The Potential Role of Nanoparticles as an Anticancer Therapy in the Treatment of Rectal Cancer
Abstract
:1. Introduction
2. Colorectal Cancer Nanocarriers
2.1. Effect of Nanoacarriers on Epithelial Cell Morphology HT-29
2.2. Nanocarriers for LCS-1 Delivery
2.3. Effects of Nanocarriers with CXCR4 Chemokine Receptor Protein
3. Colorectal Cancer NPs
RGD Peptide with Nanoparticles in Colorectal Cancer Treatment
4. Nano-Sized Anticancer Agents for Colorectal Cancer
4.1. Role of Epidermal Growth Factor Receptor (EGFR) in Colorectal Cancer Treatment
4.2. Combining Nanocarriers with Compounds in Colorectal Cancer Treatment
5. Validation Studies
6. Discussion
Solutions and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butt, A.S.; Nisar, N.; Mughal, T. A review: Therapeutics potentials of phytochemical drugs and their loading in pH specific degradable Nano-drug carrier targeting colorectal cancer. J. Pak. Med. Assoc. 2018, 68, 607–614. [Google Scholar]
- Chandran, S.P.; Natarajan, S.B.; Chandraseharan, S.; Mohd Shahimi, M.S.B. Nano drug delivery strategy of 5-fluorouracil for the treatment of colorectal cancer. J. Cancer Res. Pract. 2017, 4, 45–48. [Google Scholar] [CrossRef]
- Mohammed-Saeid, W.; Vakili, M.R.; Ethier-Hopwood, J.; Ahvazi, B.; Lavasanifar, A.; Paiva, I.M.; Sadat, S.M.; Paladino, M.; Hall, D.G.; Weinfeld, M. Characterization of Poly(Acrylic acid)-Modified CNC Based Bio-Adhesive Nano-Gel for Drug Delivery. Colorectal Cancer 2019, Abstract A1. [Google Scholar]
- Hong, Y.Y.; Rao, Y. Current status of nanoscale drug delivery systems for colorectal cancer liver metastasis. Biomed. Pharmacother. 2019, 114, 108764. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Chin, Y.-T.; Nana, A.W.; Wang, S.-H.; Liao, Y.-M.; Chen, Y.-R.; Shih, Y.-J.; Changou, C.A.; Yang, Y.-C.S.; Wang, K.; et al. Enhancement by Nano-Diamino-Tetrac of Antiproliferative Action of Gefitinib on Colorectal Cancer Cells: Mediation by EGFR Sialylation and PI3K Activation. Horm. Cancer 2018, 9, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Saber, M.M.; Al-Mahallawi, A.M.; Nassar, N.; Stork, B.; Shouman, S.A. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-Cubosomes. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef]
