Feasibility of Processing Hot-Melt Pressure-Sensitive Adhesive (HMPSA) with Solvent in the Lab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adhesive Preparation
2.3. Bond Strength Test
2.4. Viscosity Measurement
2.5. Thermal Analysis
2.6. Fourier Transform Infrared (FTIR) Characterization
2.7. Statistical Analysis
3. Results and Discussion
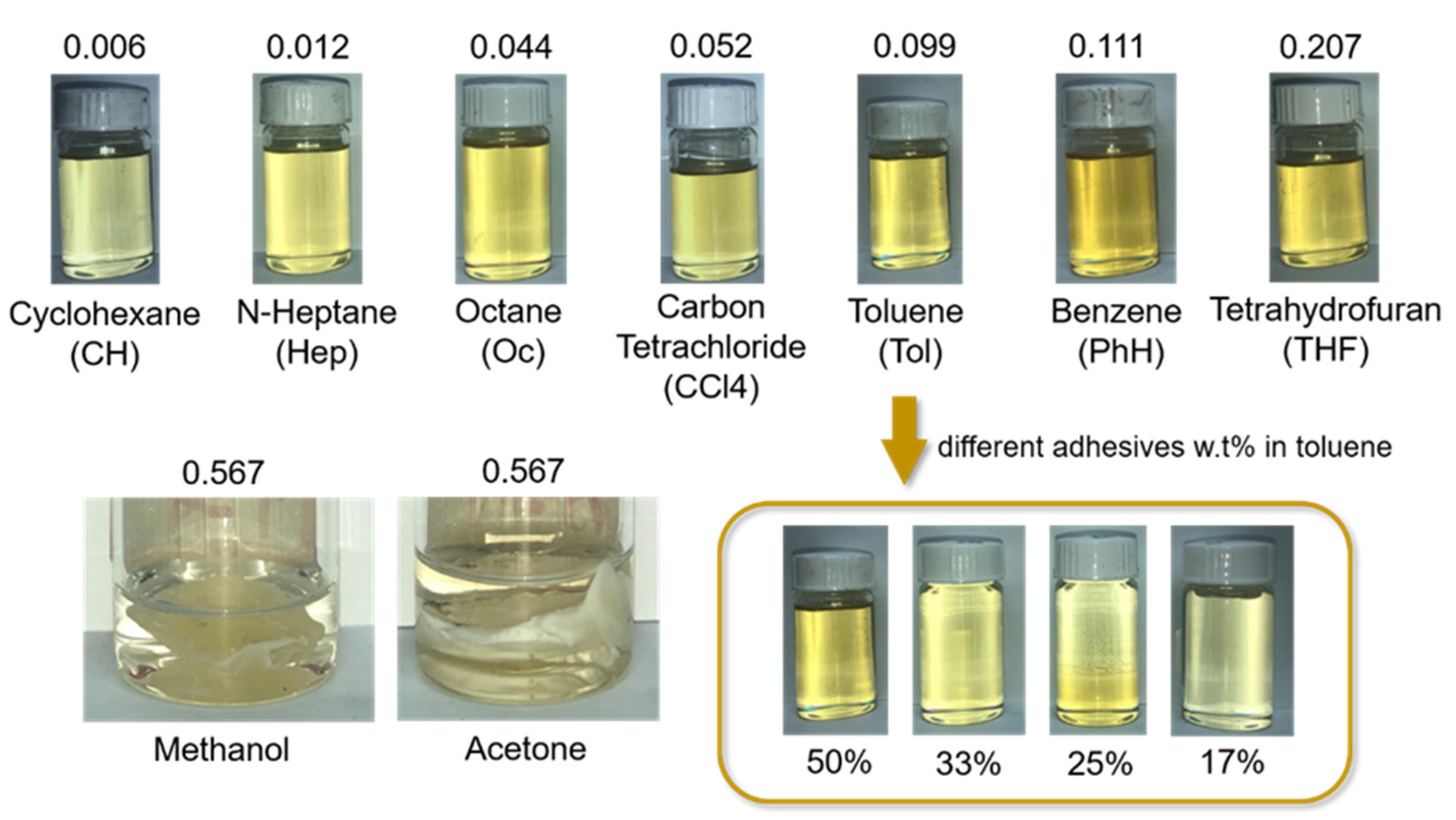
3.1. Solubility
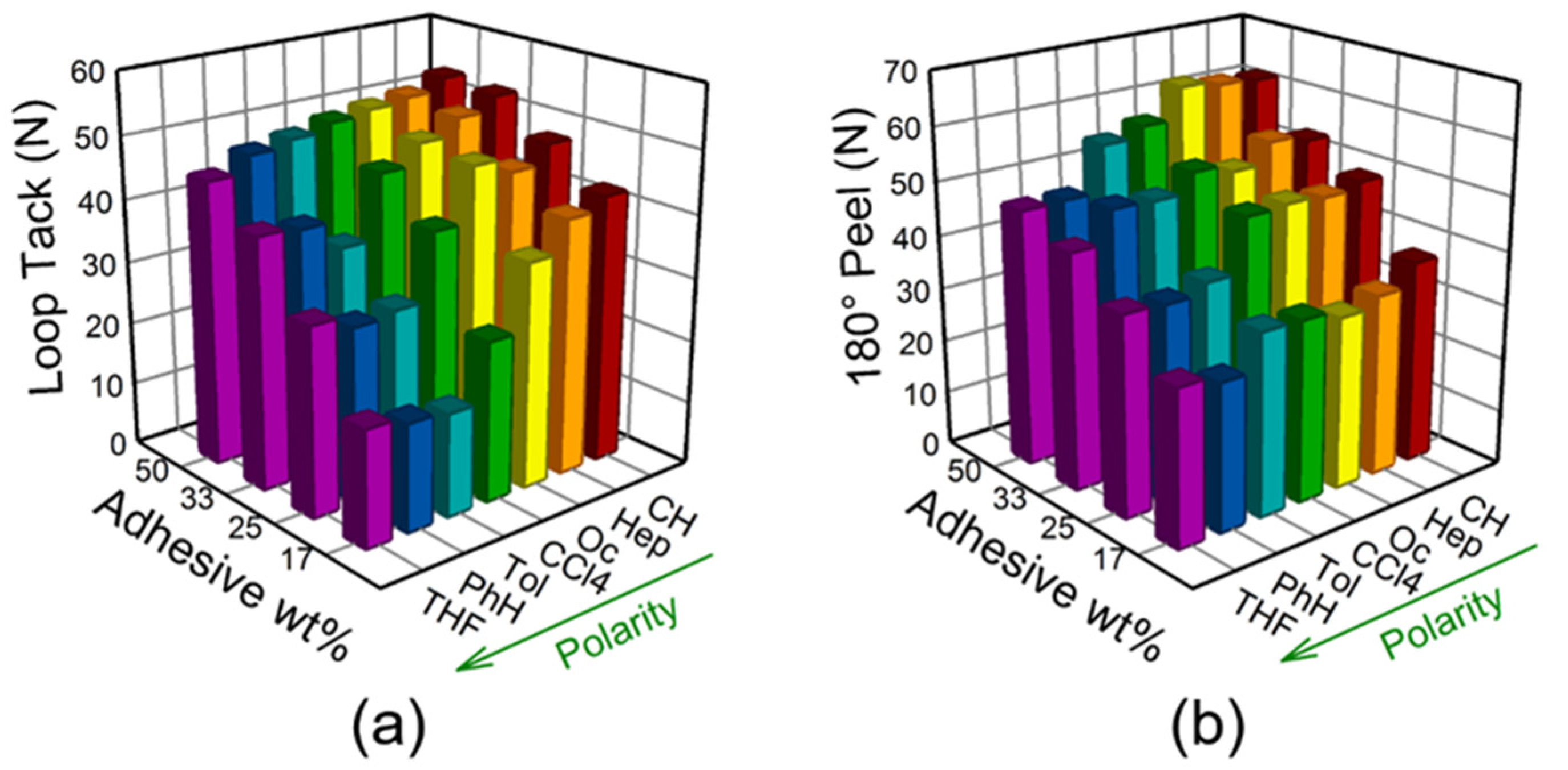
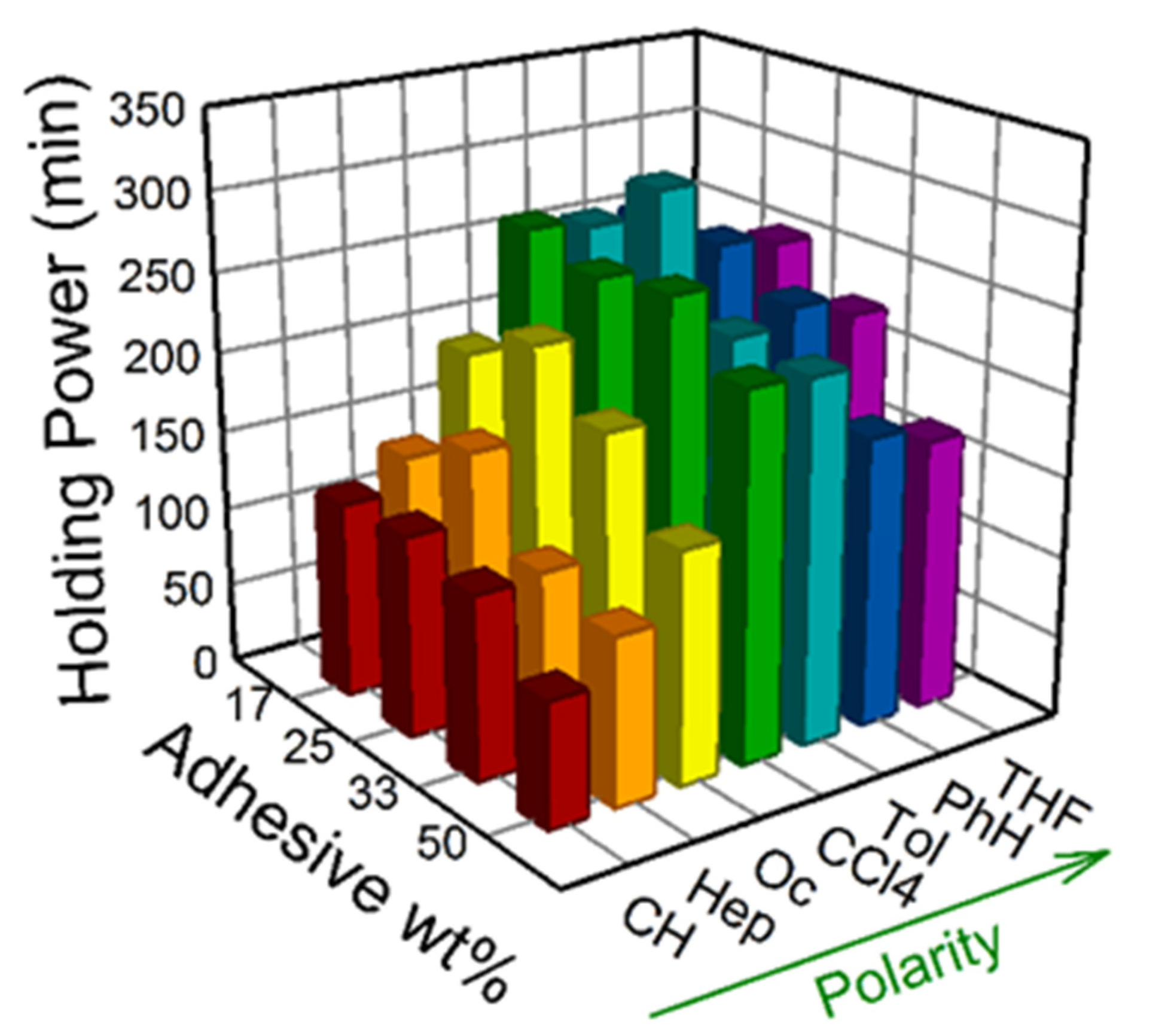
3.2. Bond Strength
3.3. Viscosity
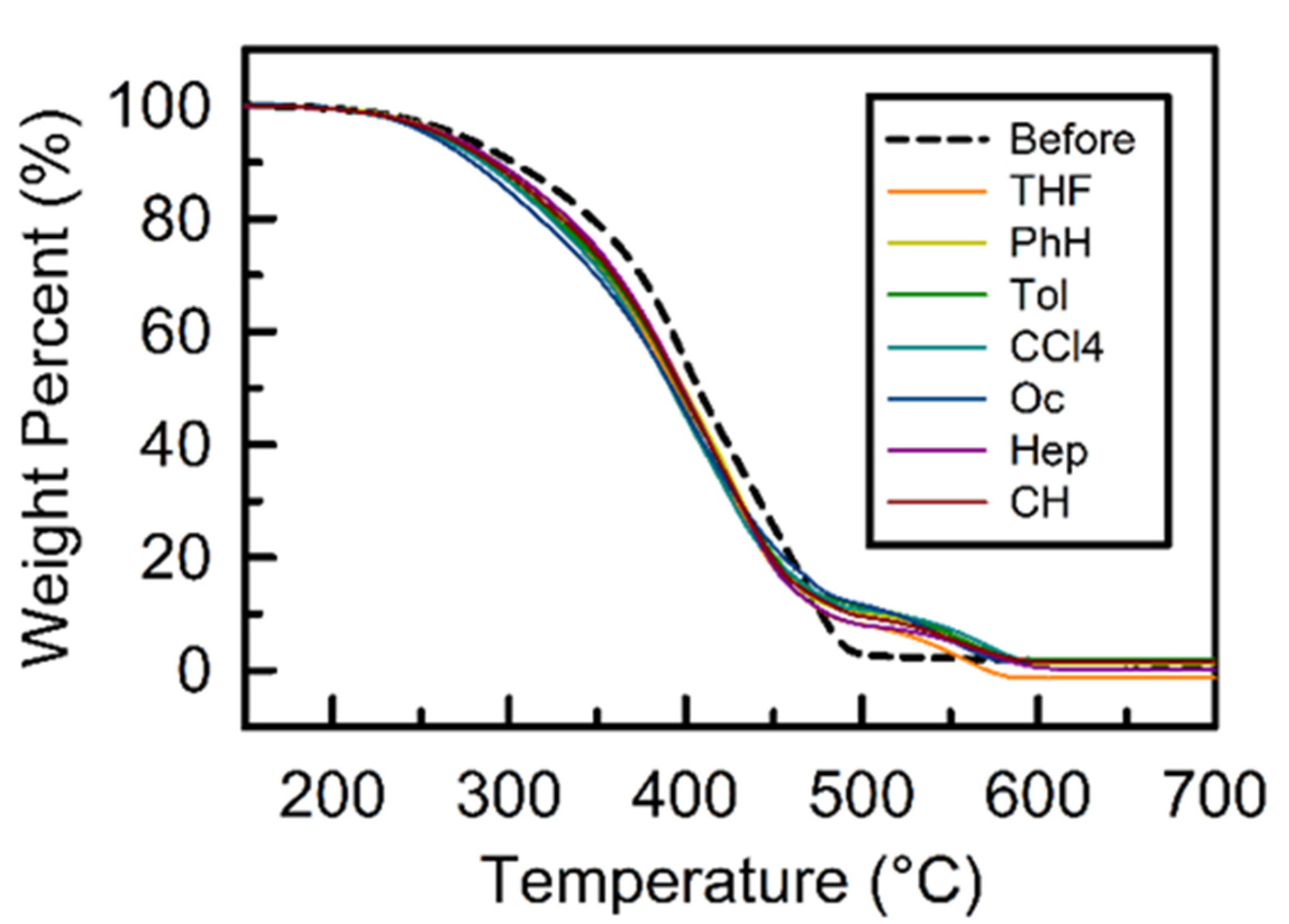
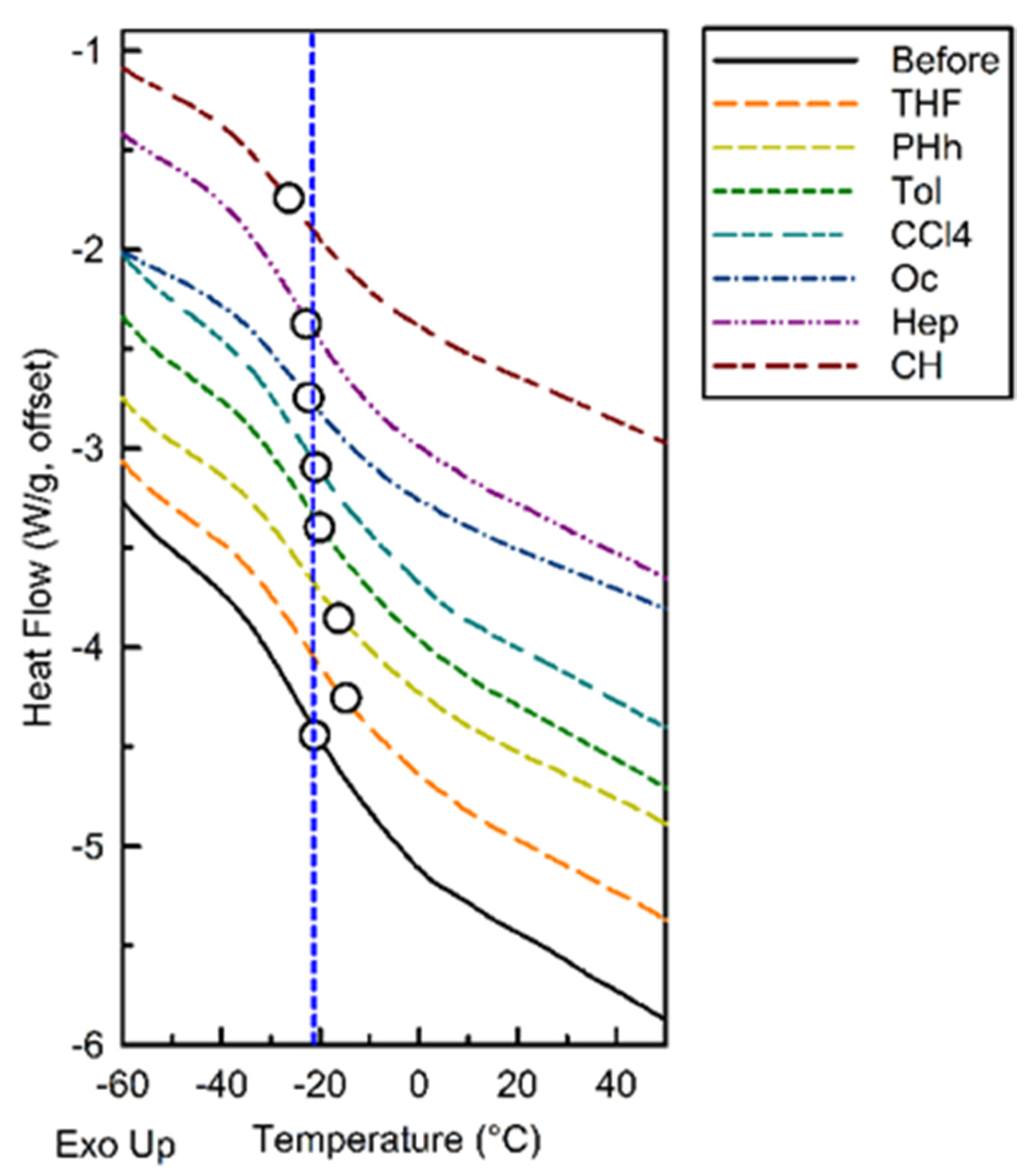
3.4. Thermal Properties
3.5. Changes in Chemical Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creton, C. Pressure-Sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef] [Green Version]
- Paul, C. Hot-melt adhesives. MRS Bull. 2003, 28, 440–444. [Google Scholar] [CrossRef]
- Czech, Z. Solvent-based pressure-sensitive adhesives for removable products. Int. J. Adhes. Adhes. 2006, 26, 414–418. [Google Scholar] [CrossRef]
- Czech, Z.; Martysz, D. UV-crosslinkable solvent-based pressure-sensitive adhesives with very low shrinkage. Int. J. Adhes. Adhes. 2004, 24, 533–534. [Google Scholar] [CrossRef]
