Polyphenolic Herbal Extract of Cistus incanus as Natural Preservatives for Sausages Enriched with Natural Colors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of C. incanus Plant Extract
2.2. Preparation of Dyes
2.3. Determination of Total Phenolics in C. incanus Using Folin-Ciocalteu Reagent
2.4. Antioxidant Activity (DPPH and ABTS)
2.5. LC-MS Analysis of Polyphenols
2.6. Evaluation of the Activity of Extracts and Dyes against Selected Bacterial Strains
2.7. Preparation of Model Sausages
2.7.1. TBARS Index
2.7.2. Color Determination
2.7.3. Microbiological Analysis
2.7.4. Sensory Analysis
2.7.5. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity C. incanus Extract and Dyes
3.2. Polyphenolic Extract Composition
3.3. Antibacterial Activity of C. incanus Extract and Dyes
3.4. Effect of Polyphenolic Extract and Dyes on Lipid Oxidation in Pork Sausages
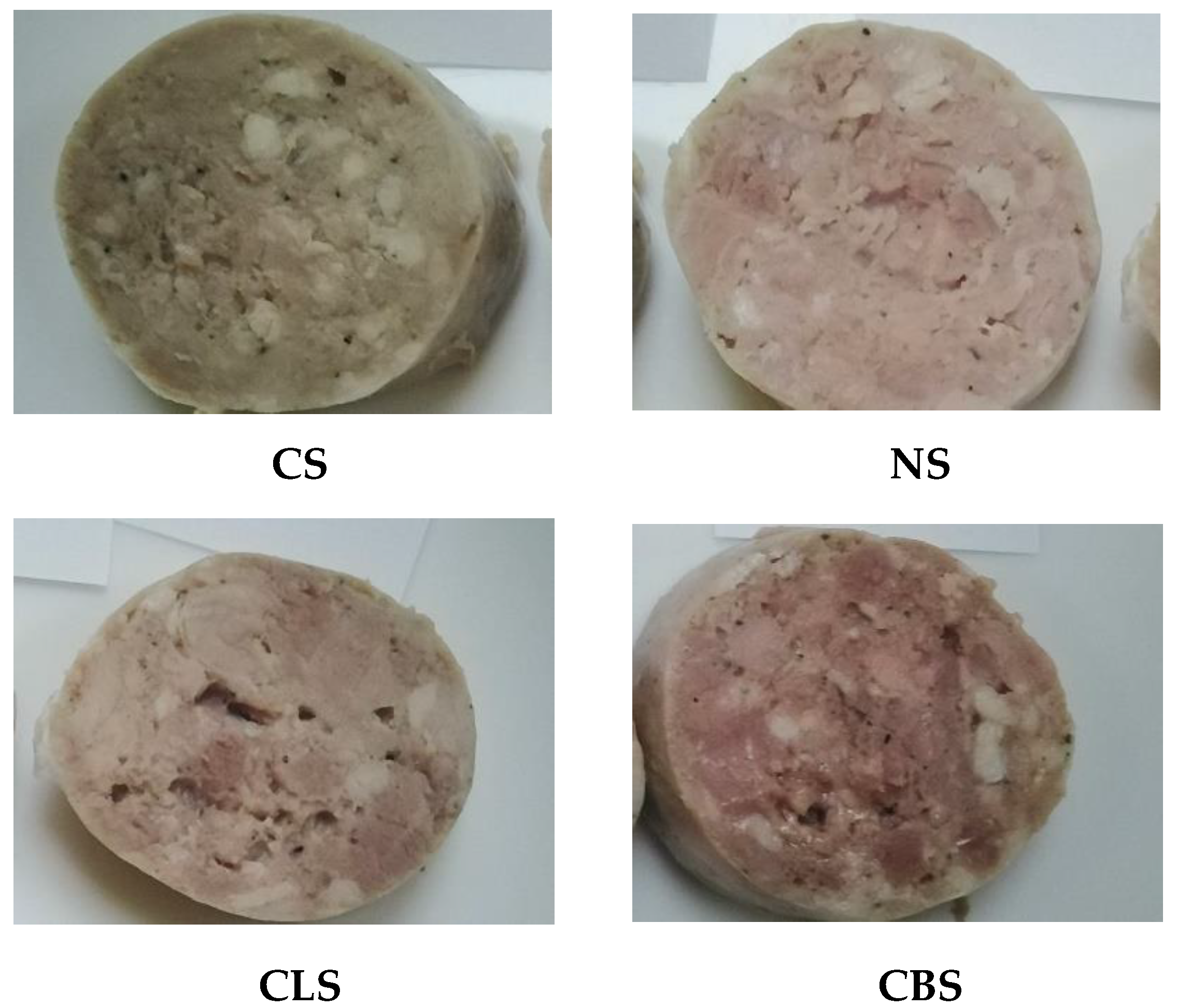
3.5. Effect of Polyphenolic Extract and Dyes on Sausage Color
3.6. Effect of Polyphenolic Extract and Dyes on Microbial Stability of Sausages
3.7. Effect of Polyphenolic Extract and Dyes on Sensory Evaluation of Sausages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, C.; Han, W.; Zhang, J.; Hou, J. Analysis of the monitoring status of residual nitrite in meat products in China from 2000 to 2011. Meat Sci. 2018, 136, 30–34. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Yang, H.S.; Park, T.S.; Yim, D.G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Int. Food Res. J. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Pateiro, M.; Agregán, R.; Lorenzo, J.M. Effect of the partial replacement of pork back fat by microencapsulated fish oil or mixed fish and olive oil on the quality of frankfurter type sausage. J. Food Sci. Technol. 2017, 54, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho de Oliveira, T.L.; Malfitano de Carvalho, S.; de Araujo Soares, R.; Apericida Andrade, M.; das Graças Cardoso, M.; Mendes Ramos, E.; Hilsdorf Piccolia, R. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT Food Sci. Technol. 2012, 45, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Šojić, B.; Pavlić, B.; Ikonić, P.; Tomović, V.; Ikonić, B.; Zeković, Z.; Kocić-Tanackova, S.; Jokanovića, M.; Škaljaca, S.; Ivića, M. Coriandere ssential oil as natural food additive improves quality and safety of cooked pork sausages with different nitrite levels. Meat Sci. 2019, 10, 78–79. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, M.; Zhou, G.; Zou, Y.; Xu, X. Prooxidant effects of the combination of green tea extract and sodium nitrite for accelerating lipolysis and lipid oxidation in pepperoni during storage. J. Food Sci. 2011, 76, C694–C700. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynail, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation phenomena and color properties of grape pomace on nitrite-reduced meat emulsion systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef]