- Tran, T.; Tran, P.H.; Wang, Y.; Li, P.; Kong, L. Nanoparticulate Drug Delivery to Colorectal Cancer: Formulation Strategies and Surface Engineering. Curr. Pharm. Des. 2016, 22, 2904–2912. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor Targeted Curcumin Delivery by Folate-Modified MPEG-PCL Self-Assembly Micelles for Colorectal Cancer Therapy. Int. J. Nanomed. 2020, 15, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Wu, J.; Lin, D. Construction of a tumor microenvironment pH-Responsive cleavable PEGylated hyaluronic acid nano-Drug delivery system for colorectal cancer treatment. Biomater. Sci. 2020, 8, 1885–1896. [Google Scholar] [CrossRef]
- Bagheri, R.; Sanaat, Z.; Zarghami, N. Synergistic Effect of Free and Nano-Encapsulated Chrysin-Curcumin on Inhibition of hTERT Gene Expression in SW480 Colorectal Cancer Cell Line. Drug Res. 2018, 68, 335–343. [Google Scholar] [CrossRef]
- Pouw, J.J.; Grootendorst, M.; Klaase, J.M.; Baarlen, J.V.; Haken, B.T. Ex vivo sentinel lymph node mapping in colorectal cancer using a magnetic nanoparticle tracer to improve staging accuracy: A pilot study. Colorec. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2016, 18, 1147–1153. [Google Scholar]
- Chattopadhyay, M.; Kodela, R.; Kashfi, K. Therapeutic potential of NOSH-Aspirin, a dual nitric oxide-And hydrogen sulfide-Donating hybrid. Rectal Cancer 2012, 72 (Suppl. S8), 3891. [Google Scholar]
- Lim, W.Q.; Phua, S.Z.F.; Chen, H.; Zhao, Y. An oxaliplatin(iv) prodrug-Based supramolecular self-Delivery nanocarrier for targeted colorectal cancer treatment. Chem. Commun. 2018, 54, 12762–12765. [Google Scholar] [CrossRef]
- Nabavizadeh, F.; Fanaei, H.; Imani, A.; Vahedian, J.; Amoli, F.A.; Ghorbi, J.; Sohanaki, H.; Mohammadi, S.M.; Golchoobian, R. Evaluation of Nanocarrier Targeted Drug Delivery of Capecitabine-PAMAM Dendrimer Complex in a Mice Colorectal Cancer Model. Acta Med. Iran. 2016, 54, 485–493. [Google Scholar]
- Wang, Z.; Zang, A.; Wei, Y.; An, L.; Hong, D.; Shi, Y.; Zhang, J.; Su, S.; Fang, G. Hyaluronic Acid Capped, Irinotecan and Gene Co-Loaded Lipid-Polymer Hybrid Nanocarrier-Based Combination Therapy Platform for Colorectal Cancer. Drug Des. Dev. Ther. 2020, 14, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Sen, K.; Banerjee, S.; Mandal, M. Dual drug loaded liposome bearing apigenin and 5-Fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf. B Biointerfaces 2019, 180, 9–22. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.E.; Jakiela, S.; Stobiecka, M. Optical Biosensing System for the Detection of Survivin mRNA in Colorectal Cancer Cells Using a Graphene Oxide Carrier-Bound Oligonucleotide Molecular Beacon. Nanomaterials 2018, 8, 510. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wei, Y.; Fang, G.; Hong, D.D.; An, L.; Jiao, T.; Shi, Y.; Zang, A. Colorectal cancer combination therapy using drug and gene co-Delivered, targeted poly(ethylene glycol)-ε-Poly(caprolactone) nanocarriers. Drug Des. Dev. Ther. 2018, 12, 3171–3180. [Google Scholar] [CrossRef] [Green Version]
- Arafa, K.; Shamma, R.N.; El-Gazayerly, O.N.; El-Sherbiny, I.M. Facile development, characterization, and optimization of new metformin-Loaded nanocarrier system for efficient rectalcancer adjunct therapy. Drug Dev. Ind. Pharm. 2018, 44, 1158–1170. [Google Scholar] [CrossRef]
- Banerjee, K.; Banerjee, S.; Mandal, M. Enhanced chemotherapeutic efficacy of apigenin liposomes in colorectal cancer based on flavone-membrane interactions. J. Colloids Interface Sci. 2017, 491, 98–110. [Google Scholar] [CrossRef]
- Lee, S.; Shieh, M.-J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef]
- Bijari, N.; Ghobadi, S.; Derakhshandeh, K. β-Lactoglobulin-Irinotecan inclusion complex as a new targeted nanocarrier for colorectal cancer cells. Res. Pharm. Sci. 2019, 14, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.C.; Hudiță, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Tanasă, E.; Nitu, S.G.; Ginghină, O.; Negrei, C.; Tsatsakis, A.M.; et al. Poly(3-hydroxybutyrate-CO-3-Hydroxyvalerate) PHBHV biocompatible nanocarriers for 5-FU delivery targeting colorectal cancer. Drug Deliv. 2019, 26, 318–327. [Google Scholar] [CrossRef]
- Bouramtane, S.; Bretin, L.; Pinon, A.; Leger, D.Y.; Liagre, B.; Richard, L.; Brégier, F.; Sol, V.; Chaleix, V. Porphyrin-Xylan-Coated silica nanoparticles for anticancer photodynamic therapy. Carbohydr. Polym. 2019, 213, 168–175. [Google Scholar] [CrossRef]
- Sevinç, S.K.; Orun, O.; Tiber, P.M.; Çıkla-Süzgün, P.; Küçükgüzel, Ş.G. Anti-Cancer Acitivity of Etodolac and Its Derivatives on Prostate and Colorectal Cancer Cell Lines. Proceedings 2018, 2, 1573. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Ahmad, A.; Singh, H.; Kaur, S.; NeethuK, M.; Ansari, M.M.; Jayamurugan, G.; Khan, R. Nanocarrier Composed of Magnetite Core Coated with Three Polymeric Shells Mediates LCS-1 Delivery for Synthetic Lethal Therapy of BLM-Defective Colorectal Cancer Cells. Biomacromolecules 2018, 19, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Gupta, A.; Ahmad, A.; Govindasamy, J. Nanoparticle mediated LCS-1 delivery for the targeted therapy of BLM-Deficient colorectal cancer cells. Arch. Cancer Res. 2018, 6, 94. [Google Scholar] [CrossRef]
- Céspedes, M.V.; Unzueta, U.; Álamo, P.; Gallardo, A.; Sala, R.; Casanova, I.; Pavón, M.A.; Mangues, M.A.; Trías, M.; López-Pousa, A.; et al. Cancer-specific uptake of a liganded protein nanocarrier targeting aggressive CXCR4+ colorectal cancer models. Nanomedicine. 2016, 12, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Sala, R.; Sánchez-García, L.; Serna, N.; Céspedes, M.V.; Casanova, I.; Roldán, M.; Sánchez-Chardi, A.; Unzueta, U.; Vázquez, E.; Mangues, R.; et al. Collaborative membrane activity and receptor-Dependent tumor cell targeting for precise nanoparticle delivery in CXCR4+ colorectal cancer. Acta Biomater. 2019, 99, 426–432. [Google Scholar] [CrossRef]
- Zhang, S.; Langer, R.; Traverso, G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today 2017, 16, 82–96. [Google Scholar] [CrossRef]
- Rajendran, S.; Geetha, G.; Venkatanarayanan, R.; Santhi, N. Amelioration of 1, 2 Dimethylhydrazine Induced Tumor Promotion Response by Novel Benzimidazole Derivative Nanoparticle in Wistar Rats. J. Young Pharm. 2018, 10, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Kong, C.; Jiang, C.; Hou, R.; Zhao, X.; Li, J.; Wang, Y.; Gao, Y.; Zhang, H.; Yang, B.; et al. Polydopa-Mine-Coated Au-Ag nanoparticle-Guided photothermal colorectal cancer therapy through multiple cell death pathways. Acta Biomater. 2019, 83, 414–424. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Wang, H.; Xu, S.; Zhang, X.; Wu, R.; Yang, G. Superparamagnetic chitosan nanocomplexes for colorectal tumor-Targeted delivery of irinotecan. Int. J. Pharm. 2020, 584, 119394. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shan, S.; Li, C.; Song, X.; Zhang, C.; Chen, J.; You, J.; Xiong, J. Application of the Tumor Site Recognizable and Dual-Responsive Nanoparticles for Combinational Treatment of the Drug-Resistant Colorectal Cancer. Pharm. Res. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Chen, J.L.; Tsai, Y.; Tsai, M.; Lee, S.; Wei, M.; Kuo, S.; Shieh, M. Prominin-1-Specific Binding Peptide-Modified Apoferritin Nanoparticle Carrying Irinotecan as a Novel Radiosensitizer for Colorectal Cancer Stem-Like Cells. Part. Part. Syst. Charact. 2017, 34, 1600424. [Google Scholar] [CrossRef]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S.S. Chitosan-Coated Cinnamon/Oregano-Loaded Solid Lipid Nanoparticles to Augment 5-Fluorouracil Cytotoxicity for Colorectal Cancer: Extract Standardization, Nanoparticle Optimization, and Cytotoxicity Evaluation. J. Agric. Food Chem. 2017, 65, 7966–7981. [Google Scholar] [CrossRef]
- Rosch, J.G.; Landry, M.R.; Thomas, C.R.; Sun, C. Enhancing chemoradiation of colorectal cancer through targeted delivery of raltitrexed by hyaluronic acid coated nanoparticles. Nanoscale 2019, 11, 13947–13960. [Google Scholar] [CrossRef]
- Moradi, Z.; Mohammadian, M.; Saberi, H.; Ebrahimifar, M.; Mohammadi, Z.; Ebrahimpour, M.; Behrouzkia, Z. Anti-Cancer effects of chemotherapeutic agent; 17-AAG, in combined with gold nanoparticles and irradiation in human colorectal cancer cells. DARU J. Pharm. Sci. 2019, 27, 111–119. [Google Scholar] [CrossRef]
- Bretin, L.; Pinon, A.; Bouramtane, S.; Ouk, C.; Richard, L.; Perrin, M.-L.; Chaunavel, A.; Carrion, C.; Bregier, F.; Sol, V.; et al. Photodynamic Therapy Activity of New Porphyrin-Xylan-Coated Silica Nanoparticles in Human Colorectal Cancer. Cancers 2019, 11, 1474. [Google Scholar] [CrossRef] [Green Version]
- Rychahou, P.; Bae, Y.; Reichel, D.; Zaytseva, Y.Y.; Lee, E.Y.; Napier, D.; Weiss, H.L.; Roller, N.; Frohman, H.; Le, A.-T.; et al. Colorectal cancer lung metastasis treatment with polymer–drug nanoparticles. J. Control Release 2018, 275, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Chakraborty, A.; Mukherjee, B.; Gupta, S. Aptamer-Conjugated Apigenin Nanoparticles to Target Colorectal Carcinoma: A Promising Safe Alternative of Colorectal Cancer Chemotherapy. ACS Appl. Bio Mater. 2018, 1, 1538–1556. [Google Scholar] [CrossRef]
- Zhang, Z.; Qian, H.; Huang, J.; Sha, H.; Zhang, H.; Yu, L.; Liu, B.; Hua, D.; Qian, X. Anti-EGFR-IRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment. Int. J. Nanomed. 2018, 13, 4961–4975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscaglia, F.; Ripani, G.; Rajendran, S.; Benna, C.; Mocellin, S.; Bocchinfuso, G.; Meneghetti, M.; Palleschi, A.; Gobbo, M. Gold Nanoparticle Aggregates Functionalized with Cyclic RGD Peptides for Targeting and Imaging of Colorectal Cancer Cells. ACS Appl. Nano Mater. 2019, 2, 6436–6444. [Google Scholar] [CrossRef]
