Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experiment Design
2.2. Oral Glucose Tolerance Test (OGTT)
2.3. Determination of Insulin, Glycated Hemoglobin (Hba1c) and Liver Function Markers
2.4. Determination of Liver Glycogen and Carbohydrate-Metabolizing Enzymes
2.5. Determination of Oxidative Stress and Inflammation Markers, and HO-1 Activity
2.6. Histological and Immunohistochemical Examinations
2.7. Gene Expression
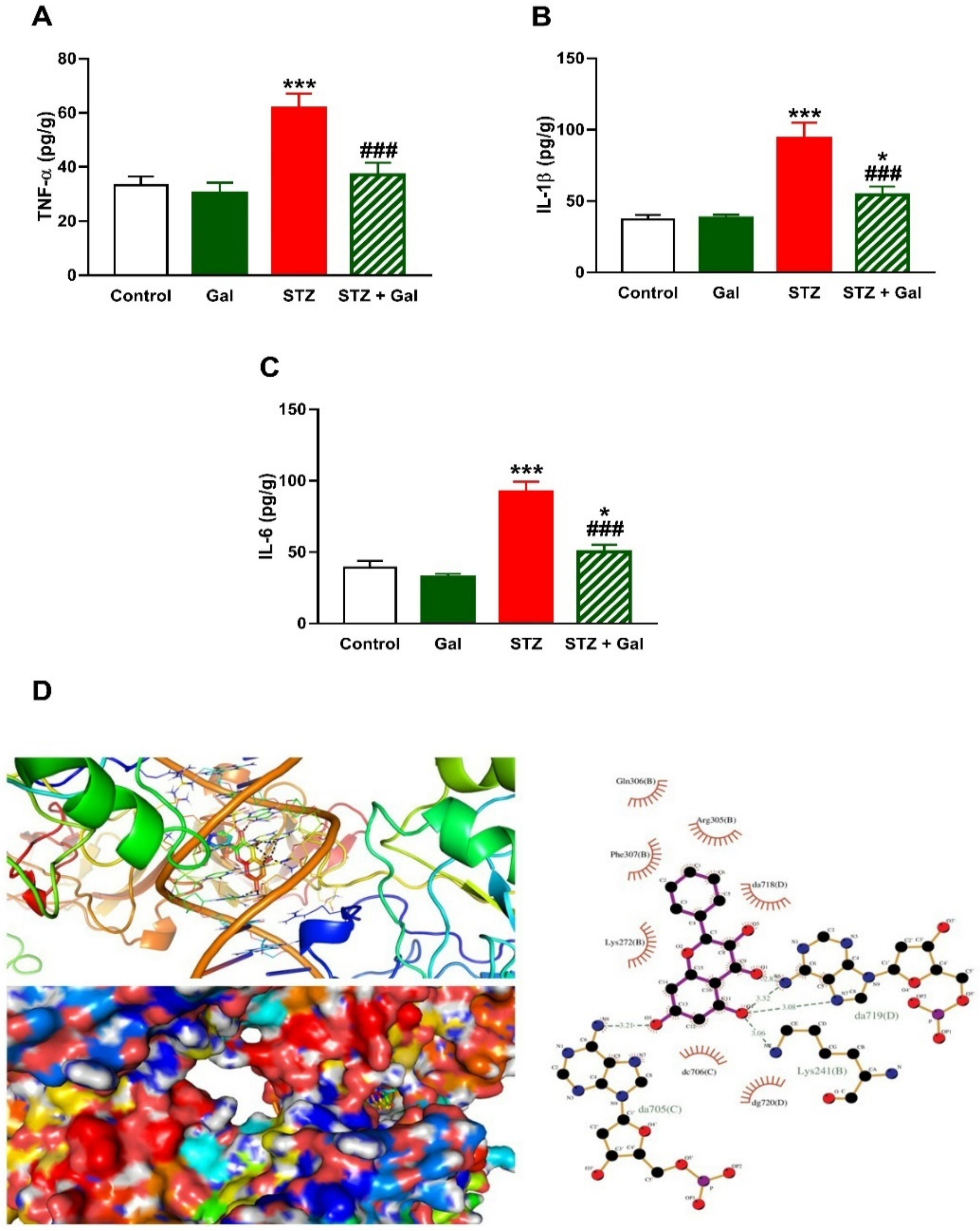
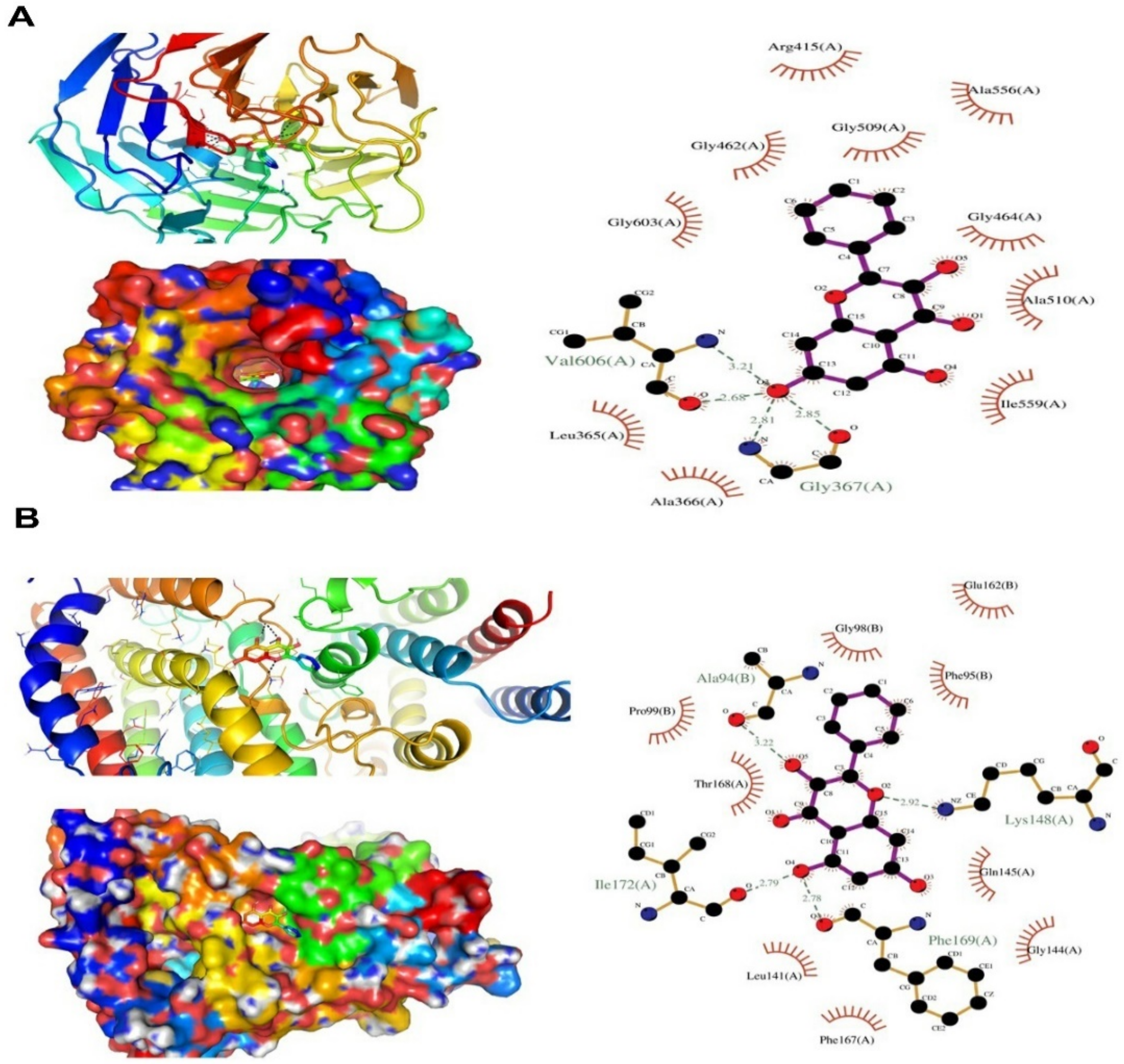
2.8. In Silico Molecular Docking Study
2.9. Statistical Analysis
3. Results
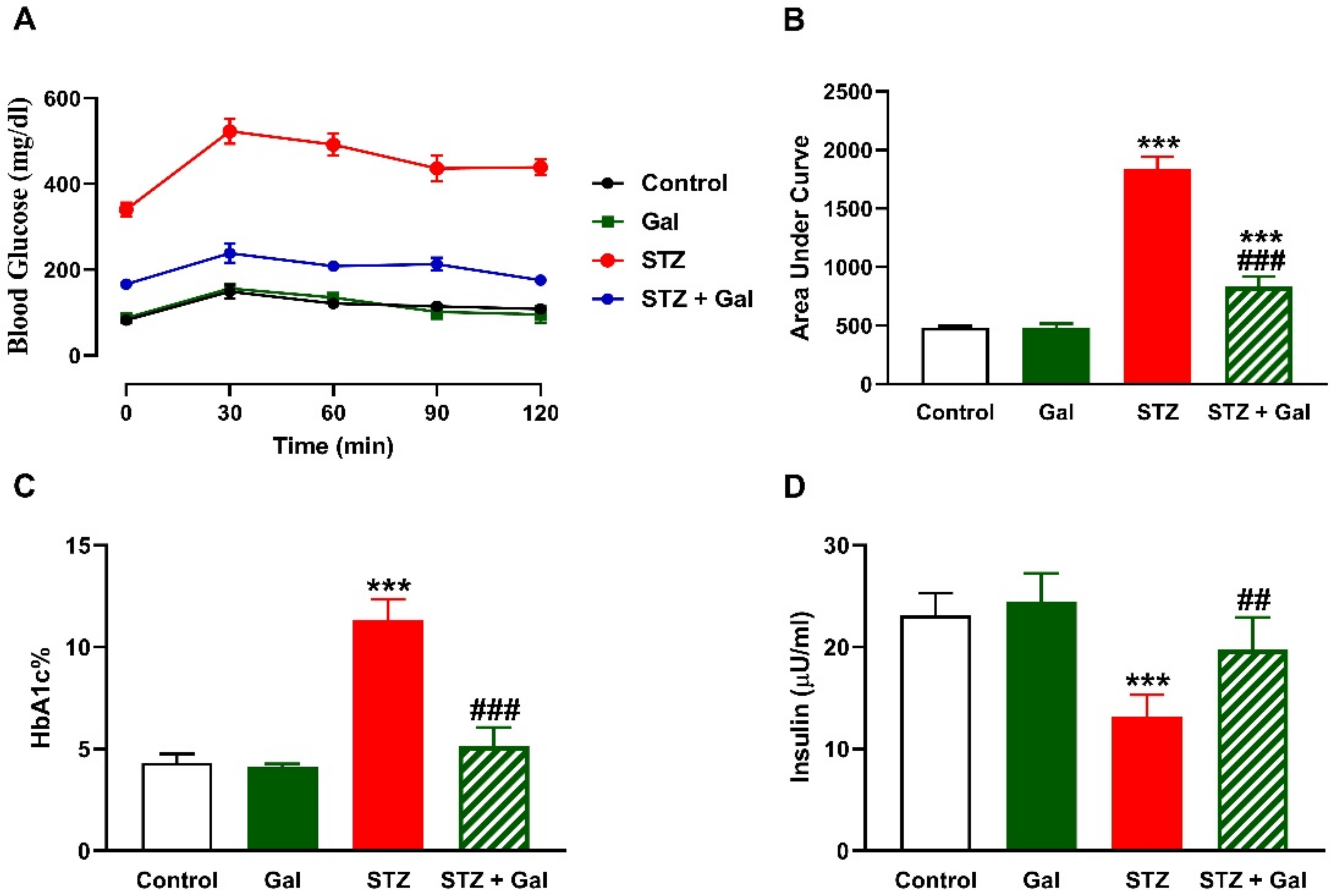
3.1. Gal Ameliorates Hyperglycemia in Diabetic Rats
3.2. Gal Alleviates Liver Glycogen and Carbohydrate Metabolizing Enzymes in Diabetic Rats
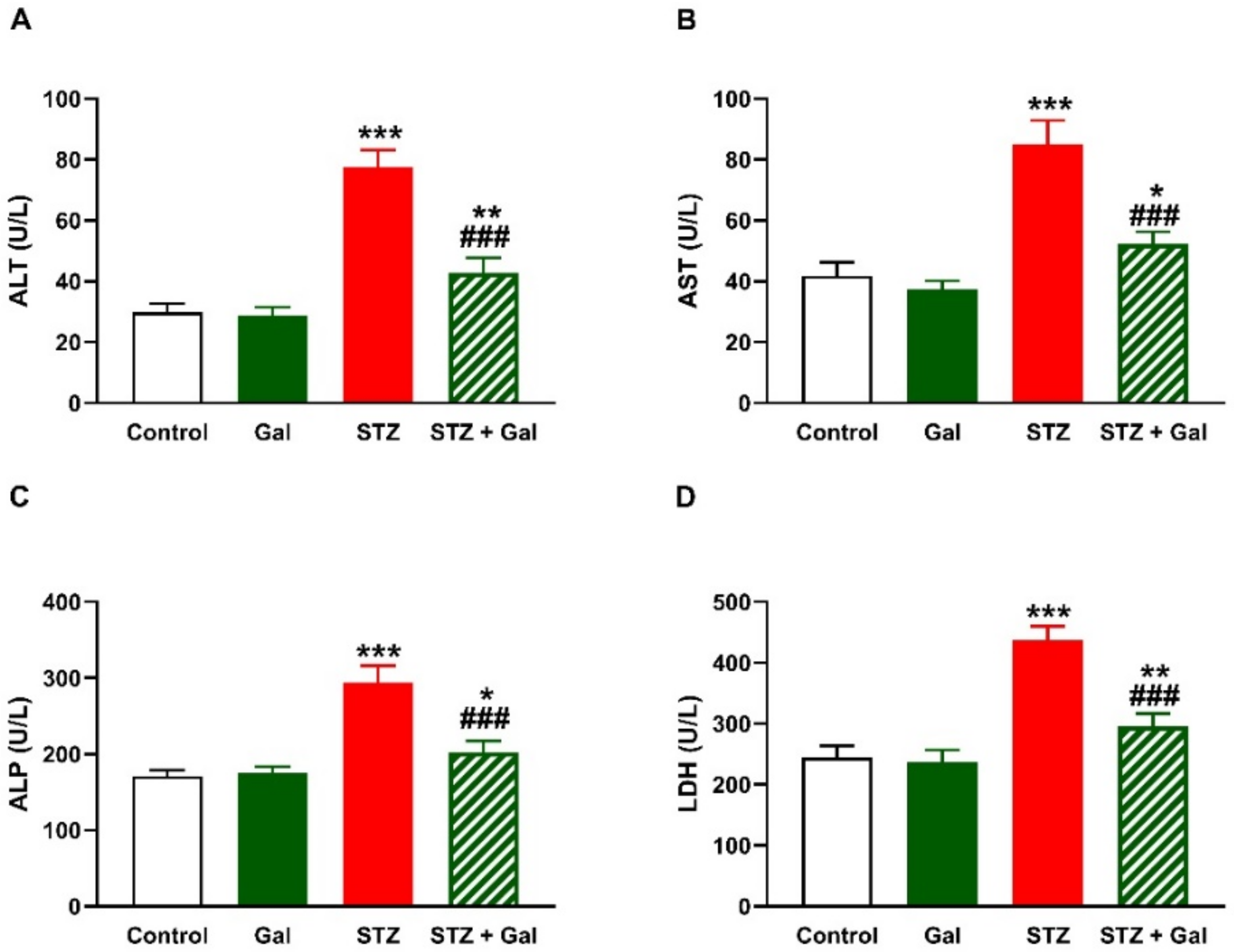
3.3. Gal Attenuates Liver Injury in Diabetic Rats
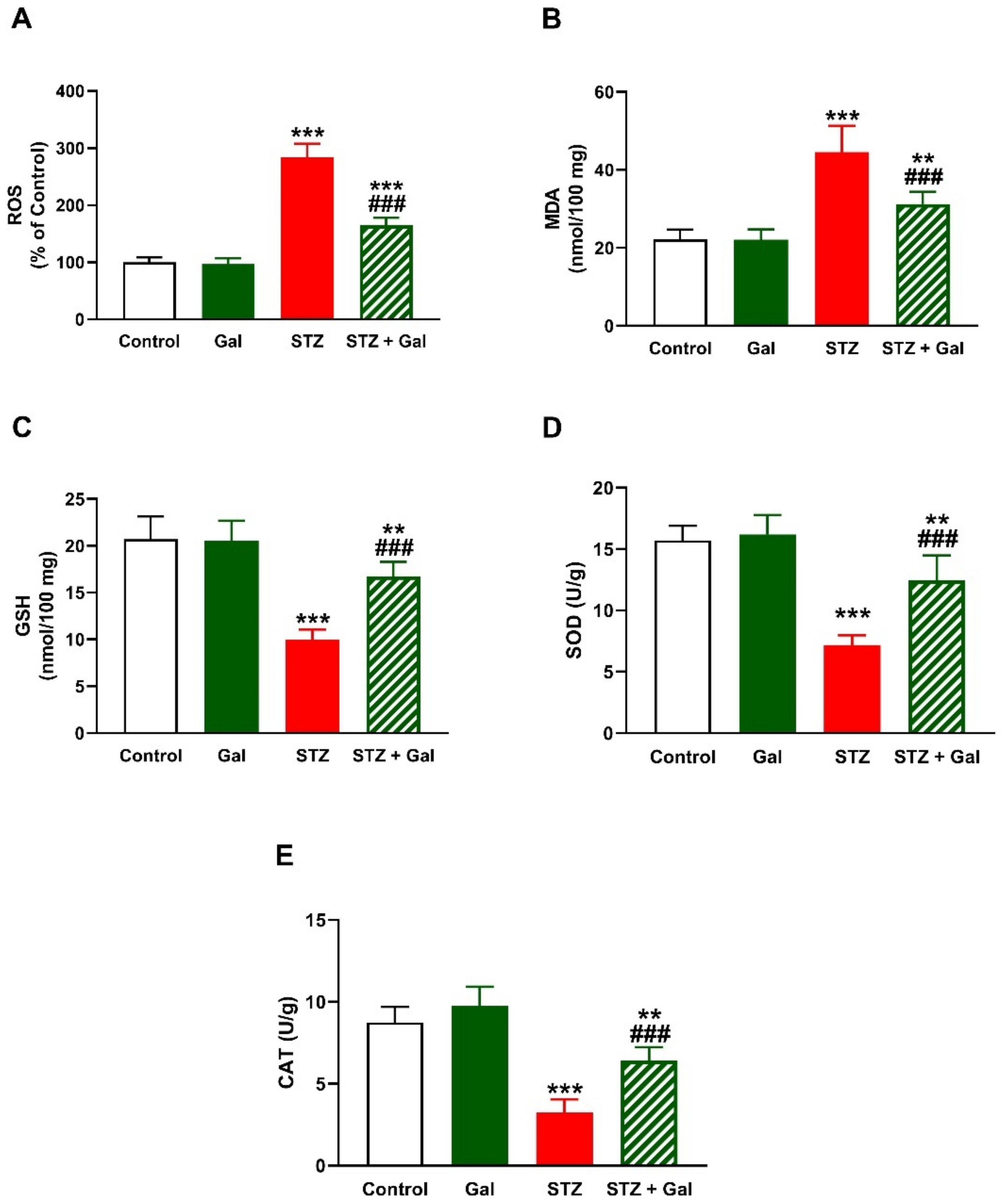
3.4. Gal Attenuates Hepatic Oxidative Stress in Diabetic Rats
3.5. Gal Mitigates Hepatic Inflammation in Diabetic Rats
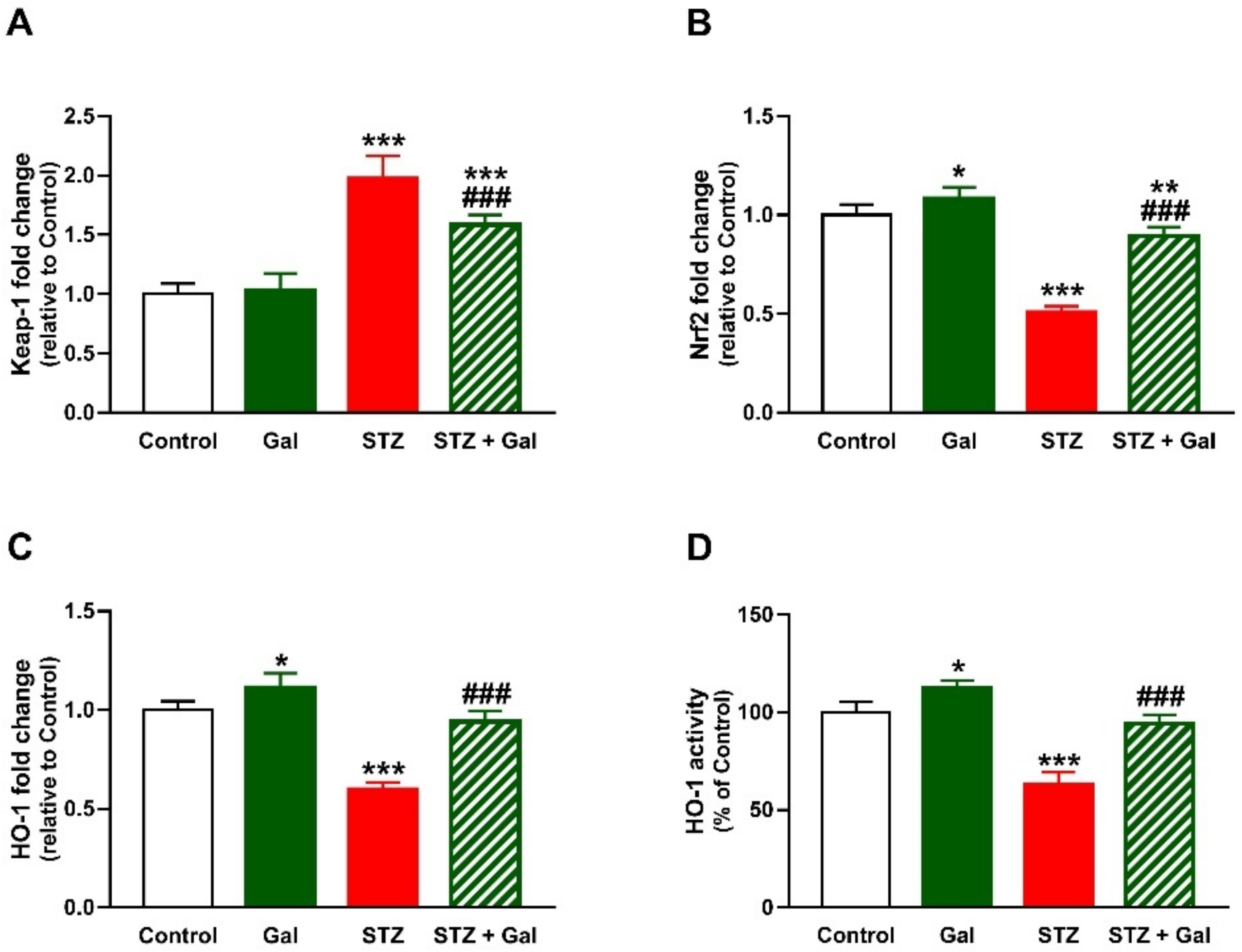
3.6. Gal Upregulates Nrf2/HO-1 Signaling in Liver of Diabetic Rats
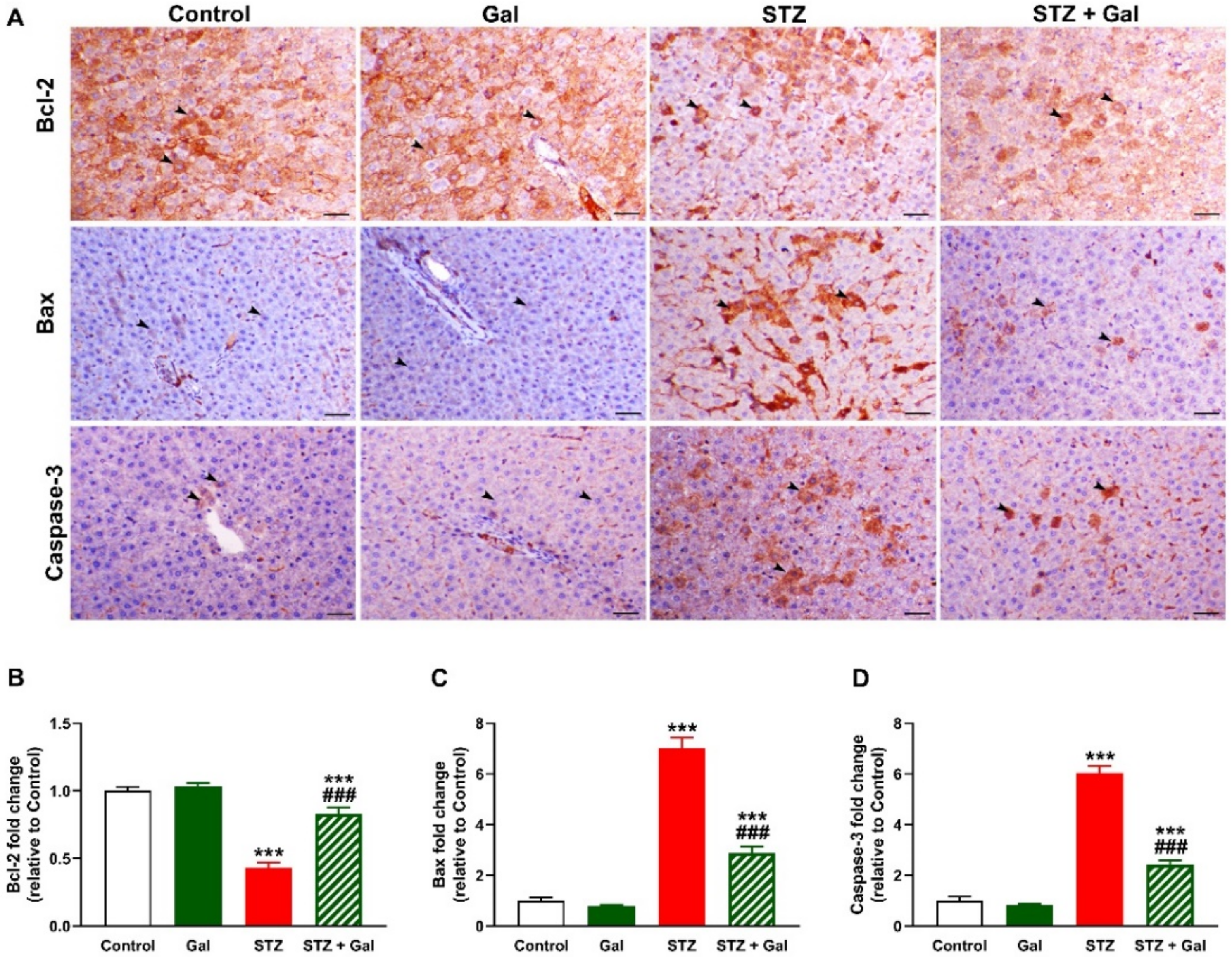
3.7. Gal Attenuates Apoptosis in Liver of Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levinthal, G.N.; Tavill, A.S. Liver Disease and Diabetes Mellitus. Clin. Diabetes 1999, 17, 73–81. [Google Scholar]
- Harrison, S.A. Liver disease in patients with diabetes mellitus. J. Clin. Gastroenterol. 2006, 40, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.; Budin, S. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Petrović, A.; Bogojević, D.; Korać, A.; Golić, I.; Jovanović-Stojanov, S.; Martinović, V.; Ivanović-Matić, S.; Stevanović, J.; Poznanović, G.; Grigorov, I. Oxidative stress-dependent contribution of HMGB1 to the interplay between apoptosis and autophagy in diabetic rat liver. J. Physiol. Biochem. 