- Semeriak, K.; Jarmoluk, A. Wpływ naturalnych antyoksydantów na barwę peklowanych przetworów mięsnych. Żywn. Nauka. Technol. Jakość 2011, 4, 138–150. [Google Scholar]
- Hammad, H.M.H.; Ma, M.; Jin, G.; Khalifa, I.; Zeng, Q.; Liu, Y. Nitroso—Hemoglobin increased the color stability and inhibited the pathogenic bacteria in a minced beef model: A combined low–field NMR study. Food Sci. Anim. Resour. 2019, 39, 704–724. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, Z. Chemia Żywności—Składniki Żywności Tom 1; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 2007; pp. 142–170. [Google Scholar]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Czajkowska, K.; Kowalska, H.; Piotrowski, D. Rola konsumenta w procesie projektowania nowych produktów spożywczych. Zesz. Probl. Post. Nauk Roln. 2013, 575, 23–32. [Google Scholar]
- Tabaka, K.; Cierach, M. Barwa modelowych produktów mięsnych z dodatkiem preparatów zawierających barwniki naturalne i o zmniejszonej zawartości azotanu III sodowego. Zesz. Probl. Post. Nauk. Roln. 2014, 576, 161–171. [Google Scholar]
- Dias, S.; Castanheira, E.M.S.; Gil Fortes, A.; Pereira, D.M.; Rodrigues, R.O.; Pereira, R.; Gonçalves, M.S.T. Application of Natural Pigments in Ordinary Cooked Ham. Molecules 2020, 25, 2241. [Google Scholar] [CrossRef]
- Østerlie, M.; Lerfall, J. Lycopene from tomato products added minced meat: Effect on storage quality and colour. Int. Food Res. J. 2005, 38, 925–929. [Google Scholar] [CrossRef]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, K. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and antiadherent activity against Streptococus mutans. Planta Med. 2015, 81, 1727. [Google Scholar] [CrossRef] [Green Version]
- Rebensburg, S.; Helfer, M.; Schneider, M.; Koppensteiner, H.; Eberle, J.; Schindler, M.; Gürtler, L.; Brack-Werner, R. Potent in vitro antiviral acitivity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Sci. Rep. Nat. 2016, 6, 20394. [Google Scholar] [CrossRef]
- Stępień, A.; Aebisher, D.; Bartusik-Aebisher, D. Biological properties of Cistus species. Eur. J. Clin. Exp. Med. 2018, 16, 127–132. [Google Scholar] [CrossRef]
- Vitali, F.; Pennisi, G.; Attaguile, G.; Savoca, F. Antiproliferative and cytotoxic activity of extracts from Cistus incanus L. and Cistus monspeliensis L. on human prostatę cel lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef]
- Kozłowska, M. Badanie zawartości polifenoli i aktywności przeciwutleniającej ekstraktów z roślin przyprawowych podczas ich przechowywania. Bromatol. Chem. Toksykol. 2012, 3, 358–360. [Google Scholar]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of ma-lonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- ISO 6887-2:2017 Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 2: Specific Rules for the Preparation of Meat and Meat Products; ISO: Geneva, Switzerland, 2017.