- Zhong, Y.; Su, T.; Shi, Q.; Feng, Y.; Tao, Z.; Huang, Q.; Li, L.; Hu, L.; Li, S.; Tan, H.; et al. Co-Administration Of iRGD Enhances Tumor-Targeted Delivery and Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment. Int. J. Nanomed. 2019, 14, 8543–8560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, H.; Zheng, N.; Li, Z.; Zhi, Y. Synergistic Effect of Tangeretin and Atorvastatin for Rectal Cancer Combination Therapy: Targeted Delivery of These Dual Drugs Using RGD Peptide Decorated Nanocarriers. Drug Des. Dev. Ther. 2020, 14, 3057–3068. [Google Scholar] [CrossRef]
- Tsai, M.; Peng, C.; Yao, C.; Shieh, M. Enhanced efficacy of chemotherapeutic drugs against colorectal cancer using ligand-decorated self-breakable agents. RSC Adv. 2015, 5, 92361–92370. [Google Scholar] [CrossRef]
- Tran, P.H.; Wang, T.; Yin, W.; Tran, T.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.; Lee, B.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydro-phobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef]
- Alasmari, A.; Ullah, Z.; Balowi, A.A.; Islam, M. In vitro determination of the efficacy of scorpion venoms as anti-Cancer agents against colorectal cancer cells: A nano-Liposomal delivery approach. Int. J. Nanomed. 2017, 12, 559–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorash, B.B.; Uyanik, B.; Kochetkova, E.Y.; Demidov, O.N. Nuclear protein phosphatase Wip1 regulate sensitivity of human colorectal cancer cells to DNA damaging anti-Cancer agents. Biopolym. Cell 2019, 35, 212–213. [Google Scholar] [CrossRef]
- Li, P.; Liu, L.; Huang, S.; Zhang, Y.; Xu, J.; Zhang, Z. Anti-cancer Effects of a Neutral Triterpene Fraction from Ganoderma lucidum and its Active Constituents on SW620 Human Colorectal Cancer Cells. Anti Cancer Agents Med. Chem. 2019, 20, 237–244. [Google Scholar] [CrossRef]
- Xu, L.; Shi, L.; Qiu, S.; Chen, S.; Lin, M.; Xiang, Y.; Zhao, C.; Zhu, J.; Shen, L.; Zuo, Z. Design, Synthesis, And Evaluation Of Cyanopyridines As Anti-Colorectal Cancer Agents Via Inhibiting STAT3 Pathway. Drug Des. Dev. Ther. 2019, 13, 3369–3381. [Google Scholar] [CrossRef] [Green Version]