2017, 73, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- De Lédinghen, V.; Vergniol, J.; Gonzalez, C.; Foucher, J.; Maury, E.; Chemineau, L.; Villars, S.; Gin, H.; Rigalleau, V. Screening for liver fibrosis by using FibroScan® and FibroTest in patients with diabetes. Dig. Liver Dis. 2012, 44, 413–418. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Alexander, M.Y.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, Y.-F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. WJG 2014, 20, 13079. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Alexander, M.Y.; Tutar, Y.; Wilkinson, F.L.; Venditti, A. Oxidative Stress in Metabolic Disorders and Drug-Induced Injury: The Potential Role of Nrf2 and PPARs Activators. Oxid. Med. Cell. Longev. 2017, 2017, 2508909. [Google Scholar] [CrossRef] [Green Version]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; El-Twab, S.M.A.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. Int. 2020, 27, 20725–20735. [Google Scholar] [CrossRef]
- Sugimoto, H.; Okada, K.; Shoda, J.; Warabi, E.; Ishige, K.; Ueda, T.; Taguchi, K.; Yanagawa, T.; Nakahara, A.; Hyodo, I. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G283–G294. [Google Scholar] [CrossRef]
- Elsayed, R.H.; Kamel, E.M.; Mahmoud, A.M.; El-Bassuony, A.A.; Bin-Jumah, M.; Lamsabhi, A.M.; Ahmed, S.A. Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem. Toxicol. 2020, 138, 111202. [Google Scholar] [CrossRef]
- Germoush, M.O.; Elgebaly, H.A.; Hassan, S.; Kamel, E.M.; Bin-Jumah, M.; Mahmoud, A.M. Consumption of Terpenoids-Rich Padina pavonia Extract Attenuates Hyperglycemia, Insulin Resistance and Oxidative Stress, and Upregulates PPARγ in a Rat Model of Type 2 Diabetes. Antioxidants 