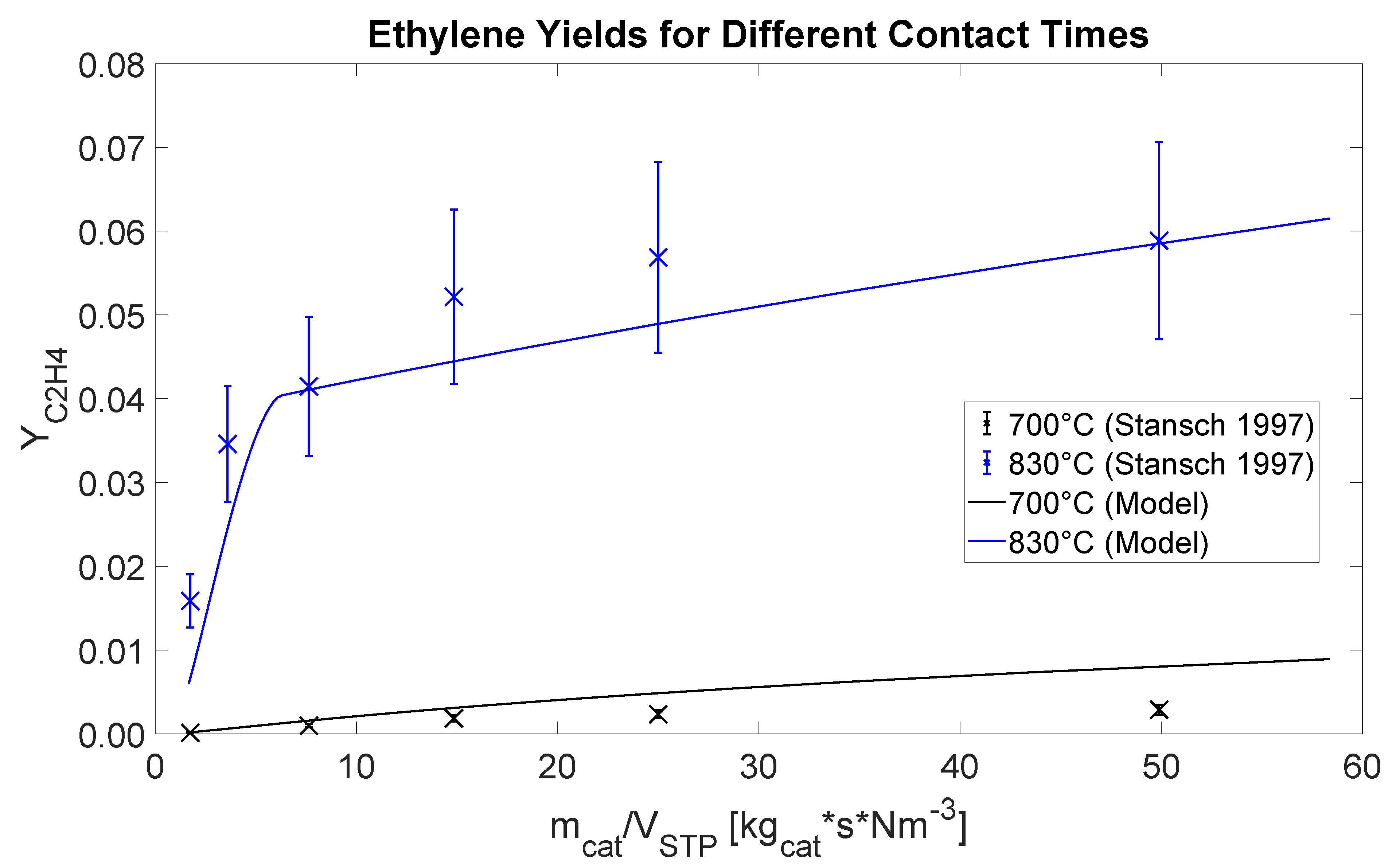
The reactors are modeled using the Plug-Flow Reactor (PFR) model in Aspen Plus (RPlug) with kinetics available in literature for La
2O
3/CaO catalyst [
7]. The reactor model implementation is validated by comparing simulation results to lab-scale isothermal experiments by [
7]. The comparison in terms of ethylene yield (defined in Equation (11)) as a function of the contact time (relation between amount of catalyst and gas flow rate) for two different temperatures is shown in
Figure 2. The simulated and experimental results are very comparable. The sharp bend occurring at 830
( 1103
) is due to oxygen extinction in the reactor.
2.2. Carbon Dioxide Removal Section Model
Two competing process structures are considered for this section, i.e., a standalone amine-absorption process and a hybrid process that applies both Gas Separation Membranes (GSM) and amine absorption. Amine absorption is used in a wide variety of industrial processes for CO
2 removal. A common benchmark amine solution is Monoethanolamine (IUPAC: 2-aminoethan-1-ol) (MEA), which is usually employed in aqueous solutions of up to 30 wt%. The solution reacts promptly with CO
2 in the absorption column, but its regeneration in a secondary desorption column consumes a significant amount of energy (up to
MJ kg
-1CO2) [
30]. The hybrid process consists in applying GSM, in this case polyimide polymeric membrane modules, to partially remove CO
2 in the upstream of the absorption column. This reduces the required amount of recirculating amine solution and, therefore the energy associated with its regeneration. However, the hybrid process may potentially require additional compression to drive separation through the membranes, higher capital investment in compressors, and lead to additional product (ethylene) losses through the membranes. To define the optimal process structure and operating conditions, a superstructure containing both alternatives is formulated to solve an economic optimization problem.
