Numerical Simulation of the Novel Coronavirus Spread in Commercial Aircraft Cabin
Abstract
:1. Introduction
2. Methods
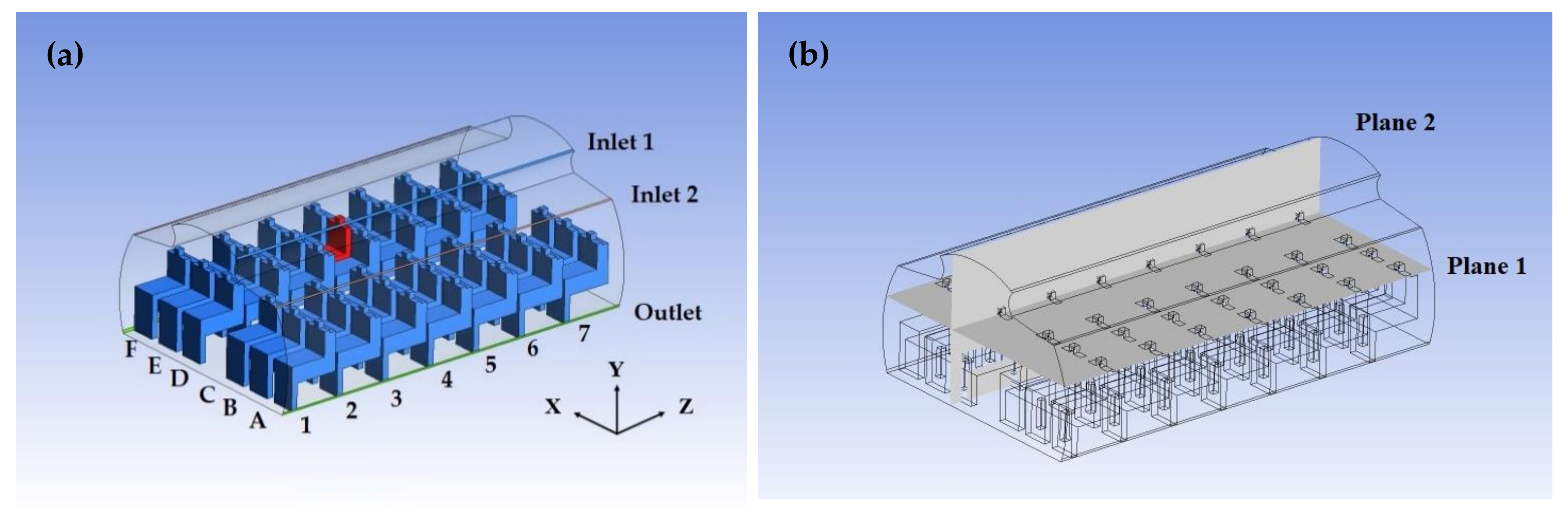
2.1. Cabin Physical Model and Meshing
2.2. Boundary Condition Setting
2.3. SARS-CoV-2 Mass Fraction Numerical Simulation
2.4. Susceptible Exposure Index (SEI) Calculation
3. Results and Discussion
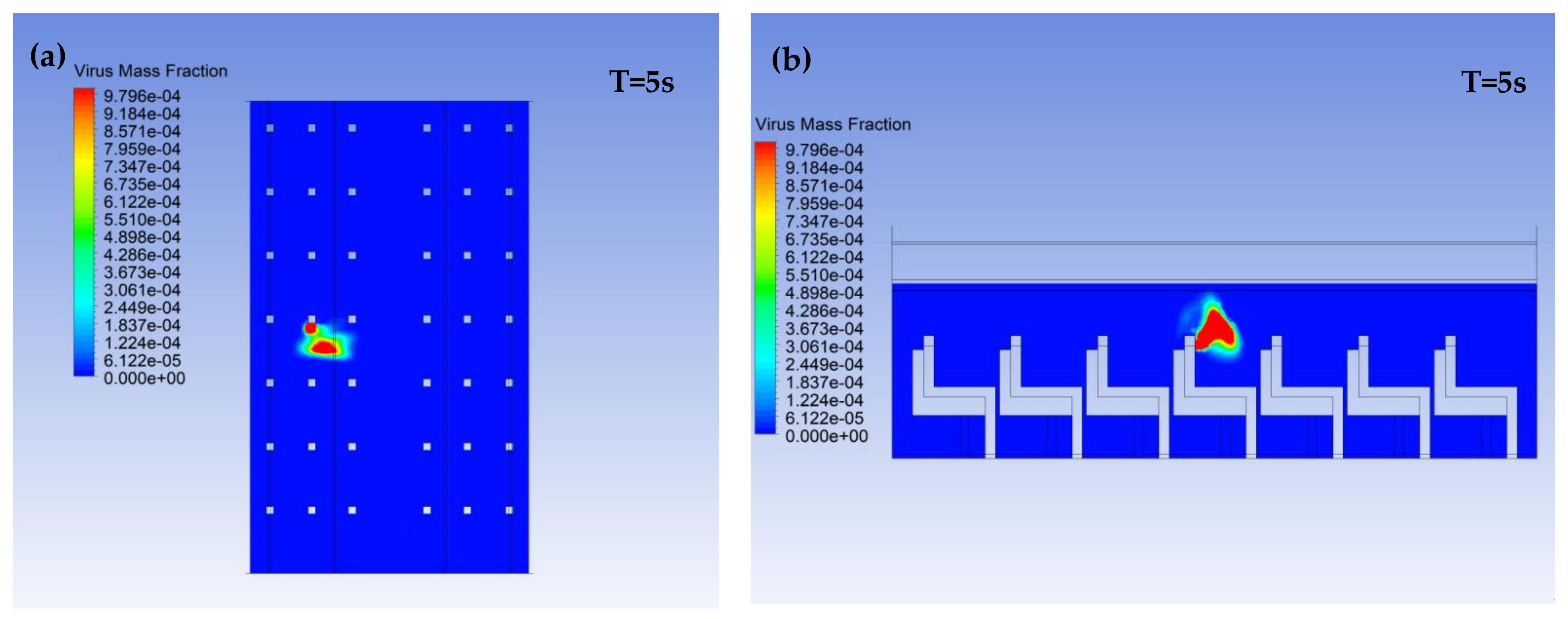
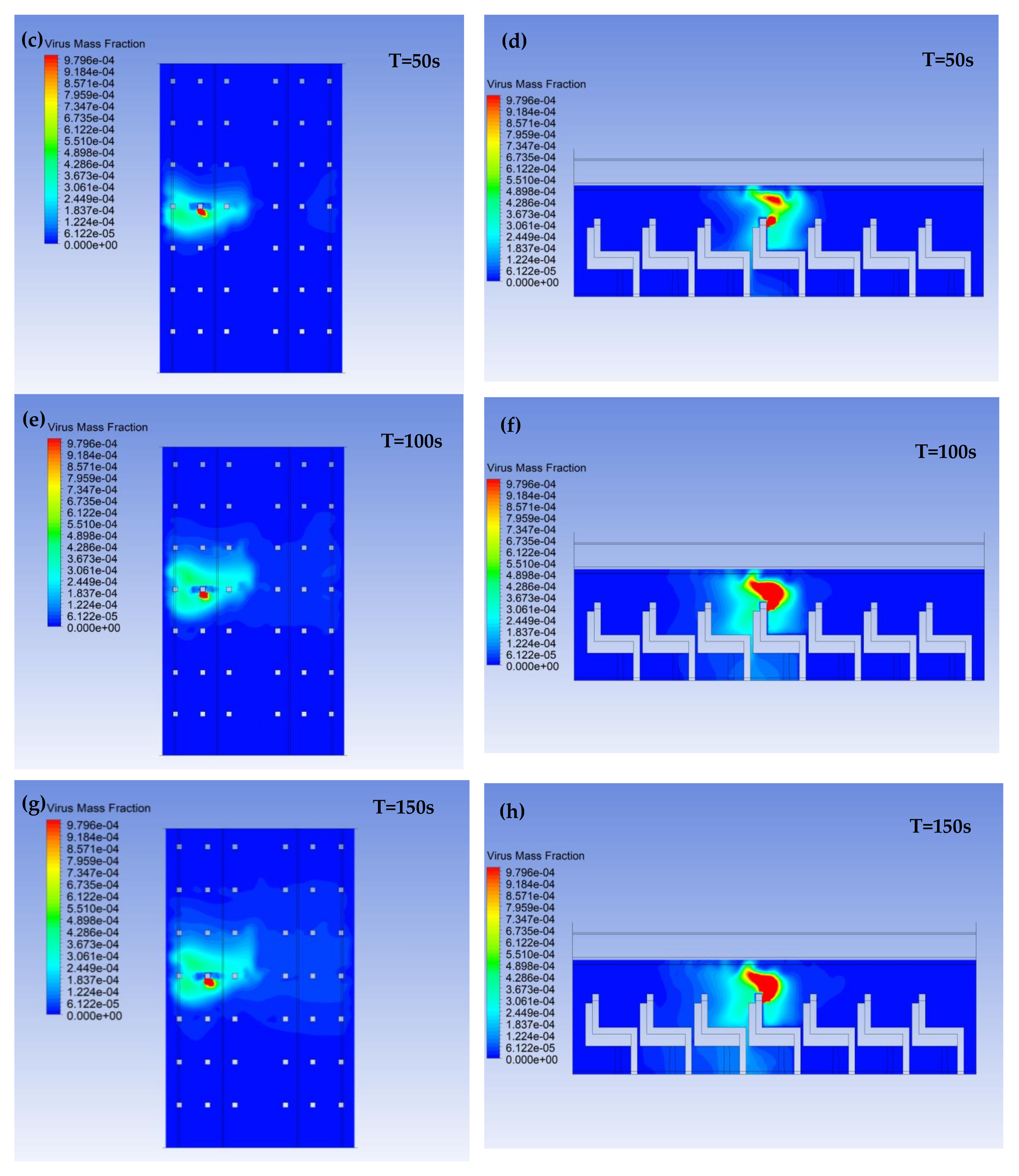
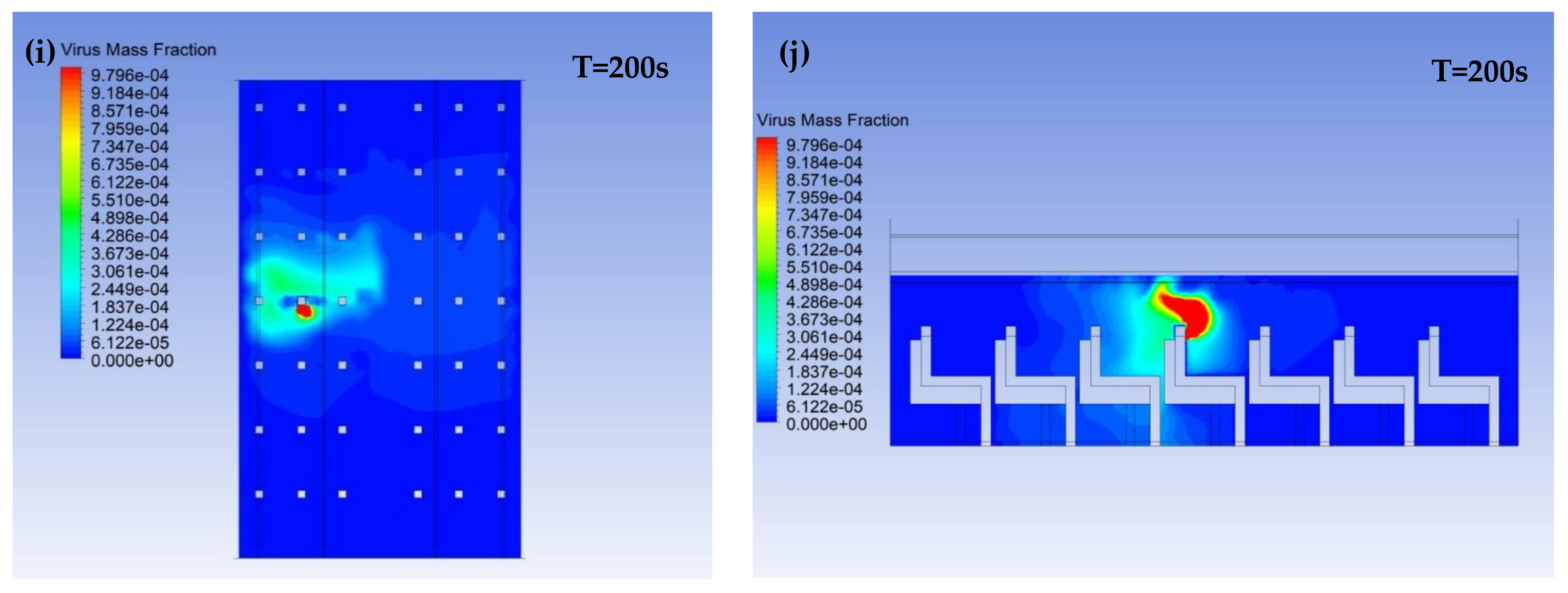
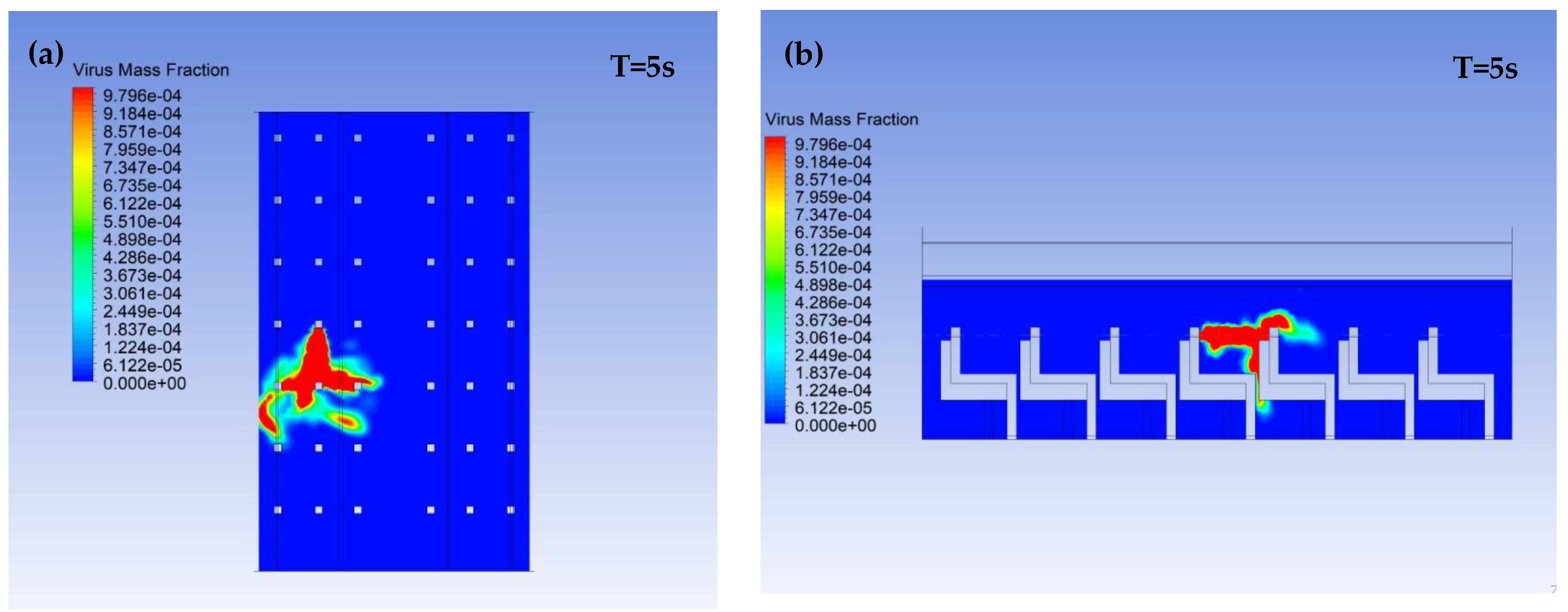
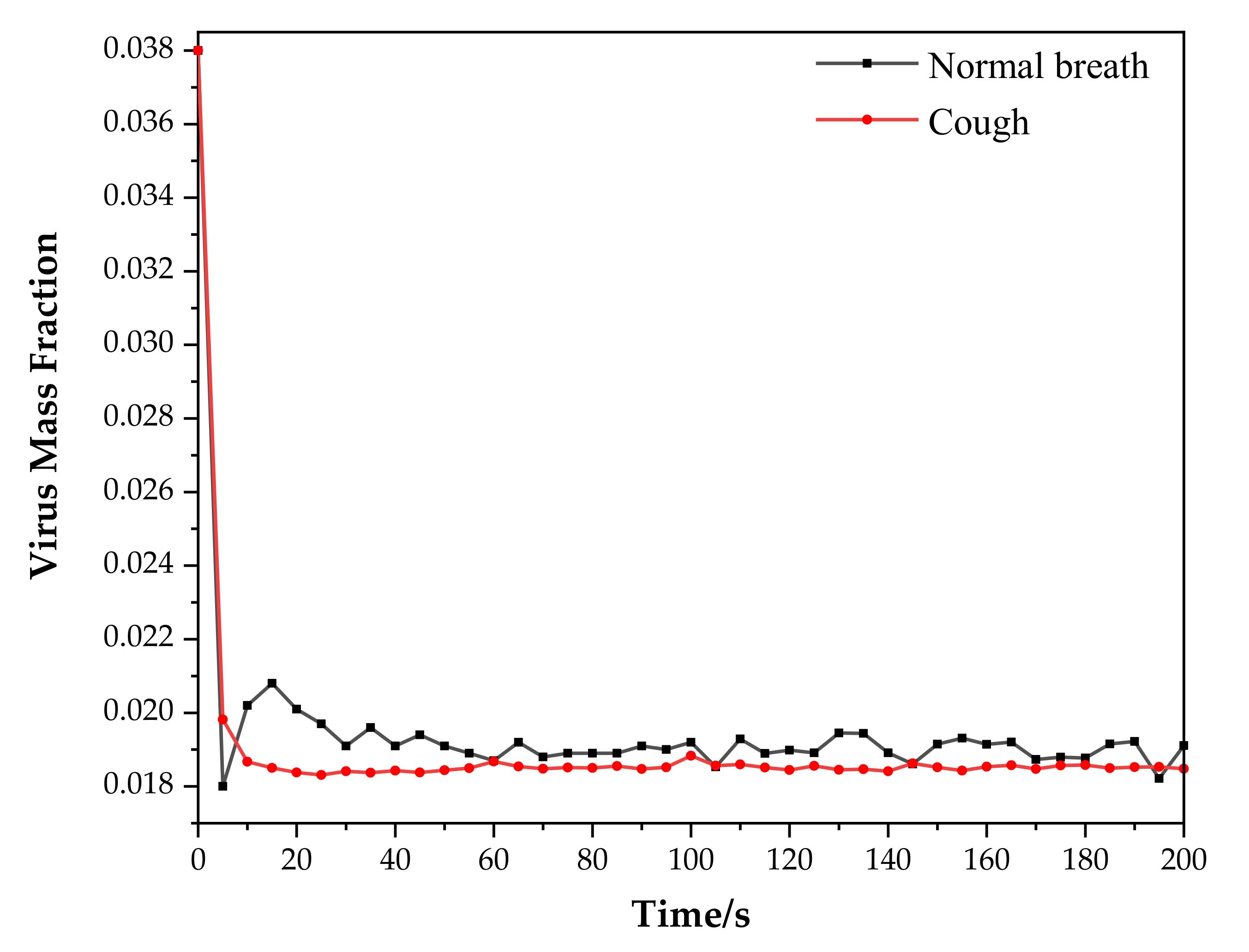
3.1. Virus Mass Fraction Dynamics
3.2. SEI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Report of the WHO—China Joint Mission on Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mboreha, C.A.; Abdallah, C.S.; Kumar, G. Risk