- Floegel, A.; Dae-Ok, K.; Sang-Jin, C.; Koo, S.I.; Chun, O.K. Comparison of ABTS /DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y. Antioxidant Activity and Inhibitory Potential of Cistus saliifolius (L) and Cistus monospeliensis (L). Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. J. Biomed. Biotechnol. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Koubaier, H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris L. conditiva) Roots and Stems Extracts. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef] [Green Version]
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plant Res. 2011, 5, 7119–7131. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 2012, 6, 3499–3509. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT Food Sci. Technol. 2020, 127, 109323. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing proces. Int. Food Res. J. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Bouamama, H.; Noel, T.; Villard, J.; Benharref, A.; Jana, M. Antimicrobial acti-vites of the leafextracts of two Moroccan cistus L. species. J. Ethnopharmacol. 2006, 104, 104–107. [Google Scholar] [CrossRef]
- Rajnbart Nedamani, A.; Rajnbart Nedemani, E. Brief report Antimicrobial Property of Lycopene Oleoresin on some Food Pathogens Running Head: Lycopene oleoresin antibacterial potent. Iran. J. Sci. Technol. Trans. A Sci. 2016, 12, 382–387. [Google Scholar]
- Efenberger-Szmechtyk, M.; Gałązka–Czarnecka, I.; Otlewska, A.; Czyżowska, A.; Nowak, A. Aronia melanocarpa (Michx.) Elliot, Chaenomeles superba Lindl. and Cornus mas L. leaf extracts as natural preservatives for pork meat products. Molecules 2021, 26, 3009. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoda, Y.; Hu, Z.-H.; Zhao, W.-H.; Shimamura, T. Different susceptibilities of Staphylococcus and gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial Activity of Punicalagin Against Staphylococcus aureus and Its Effect on Biofilm Formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2021, 28, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Viapiana, A.; Konopacka, A.; Waleron, K.; Wesolowski, M. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crop. Prod. 2017, 107, 297–304. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, X.; Li, X.; Wu, J. The application of clove extract protects Chinese-style sausages against oxidation and quality deterioration. Korean J. Food Sci. Anim. 2017, 31, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deda, M.S.; Bloukas, J.G.; Fista, G.A. Effect of tomato paste and nitrite level on processing and quality characteristics of frankfurters. Meat Sci. 2007, 76, 501–508. [Google Scholar] [CrossRef]
- Sośnicka, M.; Nowak, A.; Gałązka-Czarnecka, I. Wpływ ekstraktów z wierzbownicy drobnokwiatowej Epilobium parviflorum i czystka Cistus incanus na wybrane parametry produktów mięsnych. In Przegląd Badań z Zakresu Żywienia Technologii Żywności; Maciąg, K., Maciąg, M., Eds.; Tygiel: Lublin, Poland, 2019; pp. 20–31. ISBN 978-83-65932-88-4. [Google Scholar]