2.2.1. Absorption Model
The process model of the Bio-OCM absorption section uses the Electrolyte Non-Random Two-Liquid (eNRTL) activity model [
31,
32] and the Peng-Robinson (PR) Equation of State (EoS) [
33]. The columns are simulated using a conventional phase and chemical equilibrium approach through Aspen Plus’ block RadFrac. The solubility of carbon dioxide in an aqueous solution of 30 wt% MEA predicted by the model is compared to experimental data by [
34] in
Figure 3. A very good agreement can be observed within the relevant range of process conditions.
The solubility of hydrocarbon gases in aqueous amine solutions is usually higher than that in pure water, which several authors refer to as salting-in effect [
35]. Therefore, the parameters used to calculate the Henry constant of all gases in water and in MEA have been fitted to experimental data.
2.2.2. Gas Separation Membranes (GSM) Model
For this application, flat-sheet envelope-type membrane modules developed at Helmholtz-Zentrum Geesthacht are considered [
36]. Among the previously tested membrane materials, a polyimide-based membrane has been selected for its high CO
2 selectivity towards several hydrocarbons [
37]. For this specific application, selectivity is relatively more important than permeability, since ethylene is a valuable product and losses must be kept at minimum even if this implies large membrane areas. Since the membrane is selective for CO
2, its concentration increases in the permeate stream and reduces in the retentate stream.
For GSM, a previously published one-dimensional solution-diffusion model has been used [
37]. The component’s flux through the membrane is calculated as the product of the permeance and the driving force. The first is fitted to mini-plant experimental data [
38], and the second is given by the difference in the component’s fugacity on each side of the membrane calculated by the PR-EoS. The resulting Differential Algebraic Equation System (DAEs) is discretized by orthogonal collocation on finite elements, implemented in the software Aspen Custom Modeler, and exported as a custom unit operation into Aspen Plus.
2.3. Distillation Model
The final separation section contemplates two distillation columns, namely de-methanizer, which is responsible for recovering the light components (CH
4, H
2, CO, N
2), and C
-splitter, which is responsible for C
2H
4 and C
2H
6 separation. Two different designs are compared on an economic basis for this section. The first one is herein called traditional configuration because it has been previously adopted by several authors [
39,
40,
41]. The traditional configuration uses only external refrigeration cycles to generate the cold utility required to condense the columns’ top products. The petrochemical and natural gas processing industries, however, usually employ different schemes that expand a fraction of the hydrocarbon feed or products to produce “in-process” cold utility and reduce the load of external refrigeration cycles. To assess the potential of applying such schemes within a Bio-OCM process, a functional Recycle Split Vapor (RSV) distillation configuration is developed taking patent [
42] as a basis and compared to the traditional distillation configuration.
The columns are modeled using Aspen Plus’ RadFrac block under phase-equilibrium assumption. The PR-EoS with its original mixing rule and full set of binary parameters has been used to model this part of the process [
33]. In both columns, constraints (design-specs in Aspen Plus) are used to achieve target purities and recoveries by manipulating input variables such as the reflux and boil-up ratios. In the demethanizer bottoms, methane contamination is set to (
mol
CH4 mol
-1), while ethylene recovery is set to 99%. In the top of the C
-splitter, ethylene recovery is set to 99% while its purity is set to achieve polymer-grade, i.e.,
mol
C2H4 mol
-1.
2.4. Cost Models
This section describes the cost models used to estimate variable costs (educts, side-products, and utilities) as well as fixed cost (equipment).
2.4.1. Total Annualized Cost
The total annualized cost per mass of product (bio-ethylene), i.e.,
in
, is given by Equation (12). The
encompasses the utility cost and annualized equipment cost, while also considering product losses by placing the ethylene output mass flow in the denominator. The
is used in the process design stage as the objective function to be minimized within a process optimization problem or as the criteria to compare alternative process configurations. Throughout this study, a plant’s operating life (
N) of 30 years and an interest rate (
) of 10% have been used.
2.4.2. Ethylene Production Cost
For the economic evaluations described in Item
Section 4, the total production cost of bio-ethylene, i.e.,
in
, is calculated as per Equation (13). Besides utility cost and annualized equipment cost, the
also includes the cost of educts and revenues from side products. This is then divided by the output mass flow rate of ethylene. The equipment cost is annualized by the same formula as in Equation (12).
The calculated production cost for bio-ethylene (
) is then compared to typical market values for fossil ethylene, which can range from 0.70
to 1.50
and fluctuate significantly with the oil price [
43]. For the plant to be constructed, several other costs such as civil engineering, land and terrain, instrumentation and automation, electric engineering, operation and maintenance, taxes, depreciation, contingency, etc., would also incur. Environmental costs are also not taken into account in this economic calculations. Therefore, the bio-ethylene production cost calculated by Equation (13) must be significantly lower than ethylene’s market value to justify the economic potential for a Bio-OCM plant. The calculation of the individual components of Equation (13) are detailed in the following sub-sections.