and prevention of COVID-19 in a commercial aircraft cabin: An overview. Int. J. Eng. Appl. Sci. Technol. 2020, 5, 661–670. [Google Scholar] [CrossRef]
- Mangili, A.; Gendreau, M.A.J.L. Transmission of infectious diseases during commercial air travel. Lancet 2005, 365, 989–996. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Fang, X.; Yan, P.; Tu, J. Transmission of COVID-19 virus by cough-induced particles in an airliner cabin section. Eng. Appl. Comput. Fluid Mech. 2021, 15, 934–950. [Google Scholar] [CrossRef]
- Olsen, S.J.; Chang, H.-L.; Cheung, T.Y.-Y.; Tang, A.F.-Y.; Fisk, T.L.; Ooi, S.P.-L.; Kuo, H.-W.; Jiang, D.D.-S.; Chen, K.-T.; Lando, J.; et al. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003, 349, 2416–2422. [Google Scholar] [CrossRef]
- National Health Commission. Novel Coronavirus Pneumonia Prevention and Control Plan, 5th ed.; National Health Commission: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Lei, H.; Li, Y.; Xiao, S.; Yang, X.; Lin, C.H.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S.J.R. Logistic growth of a surface contamination network and its role in disease spread. Sci. Rep. 2017, 7, 14826. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, A.; Wiedensohler, A.; Mishra, S.K. An Overview on the Role of Relative Humidity in Airborne Transmission of SARS-CoV-2 in Indoor Environments. Aerosol Air Qual. Res. 2020, 20, 1856–1861. [Google Scholar] [CrossRef]
- Prather, K.A.; Wang, C.C.; Schooley, R.T.J.S. Reducing transmission of SARS-CoV-2. Science 2020, 368, eabc6197. [Google Scholar] [CrossRef] [PubMed]