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Aladaileh, S.; Al-Swailmi, F.; Abukhalil, M.; Shalayel, M. Galangin protects against oxidative damage and attenuates inflammation and apoptosis via modulation of NF-κB p65 and caspase-3 signaling molecules in a rat model of diabetic nephropathy. J. Physiol. Pharmacol. 2021, 72, 1. [Google Scholar] [CrossRef]
- Abukhalil, M.H.; Althunibat, O.Y.; Aladaileh, S.H.; Al-Amarat, W.; Obeidat, H.M.; Alayn’Al-marddyah, A.; Hussein, O.E.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M. Galangin attenuates diabetic cardiomyopathy through modulating oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2021, 138, 111410. [Google Scholar] [CrossRef] [PubMed]
- Aloud, A.A.; Chinnadurai, V.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin, a dietary flavonoid, ameliorates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharm. Biol. 2018, 56, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; El Newehy, A.S.; Al-Numair, K.S. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2017, 22, 290–300. [Google Scholar] [CrossRef]
- Kalhotra, P.; Chittepu, V.C.; Osorio-Revilla, G.; Gallardo-Velázquez, T. Discovery of galangin as a potential DPP-4 inhibitor that improves insulin-stimulated skeletal muscle glucose uptake: A combinational therapy for diabetes. Int. J. Mol. Sci. 2019, 20, 1228. [Google Scholar] [CrossRef] [Green Version]
- Al Hroob, M.A.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Althunibat, O.Y.; Hroob, A.M.A.; Abukhalil, M.H.; Germoush, M.O.; Bin-Jumah, M.; Mahmoud, A.M. Fisetin ameliorates oxidative stress, inflammation and apoptosis in diabetic cardiomyopathy. Life Sci. 2019, 221, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of Glucose in Blood Using Glucose Oxidase with an Alternative Oxygen Acceptor. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Seifter, S.; Dayton, S. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950, 25, 191–200. [Google Scholar]