2.4.3. Monte Carlo Simulation
There is a large degree of uncertainty in all terms of Equation (13). In some cases, even small variations can significantly alter the outcome of the analysis. To deal with this, a Monte Carlo simulation is performed. Reasonable cost ranges, i.e., bounds, are given to each one of the cost components contained in Equation (13). The bio-ethylene production cost is then computed 10,000 times with random values between bounds being assigned for each cost component. This allows for a statistical interpretation of the results. For instance, one can compute in how many of these 10,000 samples the bio-ethylene production cost results lower than a target value, i.e., the probability that the bio-ethylene production cost is below a target value. The following
Section 2.4.4,
Section 2.4.5,
Section 2.4.6 discuss, for each term of Equation (13), the considered ranges/bounds for each variable in the Monte Carlo simulation. The randomize function of Microsoft Office Excel has been used to generate the random values for the Monte Carlo sampling.
2.4.4. Utility Cost
The Bio-OCM consumes electricity and Light Pressure Steam (LPS) and Medium Pressure Steam (MPS) for heating, while exporting High Pressure Steam (HPS) produced by using the reactions’ heat release. The electricity cost for small-medium enterprises in Brazil, which is around
USD kWh
-1 (35 USD GJ
-1) [
44], is adopted. This is additionally employed to modify the cost of low, medium, and high-pressure steam from their default values in Aspen Plus. The conditions and cost for all applied utilities are summarized in
Table 1. For the Monte Carlo simulation, the ranges are set to ±30% of the nominal values in
Table 1.
2.4.5. Equipment Cost
Sizing and costing of equipment is performed using activated economics in Aspen Plus, which essentially transfers simulation data into Aspen Process Economic Analyzer (APEA) software. APEA contains automated procedures for equipment sizing and costing based on an updated data-bank. In Item
Section 4.3, a location factor of 1.4 is applied to correct the installed equipment cost estimated by APEA, as suggested in other studies for chemical plants based in Brazil [
45].
Cost estimations in conceptual and basic engineering are typically assumed to have a ±30–50% error margin. Given the difficulties in estimating cost for royalties to be paid, notably for the reactor and catalyst technologies, a range of −30% to +50% is considered in the Monte Carlo simulation as a simple and conservative solution.
2.4.6. Cost of Educts and Side Products
Biogas
The cost for producing biogas depends on a handful of parameters such as the substrate, transportation, plant capacity, and the amount and nature of contaminants. A general study reports ranges of 0.03 USD Nm
-3 to 0.05 USD Nm
-3 [
46]. Specifically for biogas derived from vinasse AD, estimates in the range of 0.022 USD Nm
-3 to 0.038 USD Nm
-3 [
47] and
USD Nm
-3 [
48] have been identified. For the Monte Carlo simulation, the entire range of 0.022 USD Nm
-3 to 0.0525 USD Nm
-3 has been adopted.
Oxygen
Oxygen is assumed to be provided by an adjacent air separation unit, which is not included in the model. Oxygen-rich streams can be obtained industrially from air via cryogenic distillation or Pressure-Swing Adsorption (PSA). Since a high oxygen purity it not required for this application, a 95 mol% oxygen stream is assumed with the contamination consisting only of inert nitrogen. For the subsequent Monte Carlo simulation, the assumed oxygen delivery price range is 0.04 USD kg
-1O2 to 0.10 USD kg
-1O2 [
49].
Ethane
Ethane is considered to be sold as a side-product with a price in the range of 0.0467 USD kg
-1C2H6 to 0.0686 USD kg
-1C2H6 based on [
43].
Light Off-Gas
The main side product of the Bio-OCM plant is a light gas stream, which contains mostly the un-reacted CH
4 (≈ 90 mol%) along with minor amounts of H
2, CO, N
2, and trace amounts of C
2H
4 and C
2H
6. A sales price close to that of pipeline natural gas in Brazil, which ranges from 5.00 USD GJ
-1HHV to 6.91 USD GJ
-1HHV [
44,
45], is assumed. For the subsequent Monte Carlo simulation, a sales price range between 2.0 USD GJ
-1HHV to 5.0 USD GJ
-1HHV is assigned to the light off-gas stream. Natural gas specification in Brazil is given by a resolution or standard ANP No. 16-2008 by the National Agency for Petroleum, Natural Gas and Biofuels.
Table 2 compares the specification of pipeline natural gas in Brazil with those of the lights stream resulting from this simulation study. As it can be seen, the lights stream almost matches the required specifications for pipeline-quality natural gas from the standard ANP 16-2008, thus justifying a similar sales price. For additional reference, characteristics of other conventional and unconventional gaseous fuels are reported. These are based on data from [
50], which include biogas from sewage sludge and gas obtained from catalytic pyrolysis of high-density poly-(ethylene), i.e., PPG.
4. Economic Evaluation
The optimal process configuration for the Bio-OCM plant led to a reaction section employing two adiabatically operated PBR in series, an amine-based standalone absorption process to remove carbon dioxide, and a low-temperature hydrocarbon distillation process applying a RSV configuration to reduce refrigeration cost. The Bio-OCM plant consumes biogas and oxygen as the main educts. The main product is bio-ethylene, while ethane and light gases are also obtained as side-products. In terms of utilities, electricity as well as low and medium pressure steam are consumed, while high pressure steam is exported.
4.1. Cost of Educts and Side Products
Table 3 summarizes all educts and products of the Bio-OCM process in terms of flow rates, assumed price ranges, and resulting cash flow rates. The yearly cash flows are the products of the flow rates of each educt/product and their respective price ranges. Worst-case cash flows occur with educt cost at upper limit and side-product prices at lower limits, respectively. In the best-case scenario, the contrary occurs. In the average scenario, the arithmetic mean between lower and upper bounds are taken for reference.