- Astuti, I.; Diabetes, Y.J. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020, 54, 635–638. [Google Scholar] [CrossRef] [Green Version]
- To, G.S.; Wan, M.P.; Chao, C.; Fang, L.; Melikov, A. Experimental Study of Dispersion and Deposition of Expiratory Aerosols in Aircraft Cabins and Impact on Infectious Disease Transmission. Aerosol Sci. Technol. 2009, 43, 466–485. [Google Scholar] [CrossRef]
- Wang, G.Q.; Ji, S.C. Summary of the analysis of the spread of germs in the cabin. Acta Aeronautica et Astronautica Sinica. 2015, 36, 2577–2587. (In Chinese) [Google Scholar] [CrossRef]
- Mazumdar, S.; Chen, Q.J. Influence of cabin conditions on placement and response of contaminant detection sensors in a commercial aircraft. J. Environ. Monit. 2008, 10, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, Q. Comparison of the Eulerian and Lagrangian methods for predicting particle transport in enclosed spaces. Atmos. Environ. 2007, 41, 5236–5248. [Google Scholar] [CrossRef]
- Davis, A.C.; Zee, M.; Clark, A.D.; Wu, T.; Olson, N.A. Computational Fluid Dynamics Modeling of Cough Transport in an Aircraft Cabin. bioRxiv 2021, 2, 15. [Google Scholar] [CrossRef]
- Talaat, K.; Abuhegazy, M.; Mahfoze, O.A.; Anderoglu, O.; Poroseva, S.V. Simulation of aerosol transmission on a Boeing 737 airplane with intervention measures for COVID-19 mitigation. Phys. Fluids 2021, 33, 033312. [Google Scholar] [CrossRef] [PubMed]
- Conceição, S.T.; Pereira, M.L.; Tribess, A. A Review of Methods Applied to Study Airborne Biocontaminants inside Aircraft Cabins. Int. J. Aerosp. Eng. 2011, 2011, 824591. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, S.; Poussou, S.B.; Lin, C.-H.; Isukapalli, S.S.; Plesniak, M.W.; Chen, Q. Impact of scaling and body movement on contaminant transport in airliner cabins. Atmos. Environ. 2011, 45, 6019–6028. [Google Scholar] [CrossRef]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Transport of expiratory droplets in an aircraft cabin. Indoor Air 2011, 21, 3–11. [Google Scholar] [CrossRef]
- AIRBUS. Aircraft Characteristics Airport and Maintenance Plannin; AIRBUS: Blagnac, France, 2005. [Google Scholar]
- Available online: https://www.ansys.com/products/meshing (accessed on 2 July 2021).