- Reabroi, S.; Saeeng, R.; Boonmuen, N.; Kasemsuk, T.; Saengsawang, W.; Suksen, K.; Zhu, W.; Piyachaturawat, P.; Chairoungdua, A. The anti-Cancer activity of an andrographolide analogue functions through a GSK-3β-Independent Wnt/β-Catenin signaling pathway in colorectal cancer cells. Sci. Rep. 2018, 8, 7924. [Google Scholar] [CrossRef]
- Tripodi, F.; Dapiaggi, F.; Orsini, F.; Pagliarin, R.; Sello, G.; Coccetti, P. Synthesis and biological evaluation of new 3-Amino-2-Azetidinone derivatives as anti-Colorectal cancer agents. Med. Chem. Comm. 2018, 9, 843–852. [Google Scholar] [CrossRef]
- Leong, S.W.; Chia, S.L.; Abas, F.; Yusoff, K. Synthesis and in-Vitro anti-Cancer evaluations of multi-Methoxylated asymmetrical diarylpentanoids as intrinsic apoptosis inducer against colorectal cancer. Bioorg. Med. Chem. Lett. 2020, 30, 127065. [Google Scholar] [CrossRef]
- Li, C.; Zhu, G.; Cui, Z.; Zhang, J.; Zhang, S.; Wei, Y. The strong inhibitory effect of combining anti-Cancer drugs AT406 and rocaglamide with blue LED irradiation on colorectal cancer cells. Photodiagnosis Photodyn. Ther. 2020, 30, 101797. [Google Scholar] [CrossRef]
- Lee, H.W.; Son, E.; Lee, K.; Lee, Y.; Kim, Y.; Lee, J.-C.; Lim, Y.; Hur, M.; Kim, D.; Nam, D.-H. Promising Therapeutic Efficacy of GC1118, an Anti-EGFR Antibody, against KRAS Mutation-Driven Colorectal Cancer Patient-Derived Xenografts. Int. J. Mol. Sci. 2019, 20, 5894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-Colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.Q.H.; Truong, T.N.; Nguyen, P.T.V. Molecular docking studies of glycyrhetinic acid derivatives as anti-Colorectal cancer agents. Curr. Comput. Drug Des. 2020, 16, 1–24. [Google Scholar] [CrossRef]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Choi, Y.J.; Won, E.; Hui, E.; Kim, H.; Cho, Y.; Yoon, T. Gene editing particle system as a therapeutic approach for drug-Resistant colorectal cancer. Nano Res. 2020, 13, 1576–1585. [Google Scholar] [CrossRef]
- Zhao, M.; Straten, D.V.; Broekman, M.; Préat, V.; Schiffelers, R. Nanocarrier-Based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- Rahiminezhad, Z.; Tamaddon, A.M.; Borandeh, S.; Abolmaali, S.S. Janus nanoparticles: New generation of multifunctional nanocarriers in drug delivery, bioimaging and theranostics. Appl. Mater. Today 2020, 18, 100513. [Google Scholar] [CrossRef]