- Czech, Z. Development of solvent-free pressure-sensitive adhesive acrylics. Int. J. Adhes. Adhes. 2004, 24, 119–125. [Google Scholar] [CrossRef]
- Czech, Z.; Butwin, A.; Mozelewska, K. Room temperature coatable solvent-free pressure-sensitive adhesives based on acrylics (SF-LVS). Coat. Int. 2017, 50, 14–18. [Google Scholar]
- Gibert, F.X.; Marin, G.; Derail, C.; Allal, A.; Lechat, J. Rheological properties of hot melt pressure-sensitive adhesives based on styrene-isoprene copolymers. Part 1: A rheological model for [sis-si] formulations. J. Adhes. 2003, 79, 825–852. [Google Scholar] [CrossRef]
- Khan, I.; Poh, B.T. Natural Rubber-Based Pressure-Sensitive Adhesives: A Review. J. Polym. Environ. 2011, 19, 793–811. [Google Scholar] [CrossRef]
- Rutherford, W.L. Developing a new tackifying resin for hot melt adhesives. Adhes. Age 1972, 15, 49–50. [Google Scholar]
- Grunlan, J.C.; Holguin, D.L.; Chuang, H.-K.; Perez, I.; Chavira, A.; Quilatan, R.; Akhave, J.; Mehrabi, A.R. Combinatorial Development of Pressure-Sensitive Adhesives. Macromol. Rapid Commun. 2004, 25, 286–291. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Zhang, C. Preparation and characterization of polarity-modulated SIS-based hot-melt pressure-sensitive adhesives. J. Adhes. Sci. Technol. 2014, 28, 1090–1102. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, R.; Zhang, C.; Wang, Q. SISO-based hot-melt pressure-sensitive adhesives for transdermal delivery of hydrophilic drugs. Int. J. Adhes. Adhes. 2017, 74, 86–91. [Google Scholar] [CrossRef]
- Akiyama, S.; Kobori, Y.; Sugisaki, A.; Koyama, T.; Akiba, I. Phase behavior and pressure sensitive adhesive properties in blends of poly(styrene-b-isoprene-b-styrene) with tackifier resin. Polymer 2000, 41, 4021–4027. [Google Scholar] [CrossRef]
- Poh, B.T.; Chee, B.C. Effect of Blend Ratio and Testing Rate on the Adhesion Properties of Epoxidized Natural Rubber (ENR 25)/Styrene–Butadiene Rubber (SBR) Blend Adhesive. J. Adhes. 2014, 91, 950–961. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Macosko, C.W. Melt versus solvent coating: Structure and properties of block-copolymer-based pressure-sensitive adhesives. J. Appl. Polym. Sci. 2002, 86, 3355–3367. [Google Scholar] [CrossRef]