- Nowak, A.; Czyżowska, A.; Efenberger- Szmechtyk, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef]
- Swastike, W.; Suryanto, E.; Rusman, R.; Hanim, C.; Jamhari, J.; Erwanto, Y.; Jumari, J. The Substitution Effects of Tapioca Starch and Beetroot Powder as Filler on The Physical and Sensory Characteristics Of Chicken Sausage. J. Ilmu dan Teknologi Hasil Ternak 2020, 15, 97–107. [Google Scholar] [CrossRef]
- Aykin-Dinçer, E.; Güngör, K.K.; Çağlar, E.; Erbaş, M. The use of beetroot extract and extract powder in sausages as natural food colorant. Int. J. Food. Eng. 2021, 17, 75–82. [Google Scholar] [CrossRef]
- Eyiler, E.; Oztan, A. Swastike Production of frankfurters with tomato powder as a natural additive. LWT Food Sci. Technol. 2011, 44, 304–307. [Google Scholar] [CrossRef]

| Ingredients | CS | NS | CLS | CBS | |
|---|---|---|---|---|---|
| Water | mL/ 100 g | 20 | 20 | 14 | 14 |
| Cistus incanus Extract | - | - | 6 | 6 | |
| Salt | g/ 100 g | 1.8 | - | 0.9 | 0.9 |
| Curing salt | - | 1.8 | 0.9 | 0.9 | |
| Pepper | 0.2 | 0.4 | 0.4 | 0.4 | |
| Granulated garlic | 0.4 | 0.4 | 0.4 | 0.4 | |
| Beetroot powder | - | - | - | 0.2 | |
| Lycopene powder | - | - | 0.2 | - |
| DPPH | ABTS | |
| mg Trolox/100 mL | ||
| C | 24.7 ± 0.6 a | 33.2 ± 0.4 a |
| L | 18.5 ± 0.3 c | 21.4 ± 0.2 c |
| B | 25.6 ± 0.5 a | 31.3 ± 0.3 b |
| C L | 23.8 ± 0.5 b | 33.3 ± 0.5 a |
| C B | 24.3 ± 0.1 a,b | 32.4 ± 0.5 a |
| Retention Time [min] | λmax | m/z | ms2 | Compound |
|---|---|---|---|---|
| 2.20 | 345; 378 | 781 | 601; 301 | Punicalin isomer |
| 2.21 | 255; 360 | 1085 | 783; 451; 425; 301 | Cornusin B |
| 2.29 | 218; 245 | 195 | 138 | Caffeine |
| 2.37 | 250; 277 | 191 | 173;171 | Quinic acid |
| 2.96 | 256; 280 | 331 | 481; 673; 779; 963 | Galloyl glucose |
| 2.96 | 279 | 913 | 305 | Gallocatechin trimer |
| 3.00 | 238; 376 | 481 | 301;275; 257;229 | Hexahydroxydiphenyl-glucose isomer (HHDP) |
| 3.02 | 260 | 117 | 73 | Succinic acid |
| 3.49 | 265 | 331 | 225; 481; 897 | Galloyl glucose |
| 3.88 | 270 | 343 | 191; 169; 125 | 3-O-galloquinic acid |
| 3.93 | 213; 261 | 169 | 125 | Gallic acid |
| 4.01 | 275 | 609 | 591; 483; 441; 423; 305 | Gallocatechin-(4α-8)-gallocatechin |
| 4.34 | 280 | 483 | 169 | Digalloyl glucose |
| 4.50 | 260; 379 | 781 | 601; 301 | Punicalin isomer |
| 5.20 | 255; 275 | 913 | 305 | Gallocatechin trimer |
| 5.21 | 260; 285 | 609 | 423; 305; 483; 591 | Gallocatechin-(4α-8)-gallocatechin |
| 5.30 | 265 | 331 | 225; 481; 897 | Galloyl glucose |
| 5.47 | 256; 288 | 913 | 305 | Gallocatechin trimer |
| 5.84 | 259; 288 | 593 | 575; 467; 425; 407; 289 | Gallocatechin-(4α-8)-catechin |
| 6.09 | 278 | 483 | 169 | Digalloyl glucose |
| 6.25 | 240; 267 | 153 | 109 | Protocatechuic acid |
| 6.38 | 260; 273; 299 | 305 | 287; 261;221; 219; 179 | Gallocatechin |
| 6.91 | 260; 278 | 609 | 423; 305; 483; 591 | Gallocatechin-(4α-8)-gallocatechin |