In terms of educt cash flows, biogas has the highest share. Since adiabatic reaction operation requires high methane to oxygen ratios, only a small amount of oxygen is consumed. Reducing biogas production cost, e.g., by improving its generation via AD or its treatment step, can yield more significant cost savings to the process.
The obtained ethylene to ethane ratio in the reactor is 1.5, so a significant production of ethane is achieved. However, revenue from ethane sales has a very limited contribution to the total cash flow due to its low sales price. Recycling ethane back to the Bio-OCM reactor could improve ethylene production and lead to additional revenue, but it is likely to reduce overall process selectivity as both C products are readily oxidized into CO2. A potentially good alternative is the addition of Ethane Dehydrogenation (EDH) thermal or catalytic reactor that can selectively convert ethane into ethylene and hydrogen. This is not evaluated further to avoid over-extending this study, but should be considered in future analyses.
The lights stream is found to be the most sensitive component of the total cash flow. Since methane conversion in the reaction is low, a significant amount of methane is exported in this side-product stream. In fact, the entire process concept can also be seen as a biogas upgrade plant producing ethylene as a side product. Therefore, the total cash flow is highly sensitive to the price adopted for the lights stream. For the nominal and best-case scenarios, a positive cash flow is obtained even without bio-ethylene sale revenues. For the worst-case scenario, however, the cash flow is negative, thus increasing the production cost of bio-ethylene.
Recycling the lights stream back to the OCM reactor is undesired because it also contains H2, CO, and N2. Treating this stream to recycle only the methane portion is possible, but energy-intensive. Another alternative is the use of a methanation (Sabatier) reactor, within which CO and H2 from the lights stream and CO2 from the absorption separation section are converted into more methane. Finally, this stream could also be reformed with carbon dioxide, e.g., methane dry reforming, for the side production of synthesis gas. These alternatives could be considered in future techno-economic evaluations as alternative pathways for valorizing the lights off-gas stream.