- Brandstrup, N.; Kirk, J.E.; Bruni, C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J. Gerontol. 1957, 12, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Koide, H.; Oda, T. Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin. Chim. Acta 1959, 4, 554–561. [Google Scholar] [CrossRef]
- Freedland, R.A.; Harper, A.E. Metabolic adaptations in higher animals. V. The study of metabolic pathways by means of metabolic adaptations. J. Biol. Chem. 1959, 234, 1350–1354. [Google Scholar] [CrossRef]
- Stalmans, W.; Hers, H.G. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. A technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur. J. Biochem. 1975, 54, 341–350. [Google Scholar] [CrossRef]
- Fiske, C.; Subbarow, Y. The colourimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed. Pharmacother. 2019, 109, 2527–2538. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Griffith, O.W.J.A.b. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. FEBS Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Abraham, N.; Lutton, J.; Levere, R.J. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Tolman, K.G.; Fonseca, V.; Dalpiaz, A.; Tan, M.H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 2007, 30, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.E.; Park, P.R.; Na, J.Y.; Jung, I.; Cho, J.H.; Lee, J.S. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV-2 microglia through regulation of IL-1β production and the NF-κB signaling pathways. Mol. Cell. Biochem. 2019, 451, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A.; Mahmoud, A.M. Simvastatin ameliorates diabetic cardiomyopathy by attenuating oxidative stress and inflammation in rats. Oxid. Med. Cell. Longev. 