| 7.04 | 305; 345 | 593 | 447;285 | Kaempferol-3-rutinoside |
| 7.10 | 310 | 355 | 195 | Cis or trans ferulic acid hexoside |
| 8.27 | 238 | 633 | 301; | Galloyl-HHDP-glucoside |
| 9.01 | 320; 365 | 1083 | 781; 601; 301 | Punicalagin isomer |
| 9.01 | 255; 370 | 1085 | 783; 451; 425; 301 | Cornusin B |
| 9.45 | 376 | 1251 | 1207; 1082; 781;601; 301 | Punicalagin-gallate isomer |
| 9.53 | 278 | 289 | 271; 245; 205; 179 | Catechin |
| 9.79 | 249;333 | 351 | Ni | |
| 9.90 | 320; 365 | 1083 | 781; 601; 301 | Punicalagin isomer |
| 9.90 | 255; 370 | 1085 | 783; 451; 425; 301 | Cornusin B |
| 9.90 | 278 | 633 | 301 | Galloyl-HHDP-glucoside |
| 10.10 | 265; 289 | 305 | 287; 261;221; 219; 179 | Gallocatechin |
| 10.37 | 238 | 481 | 301;275; 257;229 | Hexahydroxydiphenyl-glucose isomer(HHDP) |
| 10.57 | 267; 286 | 179 | 135 | Caffeic acid |
| 11.07 | 256; 375 | 461 | 415 | Diosmetin 8-C-glucoside |
| 11.22 | 258; 376 | 1251 | 1207; 1082; 781;601; 301 | Punicalagin-gallate isomer |
| 11.24 | 260; 350 | 625 | 316 | Myricetin derivative |
| 11.29 | 238; 377 | 481 | 301;275; 257;229 | Hexahydroxydiphenyl-glucose isomer(HHDP) |
| 11.70 | 260; 349 | 449 | 447 | Myricetin-pentoside |
| 12.01 | 256; 350 | 433 | 301; 271 | Myricetin derivative |
| 12.05 | 276; 289 | 441 | 169 | Epicatechin-3-gallate |
| 13.29 | 285 | 163 | 119 | p-Coumaric acid |
| 13.44 | 355 | 475 | 285 | Kaempferoldimethyletherhexoside |
| 13.75 | 280 | 483 | 169 | Digalloyl glucose |
| 13.89 | 345 | 625 | 316 | Myricetin derivative |
| 14.11 | 351 | 479 | 317; 289; 271; 179, 151 | Myricetin-galactoside |
| 14.18 | 266; 278 | 137 | 93 | Salicylic acid |
| 15.88 | 260; 351 | 449 | 447 | Myricetin-O-xyloside |
| 16.17 | 279 | 913 | 305 | Gallocatechin trimer |
| 16.21 | 260; 351 | 479 | 317; 289; 271; 179, 151 | Myricetin-3-O-galactoside |
| 16.21 | 260; 355 | 463 | 317; 289; 271; 179; 151 | Myricetin-rhamnoside |
| 16.31 | 255; 345 | 609 | 463 | Quercetin rhamno glucoside |
| 16.71 | 260; 355 | 625 | 317; 271; 179;1 51 | Myricetin-rutinoside |
| 18.21 | 270 | 289 | 245; 205; 271 | Epicatechin |
| 18.42 | 255; 345 | 447 | 301; 271; 255; 179 | Quercetin-3-O-rhamnoside |
| 18.58 | 258; 350 | 433 | 301 | Quercetin derivative |
| 18.75 | 260; 345 | 593 | 285 | Keampferol- rhamno-glucoside |
| 19.62 | 267; 350 | 447 | 285 | Kaempferol-3-O-glucoside |
| 19.66 | 281; 296 | 483 | 169 | Digalloyl glucose |
| 23.85 | 355 | 625 | 607; 479; 317; 179 | Myricetin rhamno-glucoside |
| 24.70 | 253; 284 | 301 | 151 | Quercetin |
| Escherichia coli | Pseudomonas fragi | Salmonella enterica | Brochothrix thermosphacta | Latilactobacillus sakei | Listeria monocytogenes | |
|---|---|---|---|---|---|---|
| µmax [h−1] | ||||||
| Control | 0.844 ± 0.021 a A | 0.438 ± 0.010 a E | 0.630 ± 0.035 a C | 0.542 ± 0.013 a D | 0.547 ± 0.007 a D | 0.819 ± 0.014 a A |
| C 0.5 | 0.726 ± 0.032 b B | 0.407 ± 0.005 b E | 0.593 ± 0.018 a D | 0.412 ± 0.005 e E | 0.267 ± 0.014 e F | 0.844 ± 0.025 a A |