- Kim, D.-J.; Kim, H.-J.; Yoon, G.-H. Effects of blending and coating methods on the performance of SIS (styrene-isoprene-styrene)-based pressure-sensitive adhesives. J. Adhes. Sci. Technol. 2004, 18, 1783–1797. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Li, J.; Zhang, L.; Zuo, B.; Tsui, O.K.C.; Wang, X. Conformation-Sensitive Surface Dynamics in Thin Poly(ethylene terephthalate) Film. Macromolecules 2019, 52, 2580–2588. [Google Scholar] [CrossRef]
- Donker, C. Holt melt tape formulations with new hydrocarbon tackifying resins. Gummi Fasern Kunstst 2006, 59, 421–425. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Doi, T.; Takagi, H.; Shimizu, N.; Igarashi, N.; Sakurai, S. Effects of drying temperature in solution coating process on microphase-separated structures in coated layers of pressure-sensitive adhesive composed of di- and triblock copolymer blends as revealed by small-angle X-ray scatterin. Polym. J. 2019, 170, 211–221. [Google Scholar] [CrossRef]
- Karyu, N.; Noda, M.; Fujii, S.; Nakamura, Y.; Urahama, Y. Effect of adhesive thickness on the wettability and deformability of polyacrylic pressure-sensitive adhesives during probe tack test. J. Appl. Polym. Sci. 2016, 133, 43639. [Google Scholar] [CrossRef]