2017, 2017, 1092015. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mahmoud, A.M. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des. Devel. Ther. 2016, 10, 2095–2107. [Google Scholar] [CrossRef] [Green Version]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 36, S11–S66. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, A.S.; Viswanathan, P.; Anuradha, C.V. Dose-dependent effect of galangin on fructose-mediated insulin resistance and oxidative events in rat kidney. Redox Rep. 2010, 15, 224–232. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Zhang, G.; Kong, L.; Peng, W.; Zhang, H. Galangin and pinocembrin from propolis ameliorate insulin resistance in HepG2 cells via regulating Akt/mTOR signaling. Evid.-Based Complement. Altern. Med. 2018, 2018, 7971842. [Google Scholar] [CrossRef] [Green Version]
- Prince, P.S.M.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Pandian, M.R. Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes. Fundam. Clin. Pharmacol. 2010, 24, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pilkis, S.J.; Claus, T.H. Hepatic gluconeogenesis/glycolysis: Regulation and structure/function relationships of substrate cycle enzymes. Annu. Rev. Nutr. 1991, 11, 465–515. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Bernroider, E. Hepatic glucose metabolism in humans--its role in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 365–383. [Google Scholar] [CrossRef]
- Golden, S.; Wals, P.A.; Okajima, F.; Katz, J. Glycogen synthesis by hepatocytes from diabetic rats. Biochem. J. 1979, 182, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S. Metabolic consequences of hyperglycemia and insulin resistance. Clin. Cornerstone 2007, 8, S30–S42. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of Oxidative Stress during Diabetes Mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 15. [Google Scholar] [CrossRef]