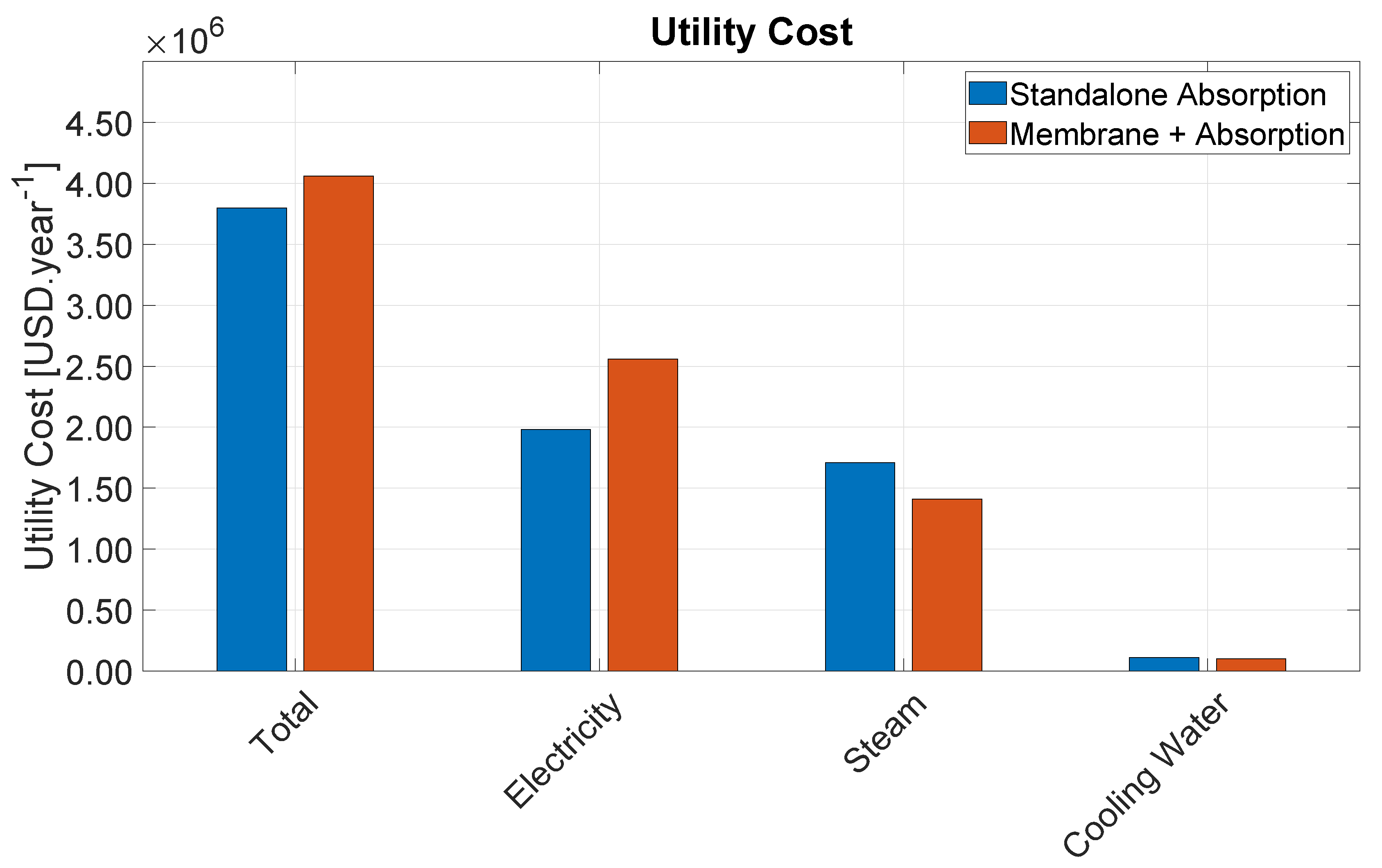
4.2. Utility Cost
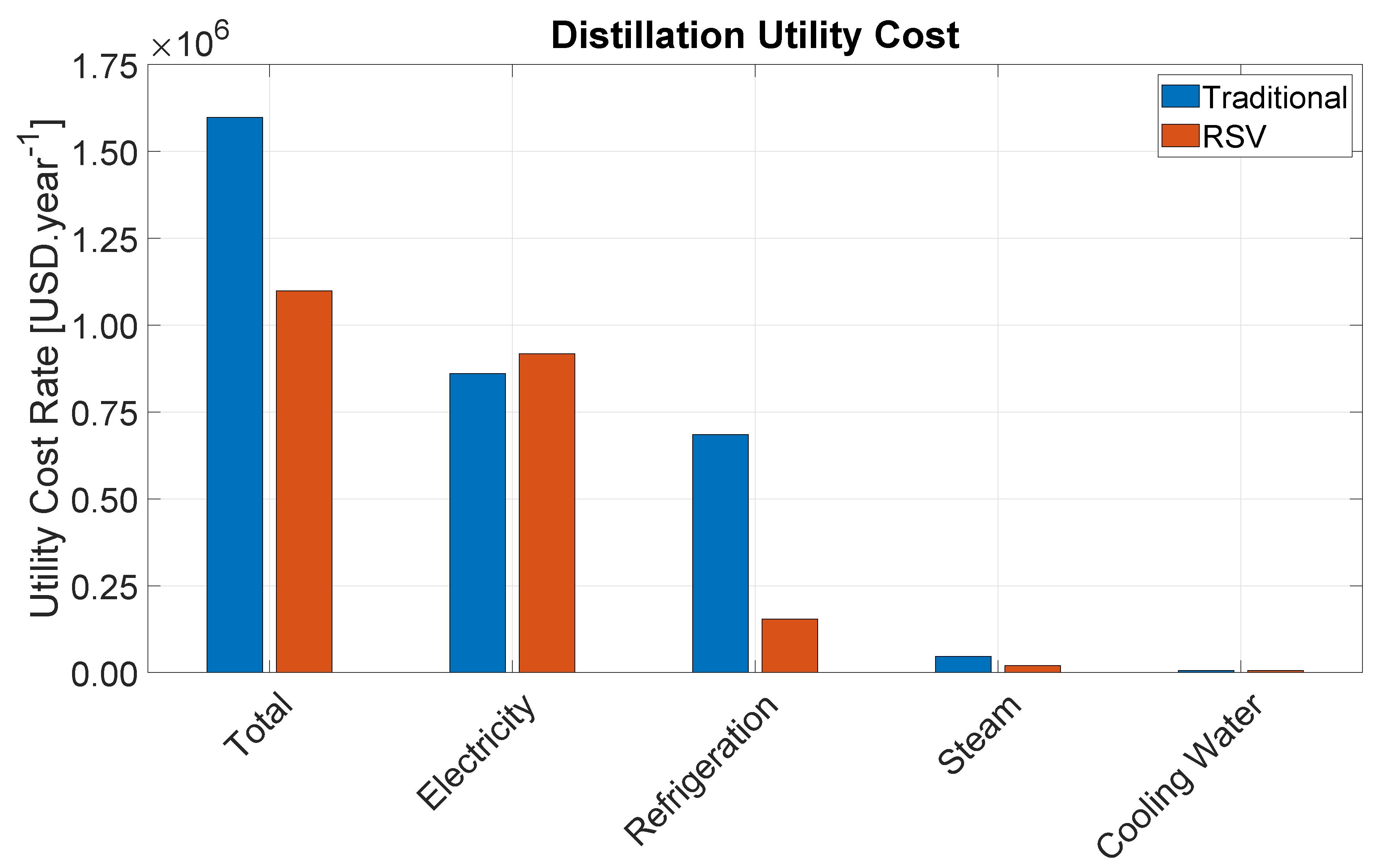
Table 4 provides the total utility cost rates for each utility category and for each section of the Bio-OCM process. Electricity consumption, which incurs mostly from gas compression, represents the major source of utility cost. Refrigeration cost is also indirectly comprised mostly of electricity to run refrigeration compressors. Altogether, these two categories amount to nearly 62% of the total utility cost rate. Therefore, the adopted RSV distillation configuration offers great economic benefit to the process by providing cost savings in the most critical utility category.
Medium Pressure Steam (MPS) represents the second most expensive utility category. MPS is used solely in the amine regeneration step, which makes this the single most expensive step in the entire process representing 52% of the total utility cost rate. This is due to the large amount of CO2 that enters the process with the biogas. This study considered the use of a benchmark 30 wt% MEA solution, which reacts extremely fast with CO2, but requires significant regeneration energy. In this sense, switching to another amine solution could potentially lead to significant cost savings.
Regarding the different process sections, the CO2 removal represents the highest share of utility cost rate. Besides MPS for amine regeneration, this section also consumes a significant amount of electricity to compress the gas from 1.3 bar to 3.13 bar as well as cooling water to run the condenser of the regeneration column, the other amine coolers, as well as the inter-stage cooling of the gas compressors. The distillation section consumes mostly electricity to run the process and refrigeration compressors, whereas the reaction section is able to export HPS to generate additional revenue.