- Winter, A.; Andorfer, L.; Herzele, S.; Zimmermann, T.; Saake, B.; Edler, M.; Griesser, T.; Konnerth, J.; Gindl-Altmutter, W. Reduced polarity and improved dispersion of microfibrillated cellulose in poly(lactic-acid) provided by residual lignin and hemicellulose. J. Mater. Sci. 2016, 52, 60–72. [Google Scholar] [CrossRef]
- Yoshie, K.; Yada, S.; Ando, S.; Ishihara, K. Effects of inner polarity and viscosity of amphiphilic phospholipid polymer aggregates on the solubility enhancement of poorly water-soluble drugs. Colloids Surf. B Biointerfaces 2020, 195, 111215. [Google Scholar] [CrossRef]
- Eng-Soon, T. Simple equipment to measure holding power of pressure sensitive adhesives. Adhes. Age 1996, 39, 36–38. [Google Scholar]
- Abboud, T.; Wutzler, A.; Radusch, H.-J. Effect of viscoelastic and surface properties on tack, peel adhesion and shear strength of polymer blends applied as hot melt pressure sensitive adhesive models comprising tackifying agents of various chemical nature. Express Polym. Lett. 2020, 14, 731–740. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, L.; Huang, Y.; Liu, B.; Hwang, K. The effect of van der Waals-based interface cohesive law on carbon nanotube-reinforced composite materials. Compos. Sci. Technol. 