- Lang, C.H.; Dobrescu, C.; Bagby, G.J. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology 1992, 130, 43–52. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Donath, M.Y.; Mandrup-Poulsen, T. Role of IL-1beta in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Nov, O.; Kohl, A.; Lewis, E.C.; Bashan, N.; Dvir, I.; Ben-Shlomo, S.; Fishman, S.; Wueest, S.; Konrad, D.; Rudich, A. Interleukin-1beta may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology 2010, 151, 4247–4256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Mooney, R.A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 2002, 51, 3391–3399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, S.; Martin, S.; Koenig, W.; Hanifi-Moghaddam, P.; Rathmann, W.; Haastert, B.; Giani, G.; Illig, T.; Thorand, B.; Kolb, H. Impaired glucose tolerance is associated with increased serum concentrations of interleukin 6 and co-regulated acute-phase proteins but not TNF-alpha or its receptors. Diabetologia 2002, 45, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Chen, J.; Weng, C.; Chen, R.; Zheng, Y.; Chen, Q.; Tang, H. Identification of the protein-protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003, 305, 989–996. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Fernández, M.; Alvarez, A.M.; Roncero, C.; Benito, M.; Gil, J.; Fabregat, I. Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor β in rat fetal hepatocytes. Hepatology 2001, 34, 548–556. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M.J.M.; Biology, C. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, 40. [Google Scholar] [CrossRef]