4.3. Equipment Cost
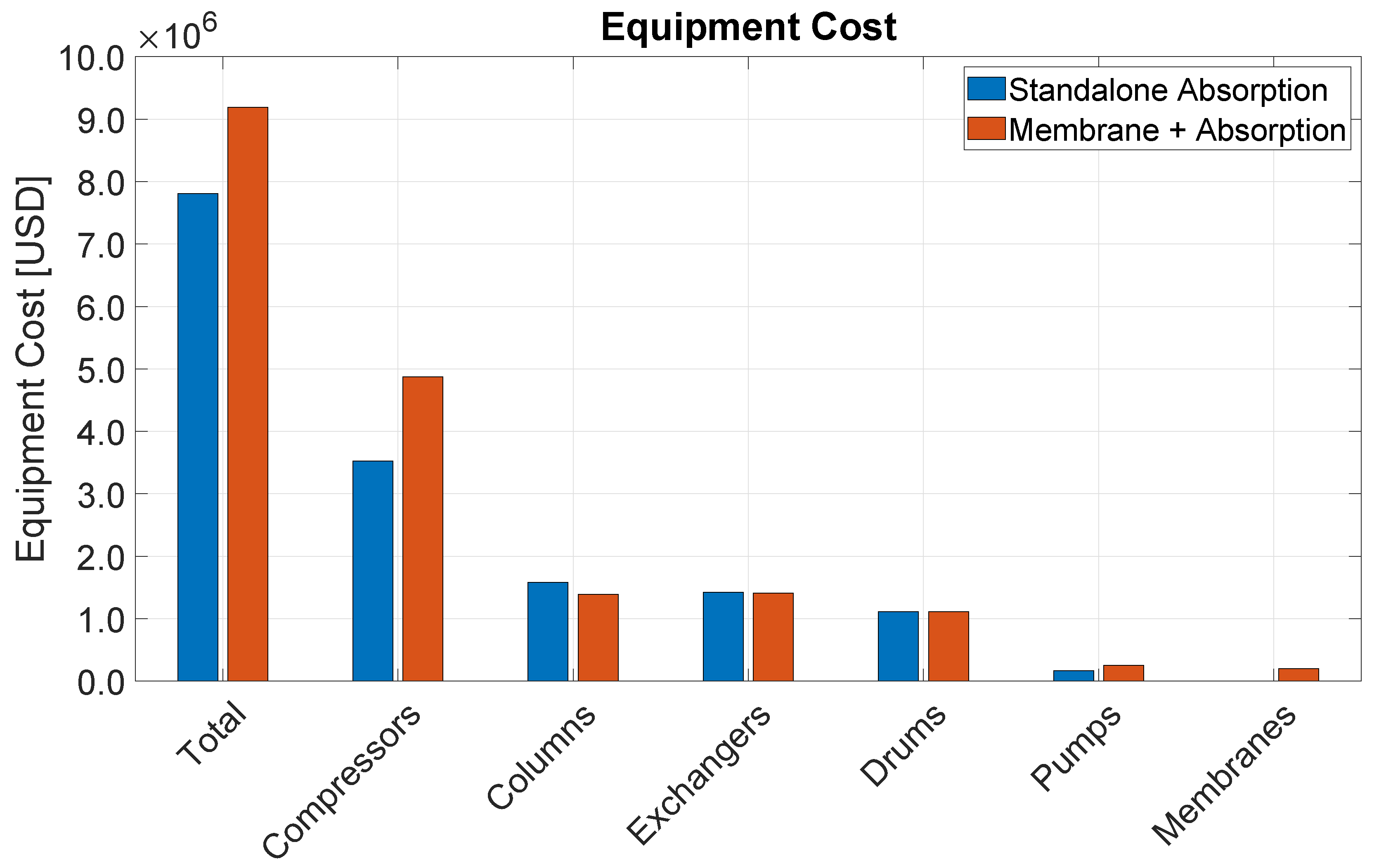
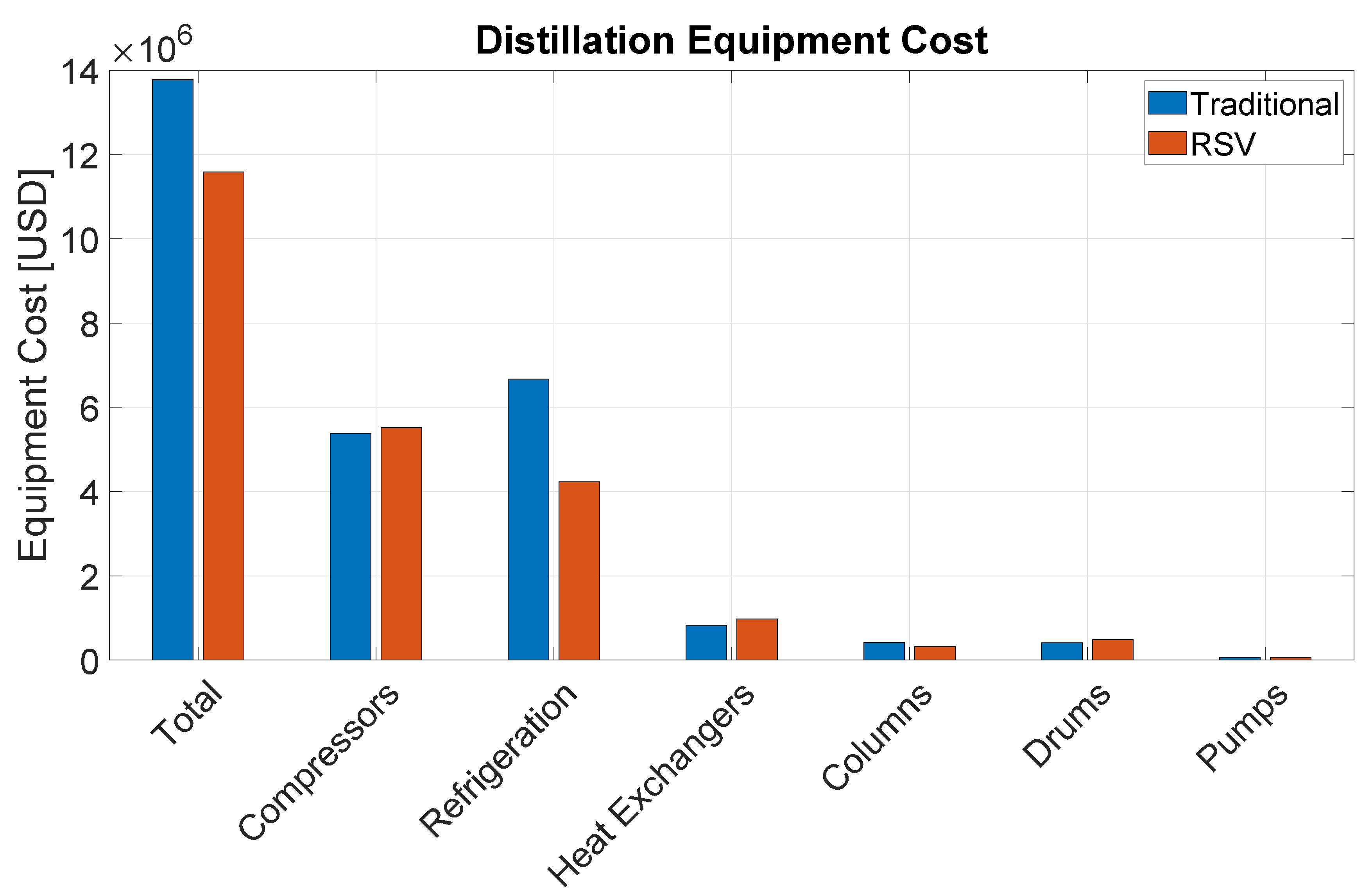
Table 5 details the cost of the different equipment types installed in each process section. Compressors represent the most expensive equipment category closely followed by refrigeration equipment, which also mostly consists of refrigeration compressors. Altogether, these two categories represent 56% of the total capital investment in equipment. The third largest category is heat exchangers, followed by columns, drums, and then by minor contributions of furnaces, reactors, and pumps.
Among the different process sections, distillation is presents the highest capital investment, accounting for 61% of the installed equipment cost. This is mainly because of process gas and refrigeration compressors. It is followed by the CO2 removal section, and finally by the reaction section.
The OCM reactors are assumed to be carbon steel vessels with a refractory lining and packed with the catalyst as described in recent patents [
42]. While the reactor by itself is not a mechanically complex and expensive equipment to manufacture, there would be additional charges to cover the cost of technological development, e.g., in the form of royalties. This uncertainty is assumed to be accounted for in the Monte Carlo simulation, wherein the equipment cost is varied between −30% and +50% as discussed in
Section 2.4.5.
4.4. Bio-Ethylene Production Cost
The total bio-ethylene product output is 4632 t
C2H4 year
-1. Based on the cost estimations obtained in
Section 4.1,
Section 4.2,
Section 4.3, the production cost per mass of bio-ethylene is calculated using Equation (13). The estimated values and ranges of each cost components are used to compute a nominal as well as a worst and a best-case scenarios. These are reported in