| C 1.0 | 0.621 ± 0.035 c B | 0.405 ± 0.011 b D | 0.483 ± 0.011 c D | 0.312 ± 0.015 g E | 0.263 ± 0.005 e F | 0.721 ± 0.008 b A |
| C 2.0 | 0.432 ± 0.024 e B | 0.395 ± 0.013 b C | 0.469 ± 0.023 c B | 0.292 ± 0.010 g D | 0.260 ± 0.022 e D | 0.582 ± 0.007 c A |
| L | 0.451± 0.005 e B | 0.410 ± 0.021 a,b B | 0.444 ± 0.007 c,d B | 0.293 ± 0.027 g C | 0.500 ± 0.021 b A | 0.429 ± 0.014 e B |
| B | 0.560 ± 0.017 d A | 0.351 ± 0.015 c C | 0.432 ± 0.021 c,d B | 0.409 ± 0.017 e B | 0.512 ± 0.004 b A | 0.567 ± 0.017 c A |
| C 0.5 L | 0.825 ± 0.034 a A | 0.455 ± 0.021 a E | 0.613 ± 0.017 a C | 0.520 ± 0.009 a D | 0.277 ± 0.013 e F | 0.493 ± 0.013 d E |
| C 1.0 L | 0.810 ± 0.021 a A | 0.445 ± 0.003 a E | 0.583 ± 0.015 a,b D | 0.454 ± 0.010 d E | 0.289 ± 0.008 e G | 0.391 ± 0.025 e,f F |
| C 2.0 L | 0.803 ± 0.019 a A | 0.426 ± 0.010 a,b E | 0.573 ± 0.021 a,b D | 0.381 ± 0.017 e,f F | 0.269 ± 0.019 e G | 0.413 ± 0.011 e,f E |
| C 0.5 B | 0.795 ± 0.024 a A | 0.435 ± 0.015 a D | 0.591 ± 0.009 a C | 0.534 ± 0.015 a C | 0.340 ± 0.011 c E | 0.456 ± 0.025 d,e D |
| C 1.0 B | 0.780 ± 0.021 a,b A | 0.345 ± 0.025 c E | 0.564 ± 0.028 a,b C | 0.505 ± 0.006 b C | 0.315 ± 0.007 d E | 0.434 ± 0.014 e D |
| C 2.0 B | 0.720 ± 0.015 b A | 0.266 ± 0.004 d E | 0.500 ± 0.010 c C | 0.495 ± 0.013 b,c D | 0.253 ± 0.023 e E | 0.437 ± 0.008 e D |
| Escherichia coli | Pseudomonas fragi | Salmonella enterica | Brochothrix thermosphacta | Latilactobacillus sakei | Listeria monocytogenes | |
|---|---|---|---|---|---|---|
| tLag [h−1] | ||||||
| Control | 2.59 ± 0.05 c C | 4.67 ± 0.31 d A | 1.82 ± 0.13 d D | 3.17 ± 0.22 g B | 3.01 ± 0.07 g B | 3.38 ± 0.25 f B |
| C 0.5 | 2.52 ± 0.07 c D | 5.33 ± 0.15 c A | 1.79 ± 0.15 d E | 4.51 ± 0.11 f B | 3.37 ± 0.15 f C | 4.29 ± 0.09 c B |
| C 1.0 | 3.09 ± 0.09 b C | 5.50 ± 0.22 c A | 2.73 ± 0.11 b D | 5.15 ± 0.07 e A | 5.38 ± 0.11 d A | 4.39 ± 0.05 c B |
| C 2.0 | 3.68 ± 0.15 a E | 6.05 ± 0.15 b B | 2.80 ± 0.16 b F | 7.18 ± 0.13 c A | 6.87 ± 0.25 b B | 4.94 ± 0.12 b D |
| L | 3.53 ± 0.03 a,b F | 4.74 ± 0.20 d E | 4.30 ± 0.13 a E | 3.82 ± 0.08 g F | 9.11 ± 0.15 a A | 5.73 ± 0.03 a D |
| B | 3.42 ± 0.09 a,b F | 4.65 ± 0.15 d E | 4.15 ± 0.08 a E | 5.65 ± 0.15 d D | 9.19 ± 0.09 a A | 5.65 ± 0.07 a D |
| C 0.5 L | 2.84 ± 0.16 b D | 4.85 ± 0.08 d B | 1.92 ± 0.20 d E | 3.47 ± 0.11 g C | 5.82 ± 0.11 c A | 3.76 ± 0.08 e C |
| C 1.0 L | 3.15 ± 0.11 b E | 6.23 ± 0.05 b B | 2.48 ± 0.11 c F | 7.45 ± 0.07 b A | 6.02 ± 0.09 c B | 3.80 ± 0.12 e E |
| C 2.0 L | 3.57 ± 0.15 a E | 6.78 ± 0.31 a B | 2.81 ± 0.09 b F | 7.47 ± 0.11 b A | 6.06 ± 0.09 c B | 4.26 ± 0.13 c D |
| C 0.5 B | 2.95 ± 0.07 b C | 4.70 ± 0.16 d A | 1.85 ± 0.08 d D | 3.49 ± 0.05 g B | 3.50 ± 0.03 f B | 3.28 ± 0.15 f B |
| C 1.0 B | 3.45 ± 0.09 a,b C | 4.78 ± 0.13 d B | 2.65 ± 0.04 b D | 5.71 ± 0.11 d A | 4.59 ± 0.21 e B | 3.88 ± 0.13 e C |
| C 2.0 B | 3.73 ± 0.10 a F | 5.28 ± 0.07 c D | 2.60 ± 0.09 b,c G | 8.11 ± 0.21 a A | 4.75 ± 0.05 e E | 4.05 ± 0.01 d E |
| Lightness L* | |||||
| Day | 0 | 7 | 14 | 21 | 28 |