- ASHRAE. Standard 161-2018. Air Quality within Commercial Aircraft (ANSI Approved); ASHRAE: Atlanta, GA, USA, 2021. [Google Scholar]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Flow dynamics and characterization of a cough. Indoor Air 2010, 19, 517–525. [Google Scholar] [CrossRef]
- Nicas, M.; Nazaroff, W.W.; Hubbard, A. Toward understanding the risk of secondary airborne infection: Emission of respirable pathogens. J. Occup. Environ. Hyg. 2005, 2, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Nielsen, P.V.; Wei, J.; Jensen, R.L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 2016, 27, 452–462. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; Tannous, Z.; Wilson, A.F.; George, S.C. Single-exhalation profiles of NO and CO2 in humans: Effect of dynamically changing flow rate. Jpn. J. Physiol. 1998, 85, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.B.; Sheu, J.J.; Huang, J.W.; Lin, Y.S.; Chang, C.C. Development of a CFD model for simulating vehicle cabin indoor air quality. Transp. Res. Part D Transp. Environ. 2018, 62, 433–440. [Google Scholar] [CrossRef]
- Scott, J.L.; Kraemer, D.G.; Keller, R.J. Occupational hazards of carbon dioxide exposure. ACS Chem. Health Saf. 2009, 16, 18–22. [Google Scholar] [CrossRef]
- Yakhot, V.; Orszag, S.A.; Thangam, S.; Gatski, T.B.; Speziale, C.G. Development of turbulence models for shear flows by a double expansion technique. Phys. Fluids 1992, 4, 1510–1520. [Google Scholar] [CrossRef] [Green Version]
- Acikgoz, M.B.; Akay, B.; Miguel, A.F.; Aydin, M. Airborne Pathogens Transport in an Aircraft Cabin. Defect Diffus. Forum 2011, 312–315, 865–870. [Google Scholar] [CrossRef]
- Zhang, Z. Modeling of Airflow and Contaminant Transport in Enclosed Environments. Ph.D. Thesis, Purdue University, West Lafayette, OH, USA, 2007. [Google Scholar]
- Shengcheng, J.; Jinglei, X.; Shanggui, C.; Zhongmin, H. Numerical Study of Virus Droplet Transport in Civil Aircraft Cabin. Int. J. Astronaut. Aeronaut. Eng. 2018, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q. Comparison of different k-ε models for indoor air flow computations. Numer. Heat Transf. Part B Fundam. 1995, 28, 353–369. [Google Scholar] [CrossRef]
- Liu, W.; Wen, J.; Lin, C.H.; Liu, J.; Long, Z.; Chen, Q.J.B. Evaluation of various categories of turbulence models for predicting air distribution in an airliner cabin. Build. Environ. 2013, 65, 118–131. [Google Scholar] [CrossRef]
- Qian, H.; Li, Y. Removal of exhaled particles by ventilation and deposition in a multibed airborne infection isolation room. Indoor Air 2010, 20, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; To, G.N.S.; Fu, S.C.; Chao, C.Y.H.; Weng, W.G.; Huang, Q.Y. Effect of human movement on airborne disease transmission in an airplane cabin: Study using numerical modeling and quantitative risk analysis. BMC Infect. Dis. 2014, 14, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Dimension | Size |
|---|---|
| Length | 6.3 m |
| Width | 3.7 m |
| Height | 2.26 m |
| Passenger breathing zone height | 1.11 m |
| Inlet width | 0.04 m |
| Outlet width | 0.04 m |
| Passenger’s mouth area | 0.03 m2 |
| Row distance | 0.85 m |
| Location | Name | Parameter |
|---|---|---|
| Ceiling air inlet | Inlet 1 | v = 1 m·s−1, T = 18 °C |
| Side wall air inlet | Inlet 2 | v = 1 m·s−1, T = 18 °C |
| Near-ground air outlet | Outlet | P = 84,475.3 Pa |
| Cabin wall | Wall 1 | T = 22 °C |
| Virus carrier | Virus | Normal breath: v = 1 m·s−1, T = 37.5 °C; Cough: v = 10 m·s−1, T = 37.5 °C |
| Non-ill passengers | Wall 2 | T = 36.5 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yu, N.; Zhang, Y.; Zhang, X.; Cui, Y. Numerical Simulation of the Novel Coronavirus Spread in Commercial Aircraft Cabin. Processes 2021, 9, 1601. https://doi.org/10.3390/pr9091601
Zhang M, Yu N, Zhang Y, Zhang X, Cui Y. Numerical Simulation of the Novel Coronavirus Spread in Commercial Aircraft Cabin. Processes. 2021; 9(9):1601. https://doi.org/10.3390/pr9091601
Chicago/Turabian StyleZhang, Mengya, Nu Yu, Yao Zhang, Xin Zhang, and Yu Cui. 2021. "Numerical Simulation of the Novel Coronavirus Spread in Commercial Aircraft Cabin" Processes 9, no. 9: 1601. https://doi.org/10.3390/pr9091601