Table 6.
Table 6 clarifies that a wide range of results are possible and that the main source of uncertainty relates to the cost of educts and and price of side-products. More specifically, the most sensitive parameter is the cost assigned to the lights stream, which depends on the cost of natural gas in Brazil. In the best-case scenario, wherein the light stream is sold at
USD GJ
-1HHV, the revenue from side products is high enough to completely cover all expenses, resulting in a negative bio-ethylene production cost. In this scenario, bio-ethylene revenue would generate a bonus. In the worst-case scenario, however, the lights stream is sold at
USD GJ
-1HHV and the resulting production cost for bio-ethylene is above typical market values for fossil ethylene. The nominal case results in a production cost below market value.
Table 6 also highlights a well-distributed share between utility and equipment cost. Scaling-up the system would typically lead to a further beneficial dilution of the equipment cost due to economies of scale, but this obviously difficult since biogas production facilities tend to be rather distributed. Therefore, large-scale bio-ethanol plants generating significant amounts of biogas through vinasse AD are the best candidates for this type of project. The ethylene production capacity is, however, still tiny compared to typical fossil-based naphtha or ethane steam cracking plants.
4.5. Monte Carlo Simulation
The Monte Carlo simulation with 10,000 samples resulted in a production cost of 0.53 ± USD kg-1C2H4. The average bio-ethylene production cost is, therefore below typical market values for fossil ethylene. However, the standard deviation clarifies again how wide the range of outcomes is.