| CS | 64.82 ± 0.25 c A | 64.22 ± 0.09 c A | 64.67 ± 0.30 c A | 66.04 ± 0.42 c B | 65.21 ± 0.28 d A,B |
| NS | 64.71 ± 0.07 c A | 64.77 ± 0.23 c A | 65.22 ± 0.19 c B | 65.46 ± 0.21 c B | 65.29 ± 0.37 d B |
| CLS | 61.41 ± 0.16 b A | 62.60 ± 0.29 b B | 63.07 ± 0.26 b B | 62.75 ± 0.36 b B | 62.94 ± 0.15 b B |
| CBS | 60.01 ± 0.28 a A | 60.61 ± 0.15 a A | 61.30 ± 0.24 a B | 60.30 ± 0.45 a A | 60.67 ± 0.21 a A |
| Redness a* | |||||
| Day | 0 | 7 | 14 | 21 | 28 |
| CS | 4.44 ± 0.03 a C | 4.53 ± 0.16 a C | 5.04 ± 0.39 a C | 3.64 ± 0.24 a B | 3.13 ± 0.21 a A |
| NS | 8.91 ± 0.07 c B,C | 8.47 ± 0.45 c B | 9.48 ± 0.22 b C | 9.58 ± 0.10 b C | 7.86 ± 0.18 b A |
| CLS | 9.38 ± 0.11 c A | 9.61 ± 0.20 c A | 10.43 ± 0.14 c B | 10.39 ± 0.17 c B | 9.29 ± 0.23 c A |
| CBS | 11.65 ± 0.09 d C | 10.41 ± 0.28 d A | 11.44 ± 0.03 c B | 11.96 ± 0.16 d C | 10.31 ± 0.03 d A |
| Yellowness b* | |||||
| Day | 0 | 7 | 14 | 21 | 28 |
| CS | 15.36 ± 0.29 c A | 15.77 ± 0.11 c A | 15.74 ± 0.18 d A | 15.77 ± 0.03 b A | 16.23 ± 0.08 d B |
| NS | 12.74 ± 0.08 a A | 13.65 ± 0.16 a B | 12.79 ± 0.33 a A | 13.34± 0.17 a B | 13.53 ± 0.16 a B |
| CLS | 14.69 ± 0.35 b A | 15.88 ± 0.27 c B | 15.03 ± 0.19 d A | 15.27 ± 0.37 b A | 15.38 ± 0.22 c A,B |
| CBS | 12.92 ± 0.16 a A | 14.46 ± 0.14 b B | 14.02 ± 0.30 c A,B | 13.46 ± 0.42 a A | 14.00 ± 0.26 a A,B |
| Δ E | |||||
| Day | 0 | 7 | 14 | 21 | 28 |
| CS | - | 0.73 ± 0.05 a A | 0.67 ± 0.18 a A | 1.96 ± 0.12 c C | 1.07 ± 0.10 a B |
| NS | - | 1.01 ± 0.11 a B | 1.40 ± 0.04 b C | 0.61 ± 0.15 a A | 1.74 ± 0.04 b D |
| CLS | - | 1.70 ± 0.03 b C | 1.27 ± 0.09 b B | 0.40 ± 0.03 a A | 1.11 ± 0.09 a B |
| CBS | - | 2.07 ± 0.06 c C | 1.31 ± 0.20 b A | 1.26 ± 0.08 b A | 1.78 ± 0.03 b B |
| TMC [cfu/g] | |||||||
| day | 0 | 3 | 5 | 7 | 14 | 21 | 28 |
| CS | 2.43 ± 0.05 b A | 3.85 ± 0.12 B b | 4.64 ± 0.13 C b | 6.72 ± 0.06 D a | 6.94 ± 0.04 D a | 7.91 ± 0.14 E b | 7.70 ± 0.10 E |
| NS | 2.38 ± 0.08 b A | 2.48 ± 0.03 a A | 3.90 ± 0.04 a B | 6.81 ± 0.10 a E | 6.72 ± 0.07 a E | 6.95 ± 0.03 a E | 6.59 ± 0.05 a E |
| CLS | 1.78 ± 0.11 a A | 2.97 ± 0.15 a B | 4.40 ± 0.17 b D | 6.15 ± 0.13 a F | 6.80 ± 0.10 a F | 7.53 ± 0.05 b G | 7.34 ± 0.07 b G |
| CBS | 2.30 ± 0.05 b A | 3.30 ± 0.07 b B | 5.12 ± 0.02 c D | 6.93 ± 0.11 a E | 6.54 ± 0.08 a E | 7.40 ± 0.02 b F | 7.56 ± 0.17 b F |
| LAB [cfu/g] | |||||||
| day | 0 | 3 | 5 | 7 | 14 | 21 | 28 |
| CS | 2.28 ± 0.09 b A | 3.01 ± 0.05 b B | 4.63 ± 0.09 b C | 6.76 ± 0.14 a E | 7.21 ± 0.12 b F | 8.15 ± 0.06 c G | 8.48 ± 0.04 c G |
| NS | 2.51 ± 0.03 b A | 2.11 ± 0.18 a A | 3.70 ± 0.15 a B | 6.72 ± 0.03 a E | 6.77 ± 0.07 a E | 7.90 ± 0.02 b F | 7.34 ± 0.09 b F |
| CLS | 1.90 ± 0.14 a A | 2.65 ± 0.07 a B | 4.15 ± 0.04 b D | 6.23 ± 0.10 a F | 7.12 ± 0.15 b G | 6.57 ± 0.13 a F | 7.02 ± 0.11 b G |
| CBS | 1.95 ± 0.11 a A | 2.93 ± 0.14 b B | 5.19 ± 0.08 c D | 6.89 ± 0.06 a E | 7.19 ± 0.08 b F | 6.67 ± 0.05 a E | 6.86 ± 0.16 a E |
| TPC [cfu/g] | |||||||
| day | 0 | 3 | 5 | 7 | 14 | 21 | 28 |