- Liu, J.; Wu, K.C.; Lu, Y.F.; Ekuase, E.; Klaassen, C.D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid. Med. Cell Longev. 2013, 2013, 305861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Gomes, S.; Santos, A.G.; Caldas, C.; Silva, C.M.; Neves, J.V.; Lopes, J.; Carneiro, F.; Rodrigues, P.N.; Duarte, T.L. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J. Hepatol. 2014, 60, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.-J.; Lee, E.-J.; Park, J.-S.; Kim, S.-N.; Park, E.-M.; Kim, H.-S. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. Biochem. Pharmacol. 2017, 144, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Abukhalil, M.H.; Hussein, O.E.; Bin-Jumah, M.; Saghir, S.A.; Germoush, M.O.; Elgebaly, H.A.; Mosa, N.M.; Hamad, I.; Qarmush, M.M.; Hassanein, E.M.; et al. Farnesol attenuates oxidative stress and liver injury and modulates fatty acid synthase and acetyl-CoA carboxylase in high cholesterol-fed rats. Environ. Sci. Pollut. Res. 2020, 27, 30118–30132. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.M.; Lamsabhi, A.M.J.O.; Chemistry, B. The quasi-irreversible inactivation of cytochrome P450 enzymes by paroxetine: A computational approach. Org. Biomol. Chem. 2020, 18, 3334–3345. [Google Scholar] [CrossRef]
- Kubinyi, H. Hydrogen bonding: The last mystery in drug design. In Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical, and Computational Strategies; John Wiley & Sons: Zürich, Switzerland, 2001; pp. 513–524. [Google Scholar]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric acid ameliorates lipopolysaccharide-induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Salerno, L.; Amata, E.; Romeo, G.; Marrazzo, A.; Prezzavento, O.; Floresta, G.; Sorrenti, V.; Barbagallo, I.; Rescifina, A.; Pittalà, V.J. Potholing of the hydrophobic heme oxygenase-1 western region for the search of potent and selective imidazole-based inhibitors. Eur. J. Med. Chem. 2018, 148, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gobec, M.; Tomašič, T.; Markovič, T.; Mlinarič-Raščan, I.; Dolenc, M.S.; Jakopin, Ž. Antioxidant and anti-inflammatory properties of 1, 2, 4-oxadiazole analogs of resveratrol. Chem.