The cumulative distribution for the bio-ethylene production cost resulting from the Monte Carlo simulation is given in
Figure 15. The line shows in how many of the Monte Carlo samples (percent-wise) the calculated bio-ethylene production is less or equal to the values given in the x axis. It can be understood as the confidence that the production cost is less or equal to the given value. For instance, a bio-ethylene production costs lower than fossil ethylene’s lowest market value of
USD kg
-1C2H4 is achievable with a 55.2% confidence, whereas a production cost lower than ethylene’s highest market value of
USD kg
-1C2H4 can be achieved with 87.0% confidence. For confidence levels higher than 95%, the bio-ethylene production cost is way above the market value. Overall, it becomes clear the bio-ethylene cannot provide an economic alternative to fossil ethylene with a high degree of confidence.
4.6. Discussion
Brazil produces ethylene mainly from steam cracking of naphtha. Even though the country has recently become a net oil exporter [
53], it imports light oil for petrochemical production (≈113 million Barrels of Oil Equivalent (BOE) in 2012) given that internal production consists mostly (≈70%) of heavy oil that cannot be processed effectively in the domestic refinery park [
54]. Hence, production cost of fossil ethylene in the country is speculated to be on the higher end of the spectrum.
Brazil also hosts a 200 kt year
-1 bio-ethylene plant, which is obtained by catalytic dehydrogenation of bio-ethanol [
55]. It is estimated that the cost of bio-ethylene production from first generation bio-ethanol in Brazil is in the range of 1.13 USD kg
C2H4-1 to 1.17 USD kg
C2H4-1 (considering the average December of 2019 exchange rate of
USD EUR
-1) [
55], which is in accordance with the cost range estimated in this work. The manufacturer, i.e., Braskem, uses it to make polyethylene sold under a seal denominated “I am green”. The products holding this seal must have their bio-based carbon content determined via radiocarbon analysis (ASTM D6866) [
56]. It is unclear, yet highly possible, that customers would accept paying a higher price for a certified bio-based polyethylene containing the seal. This type of certification and market strategy could also enable bio-ethylene produced via Bio-OCM to reach higher market values.
The depletion of resources and introduction of restrictive measures such as carbon taxation is also likely to gradually raise the price for fossil commodities, including ethylene and natural gas, in short-to-medium term. Fully replacing fossil-based ethylene by biogas-based ethylene is unthinkable due to the limited production volumes achievable. Bio-OCM can, however, similarly to catalytic bioethanol dehydration, act as a bridge technology. It immediately allows for the use of a renewable feedstock to produce traditional platform chemicals with established processing and transportation infrastructure until the production of new bio-based platform chemicals is fully developed and scaled-up.
This study also highlighted the importance of the lights stream as a side-product for the overall economic viability of the process. Several OCM researchers are trying to improve yield of C
products via new catalysts and novel reactor concepts, e.g., chemical looping [
57]. However, industrial implementation of such technologies only seem feasible in the medium-to-long term future, while developing multi-product systems that efficiently integrate OCM can offer significant potential for industrial deployment today. Future studies should, thus focus in making the most out of the off-gas stream, e.g., via methanation and methane dry reforming. A combination of these process routes would be a solution to increase flexibility in the production of bio-ethylene, bio-syngas, and bio-energy based on fluctuating market conditions.
The vinasse effluent is currently employed within the agricultural sector for soil and water amendment in a technique called fertigation. However, if its high organic load is not properly treated prior to this, significant GHG (particularly methane) emissions incur. Providing alternative pathways for monetizing biogas produced through vinasse AD adds incentive for its treatment and for emission reductions. Based on the yields obtained in this study and the biogas production potential of the state of São Paulo, Brazil [
23], it has been estimated that ethylene produced through Bio-OCM could replace as much as 900 kt of fossil ethylene yearly in this state alone. This could potentially also lead to significant environmental benefits, which are best addressed through more holistic studies, i.e., Life Cycle Assessment.
5. Conclusions and Outlook
This contribution designs a process and investigates the economic potential of bio-ethylene production via Oxidative Coupling of Methane (OCM) contained in biogas derived from the Anaerobic Digestion (AD) of vinasse effluent from bioethanol refineries in Brazil. The optimized process structure consists in a reaction section employing Packed Bed Reactors (PBRs) in adiabatic regime, a CO2 removal section employing amine-absorption, and a distillation section employing a Recycle Split Vapor (RSV) configuration. A C reaction product yield of 16.12% is achieved in the simulations by optimizing process conditions. However, technical challenges regarding long-term catalyst and operation stability under the high temperature and CO2 dilution involved still need to be addressed, and the potential use of low-temperature catalysts should be considered in future studies aiming at industrial implementation. The proposed hybrid membrane-absorption configuration for the CO2 removal section is found to be uneconomical. The proposed RSV distillation configuration, on the other hand, provides a 24% lower total annualized cost than the traditional distillation configuration and, thus, has a great potential to be employed industrially.
The bio-ethylene production cost is estimated under a wide range of possible costs for educts, products, utilities, and equipment by means of a Monte Carlo simulation. The resulting average production cost of bio-ethylene is 0.53 ± USD kg-1C2H4 and is lower than typical ethylene market values. The bio-ethylene production cost is very sensitive towards the price assigned to the lights stream as a side-product, which in this study is based on the natural gas price. Under the assumed range of price scenarios, a bio-ethylene production cost under USD kg-1C2H4 is only achieved with a 55.2% probability and in those cases where natural gas, ethylene, and/or electricity prices are high. Further research should focus on reducing the uncertainty on the price assigned to the lights stream as well as investigating different process integration options to exploit this stream.