2007, 67, 2941–2946. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, M.; Liu, A. A review on mechanical properties of pressure sensitive adhesives. Int. J. Adhes. Adhes. 2013, 41, 98–106. [Google Scholar] [CrossRef]
- Peykova, Y.; Lebedeva, O.V.; Diethert, A.; Müller-Buschbaum, P.; Willenbacher, N. Adhesive properties of acrylate copolymers: Effect of the nature of the substrate and copolymer functionality. Int. J. Adhes. Adhes. 2012, 34, 107–116. [Google Scholar] [CrossRef]
- Durand, A.; Dellacherie, E. Aqueous Solutions of Native and Hydrophobically Modified Polysaccharides: Temperature Effect. Biomacromolecules 2006, 7, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.; Lee, G. Effect of residual solvent in polymer adhesive matrix on release and skin permeation of scopolamine. Int. J. Pharm. 2015, 491, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.S.; Yoon, S.H.; Hwang, H.Y. Effects of residual oils on the adhesion characteristics of metal-CFRP adhesive joints. Compos. Struct. 2019, 207, 240–254. [Google Scholar] [CrossRef]
- Frübing, P.; Wang, F.; Wegener, M. Relaxation processes and structural transitions in stretched films of polyvinylidene fluoride and its copolymer with hexafluoropropylene. Appl. Phys. A 2012, 107, 603–611. [Google Scholar] [CrossRef]
- Özdemir, C.; Güner, A. Solubility profiles of poly(ethylene glycol)/solvent systems, I: Qualitative comparison of solubility parameter approaches. Eur. Polym. J. 2007, 43, 3068–3093. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.-G. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef] [PubMed]




| Adhesive wt.% | Solvent | η0, cP | r2 | Eη (298 K), kJ/mol | η (298 K), cP |
|---|---|---|---|---|---|
| 50 | THF | 0.755 | 0.9991 | 16.737 | 648.115 |
| PhH | 0.329 | 0.9991 | 19.236 | 774.067 | |
| Tol | 1.814 | 0.9992 | 14.158 | 550.171 | |
| CCl4 | 0.047 | 0.9994 | 30.031 | 8681.116 | |
| Oc | 4.42 × 10−15 | 0.9990 | 103.399 * | 5875.483 | |
| Hep | 1.21 × 10−11 | 0.9990 | 81.745 | 2563.892 | |
| CH | 0.013 | 0.9994 | 29.557 | 1914.276 | |
| 33 | THF | 2.477 | 0.9935 | 9.015 | 94.257 |
| PhH | 0.734 | 0.9976 | 12.502 | 114.058 | |
| Tol | 0.115 | 0.9966 | 15.564 | 61.363 | |
| CCl4 | 0.299 | 0.9983 | 21.731 | 1931.933 | |
| Oc | 5.72 × 10−17 | 0.9949 | 112.721 * | 3273.596 | |