-Biol. Interact. 2015, 240, 200–207. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| Keap1 | TCAGCTAGAGGCGTACTGGA | TTCGGTTACCATCCTGCGAG |
| Nrf2 | TTGTAGATGACCATGAGTCGC | TGTCCTGCTGTATGCTGCTT |
| HO-1 | GTAAATGCAGTGTTGGCCCC | ATGTGCCAGGCATCTCCTTC |
| Actb | AGGAGTACGATGAGTCCGGC | CGCAGCTCAGTAACAGTCCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Amarat, W.; Abukhalil, M.H.; Althunibat, O.Y.; Alfwuaires, M.A.; Alnamshan, M.M.; Alqosaibi, A.I.; Ahmeda, A.F.; Kamel, E.M.; Arab, H.H.; Mahmoud, A.M. Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats. Processes 2021, 9, 1562. https://doi.org/10.3390/pr9091562
Al-Amarat W, Abukhalil MH, Althunibat OY, Alfwuaires MA, Alnamshan MM, Alqosaibi AI, Ahmeda AF, Kamel EM, Arab HH, Mahmoud AM. Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats. Processes. 2021; 9(9):1562. https://doi.org/10.3390/pr9091562
Chicago/Turabian StyleAl-Amarat, Wesam, Mohammad H. Abukhalil, Osama Y. Althunibat, Manal A. Alfwuaires, Mashael M. Alnamshan, Amany I. Alqosaibi, Ahmad F. Ahmeda, Emadeldin M. Kamel, Hany H. Arab, and Ayman M. Mahmoud. 2021. "Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats" Processes 9, no. 9: 1562. https://doi.org/10.3390/pr9091562
APA StyleAl-Amarat, W., Abukhalil, M. H., Althunibat, O. Y., Alfwuaires, M. A., Alnamshan, M. M., Alqosaibi, A. I., Ahmeda, A. F., Kamel, E. M., Arab, H. H., & Mahmoud, A. M. (2021). Galangin Attenuates Liver Injury, Oxidative Stress and Inflammation, and Upregulates Nrf2/HO-1 Signaling in Streptozotocin-Induced Diabetic Rats. Processes, 9(9), 1562. https://doi.org/10.3390/pr9091562