| CS | 2.26 ± 0.16 b A | 3.30 ± 0.11 b B | 3.85 ± 0.07 a B | 6.91 ± 0.14 a E | 7.45 ± 0.08 b F | 7.42 ± 0.11 b F | 7.57 ± 0.07 b F |
| NS | 2.00 ± 0.04 b A | 2.42 ± 0.02 a A | 3.00 ± 0.05 a B | 6.83 ± 0.18 a D | 6.93 ± 0.06 a D | 6.78 ± 0.03 a D | 6.28 ± 0.17 a D |
| CLS | 1.48 ± 0.09 a A | 2.40 ± 0.16 a B | 3.30 ± 0.12 a C | 6.26 ± 0.03 a E | 7.06 ± 0.10 a F | 7.41 ±0.14 b F | 7.21 ± 0.02 b F |
| CBS | 1.30 ± 0.05 a A | 3.13 ± 0.13 b C | 3.70 ± 0.06 a D | 6.90 ± 0.09 a E | 7.48 ± 0.04 b F | 7.37 ± 0.12 b F | 7.60 ± 0.10 b F |
| Enterobacteriaceae [cfu/g] | |||||||
| day | 0 | 3 | 5 | 7 | 14 | 21 | 28 |
| CS | <1 | 1.70 ± 0.05 a A | 2.32 ± 0.16 a B | 4.38 ± 0.04 b D | 5.95 ± 0.07 c E | 5.58 ± 0.03 b E | 5.85 ± 0.17 b E |
| NS | <1 | 1.30 ± 0.09 a A | 2.04 ± 0.02 a B | 4.20 ± 0.11b D | 4.23 ± 0.14 b D | 4.28 ± 0.05 a D | 4.81 ± 0.02 a D |
| CLS | <1 | 2.00 ± 0.14 a,b A | 2.46 ± 0.07 a A | 3.48 ± 0.15 a B | 3.00 ± 0.03 a B | 4.72 ± 0.08 a C | 4.69 ± 0.14 a C |
| CBS | <1 | 2.79 ± 0.06 b A | 2.85 ± 0.08 a A | 3.85 ± 0.09 a B | 4.28 ± 0.12 b C | 4.72 ± 0.04 a C | 5.16 ± 0.10 b D |
| Storage Time [Day] | 0 | 5 | 14 | 21 | 28 | 0 | 5 | 14 | 21 | 28 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sausage variant | Taste | Odor | ||||||||
| CS | 4.5 | 3.4 | n.t. | n.t. | n.t. | 4.8 | 3.6 | 3.0 | 2.4 | 1.0 |
| NS | 5.0 | 3.5 | n.t. | n.t. | n.t. | 5.0 | 4.0 | 3.2 | 2.7 | 1.4 |
| CLS | 5.0 | 4.0 | n.t. | n.t. | n.t. | 5.0 | 3.8 | 3.4 | 2.5 | 1.0 |
| CBS | 4.8 | 3.6 | n.t. | n.t. | n.t. | 4.6 | 3.8 | 2.7 | 2.1 | 1.0 |
| Sausage variant | Color | Appearance | ||||||||
| CS | 4.5 | 4.0 | 3.5 | 2.2 | 1.0 | 4.6 | 4.0 | 3.3 | 2.2 | 1.0 |
| NS | 5.0 | 4.5 | 4.0 | 3.6 | 2.5 | 5.0 | 4.5 | 4.0 | 3.4 | 2.6 |
| CLS | 5.0 | 4.5 | 4.2 | 3.5 | 2.4 | 5.0 | 4.5 | 4.0 | 3.3 | 2.5 |
| CBS | 4.6 | 4.0 | 3.8 | 2.6 | 1.5 | 4.8 | 4.2 | 3.5 | 2.6 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sośnicka, M.; Nowak, A.; Czyżowska, A.; Gałązka-Czarnecka, I.; Czerbniak, A. Polyphenolic Herbal Extract of Cistus incanus as Natural Preservatives for Sausages Enriched with Natural Colors. Processes 2021, 9, 1556. https://doi.org/10.3390/pr9091556
Sośnicka M, Nowak A, Czyżowska A, Gałązka-Czarnecka I, Czerbniak A. Polyphenolic Herbal Extract of Cistus incanus as Natural Preservatives for Sausages Enriched with Natural Colors. Processes. 2021; 9(9):1556. https://doi.org/10.3390/pr9091556
Chicago/Turabian StyleSośnicka, Marta, Agnieszka Nowak, Agata Czyżowska, Ilona Gałązka-Czarnecka, and Aleksandra Czerbniak. 2021. "Polyphenolic Herbal Extract of Cistus incanus as Natural Preservatives for Sausages Enriched with Natural Colors" Processes 9, no. 9: 1556. https://doi.org/10.3390/pr9091556
APA StyleSośnicka, M., Nowak, A., Czyżowska, A., Gałązka-Czarnecka, I., & Czerbniak, A. (2021). Polyphenolic Herbal Extract of Cistus incanus as Natural Preservatives for Sausages Enriched with Natural Colors. Processes, 9(9), 1556. https://doi.org/10.3390/pr9091556