| Hep | 1.25 × 10−16 | 0.9943 | 103.866 | 201.304 | |
| CH | 0.054 | 0.9977 | 20.078 | 178.239 | |
| 25 | THF | 0.717 | 0.9966 | 9.686 | 35.756 |
| PhH | 0.304 | 0.9972 | 10.064 | 17.661 | |
| Tol | 0.174 | 0.9965 | 11.692 | 19.569 | |
| CCl4 | 3.892 | 0.9970 | 11.011 | 331.501 | |
| Oc | 7.36 × 10−19 | 0.9960 | 123.063 * | 2746.660 | |
| Hep | 1.31 × 10−12 | 0.9923 | 88.153 | 3709.072 | |
| CH | 0.067 | 0.9961 | 16.929 | 61.844 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Wang, J.; Huang, H.; Wang, M.; Zhang, Y.; Zhang, L. Feasibility of Processing Hot-Melt Pressure-Sensitive Adhesive (HMPSA) with Solvent in the Lab. Processes 2021, 9, 1608. https://doi.org/10.3390/pr9091608
Xue J, Wang J, Huang H, Wang M, Zhang Y, Zhang L. Feasibility of Processing Hot-Melt Pressure-Sensitive Adhesive (HMPSA) with Solvent in the Lab. Processes. 2021; 9(9):1608. https://doi.org/10.3390/pr9091608
Chicago/Turabian StyleXue, Jing, Jing Wang, Haofei Huang, Ming Wang, Yali Zhang, and Lijuan Zhang. 2021. "Feasibility of Processing Hot-Melt Pressure-Sensitive Adhesive (HMPSA) with Solvent in the Lab" Processes 9, no. 9: 1608. https://doi.org/10.3390/pr9091608