- Gokduman, K. Strategies Targeting DNA Topoisomerase I in Cancer Chemotherapy: Camptothecins, Nanocarriers for Camptothecins, Organic Non-Camptothecin Compounds and Metal Complexes. Curr. Drug Targets 2016, 17, 1928–1939. [Google Scholar] [CrossRef]
- Mendes, E.; Cadoni, E.; Carneiro, F.; Afonso, M.B.; Brito, H.; Lavrado, J.; Santos, D.J.; Vítor, J.; Neidle, S.; Rodrigues, C.M.; et al. Combining 1,3-Ditriazolylbenzene and Quinoline to Discover a New G-Quadruplex-Interactive Small Molecule Active against Cancer Stem-Like Cells. Chem. Med. Chem. 2019, 14, 1325–1328. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Farokhirad, S.; Eckmann, D.M.; Ayyaswamy, P.S. Nanoparticle transport phenomena in confined flows. Adv. Heat Transf. 2019, 51, 55–129. [Google Scholar] [CrossRef]
- You, S.; Rui, W.; Chen, S.; Chen, H.; Liu, X.; Huang, J.; Chen, H. Process of immunogenic cell death caused by di-Sulfiram as the anti-Colorectal cancer candidate. Biochem. Biophys. Res. Commun. 2019, 513, 891–897. [Google Scholar] [CrossRef]
- Krishnan, P.; Rajan, M.; Kumari, S.; Sakinah, S.; Priya, S.P.; Amira, F.; Danjuma, L.; Ling, M.P.; Fakurazi, S.; Arulselvan, P.; et al. Efficiency of newly formulated camptothecin with β-Cyclodextrin-EDTA-Fe3O4 nanoparticle-Conjugated nanocarriers as an anti-Rectalcancer (HT29) drug. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Ruhnau, J.; Parczyk, J.; Danker, K.; Eickholt, B.; Klein, A. Synergisms of genome and metabolism stabilizing anti-Tumor therapy (GMSAT) in human breast and rectalcancer cell lines: A novel approach to screen for synergism. BMC Cancer 2020, 20, 617. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Zhou, G.; Ling, J.; Tian, A.; Sun, N. Silencing Bag-1 gene via magnetic gold nanoparticle-Delivered siRNA plasmid for colorectal cancer therapy in vivo and in vitro. Tumor Biol. 2016, 37, 10365–10374. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Mirhosseini, S.A.; Panahi, M.R.; Hosseini, H.M. Characterization of Biosynthesized Silver Nanoparticles Using Lactobacillus rhamnosus GG and its In Vitro Assessment Against Colorectal Cancer Cells. Probiotics Antimicrob. Protein 2020, 12, 740–746. [Google Scholar] [CrossRef] [PubMed]
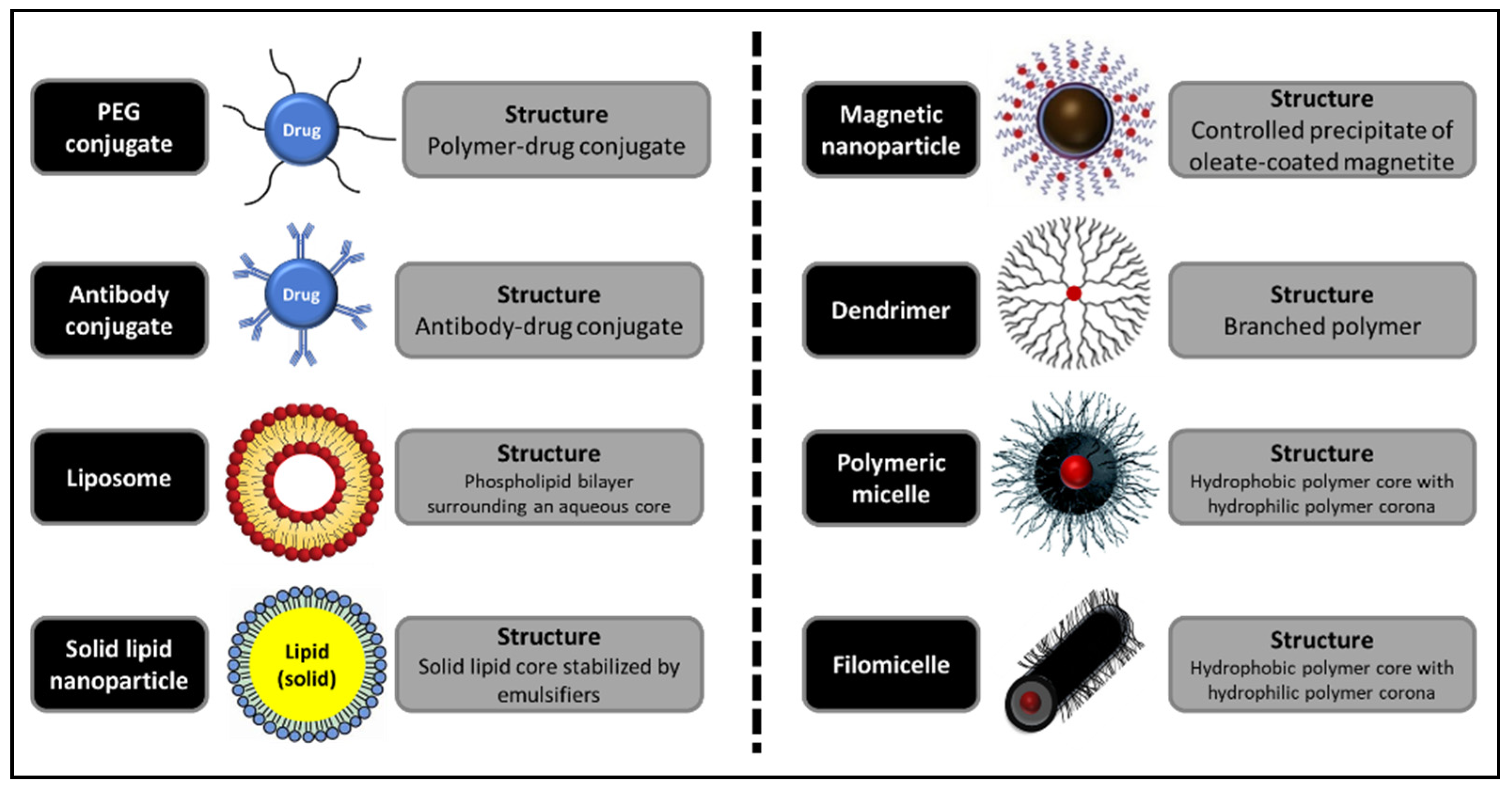
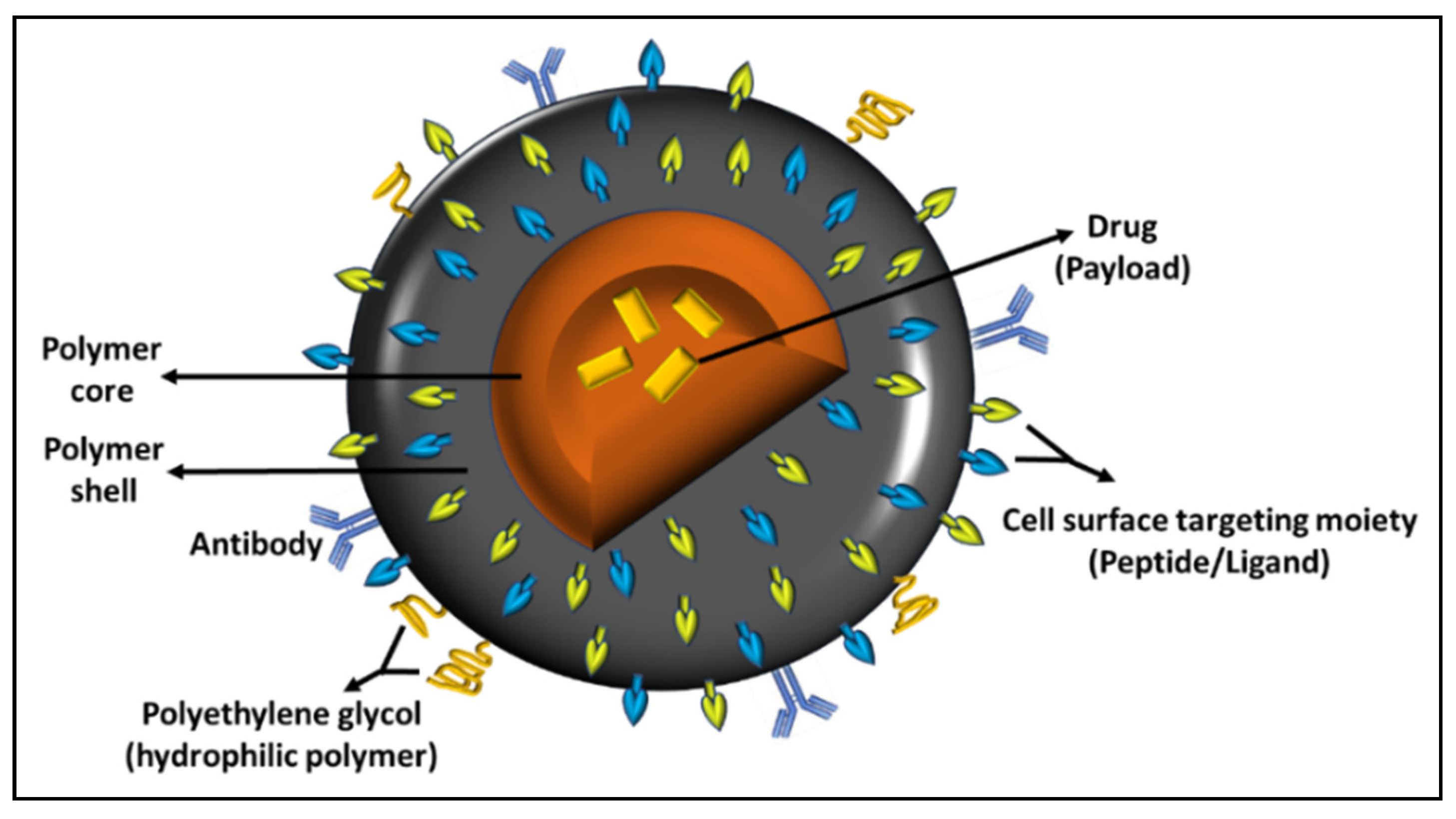
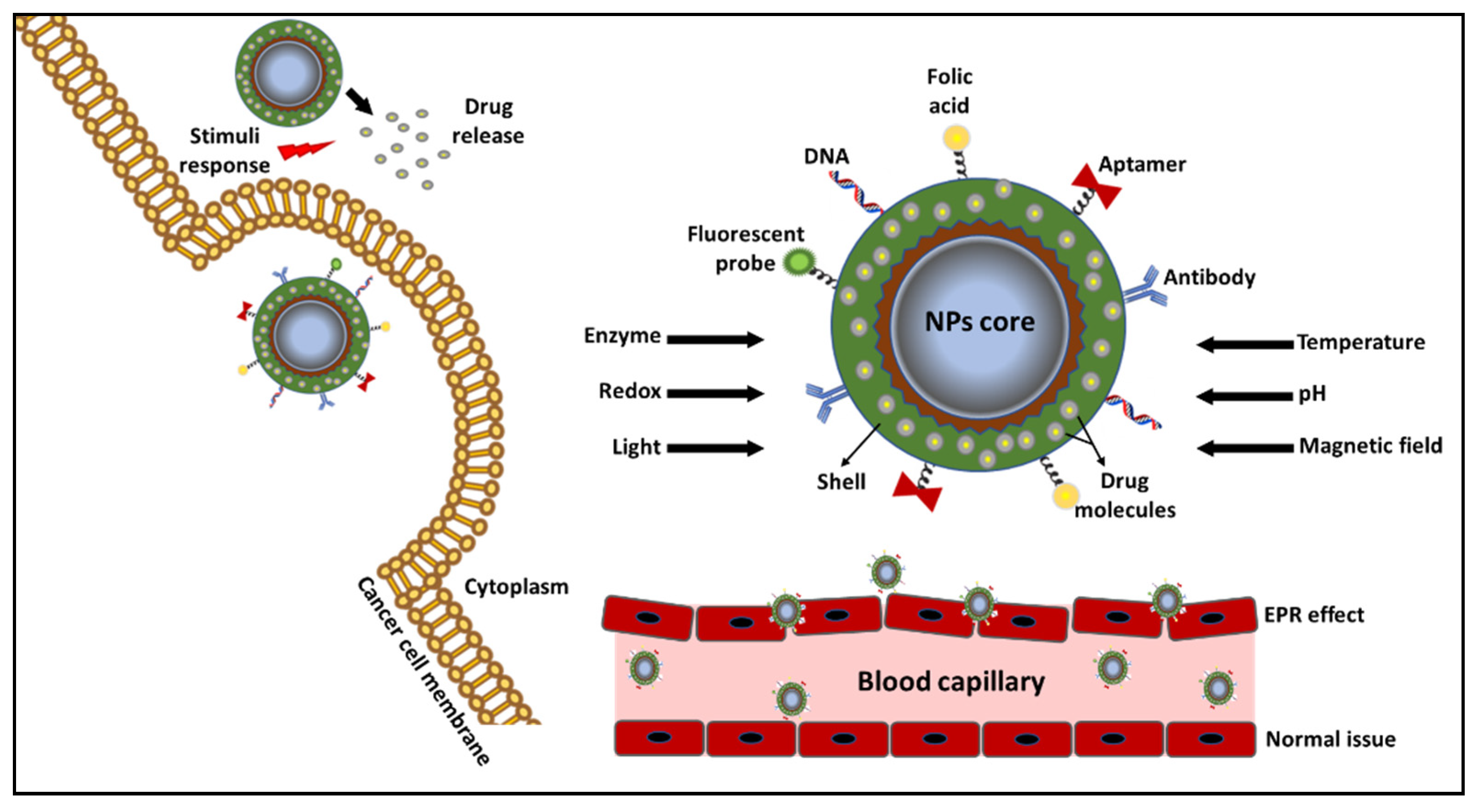
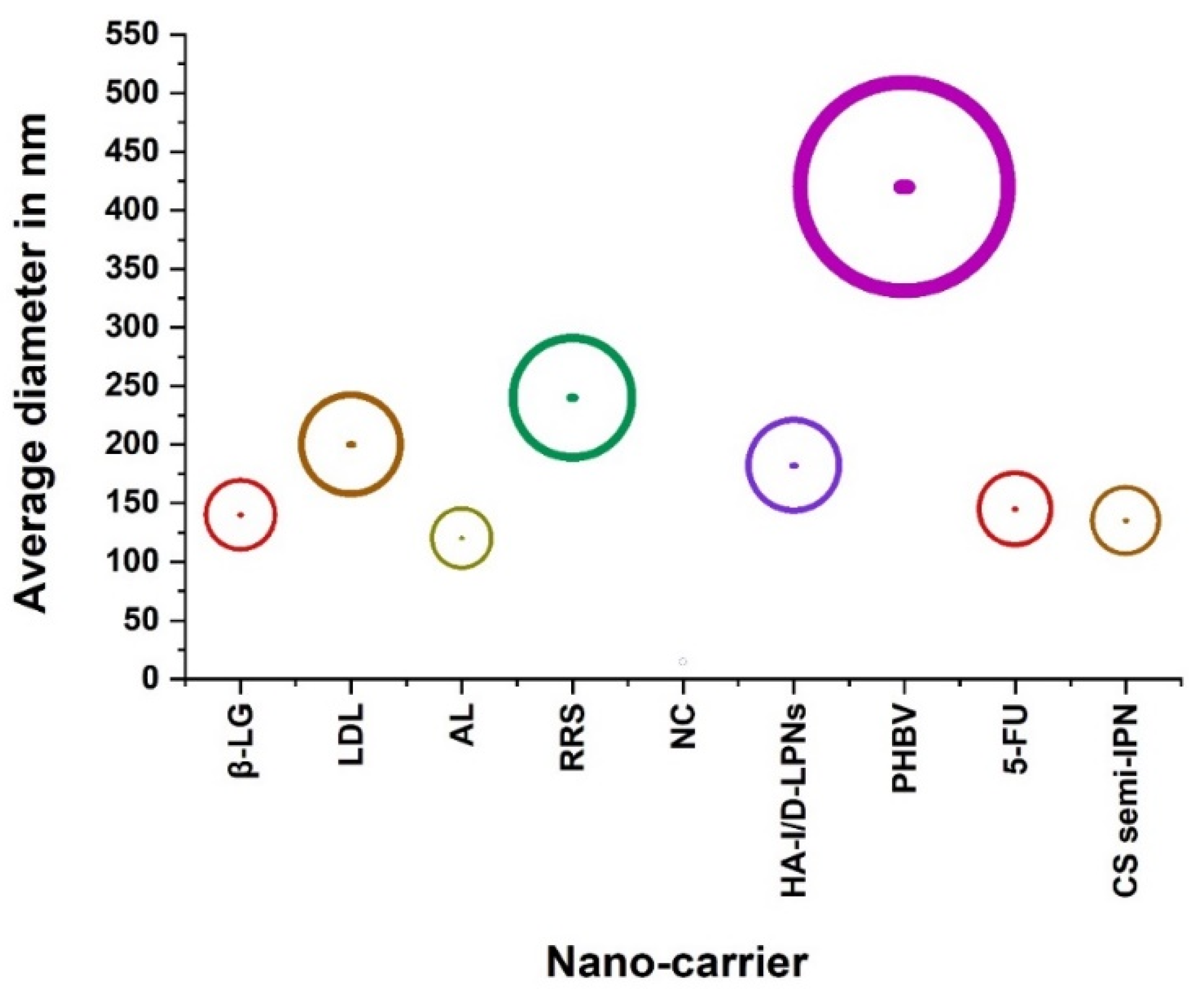
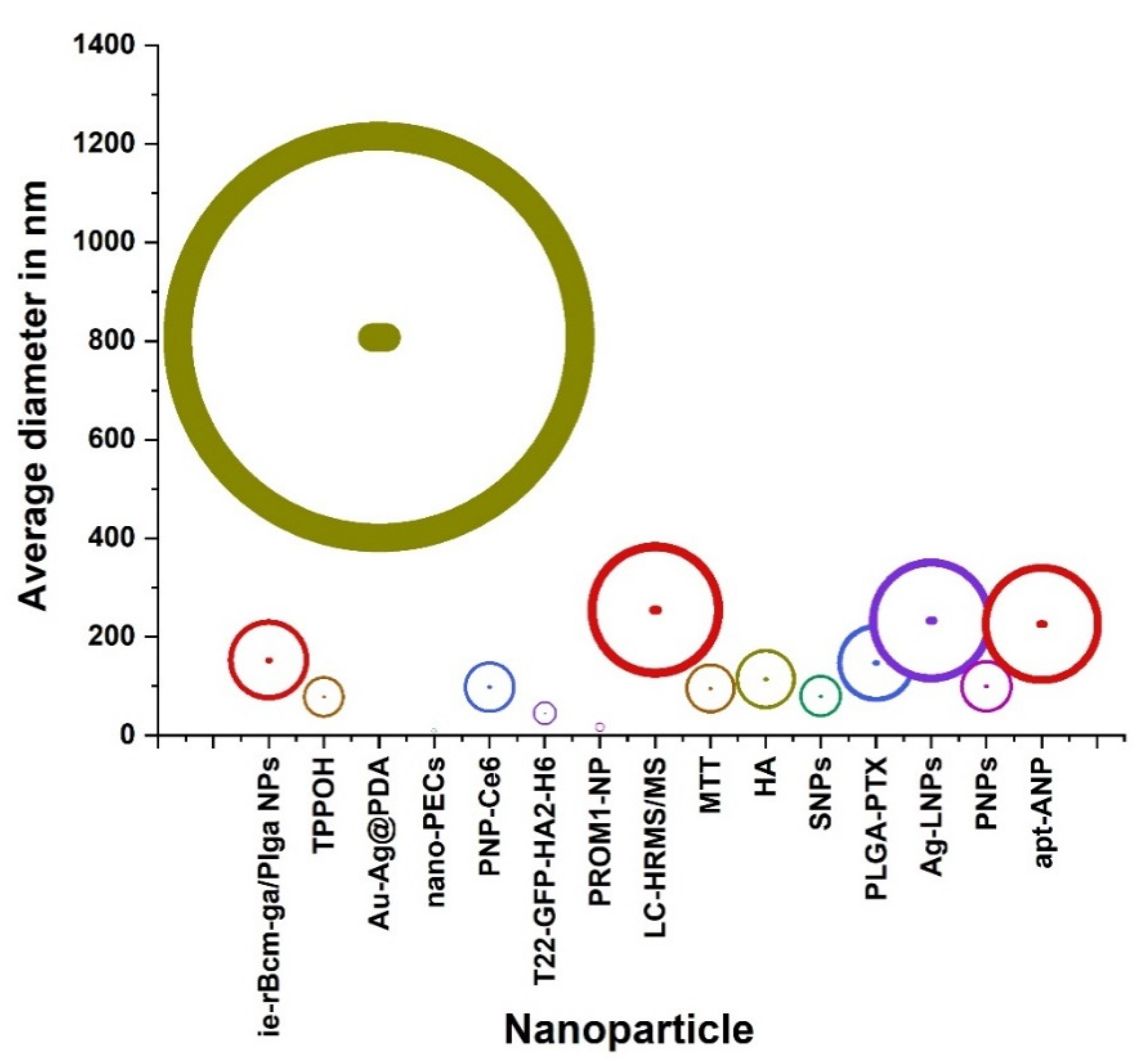
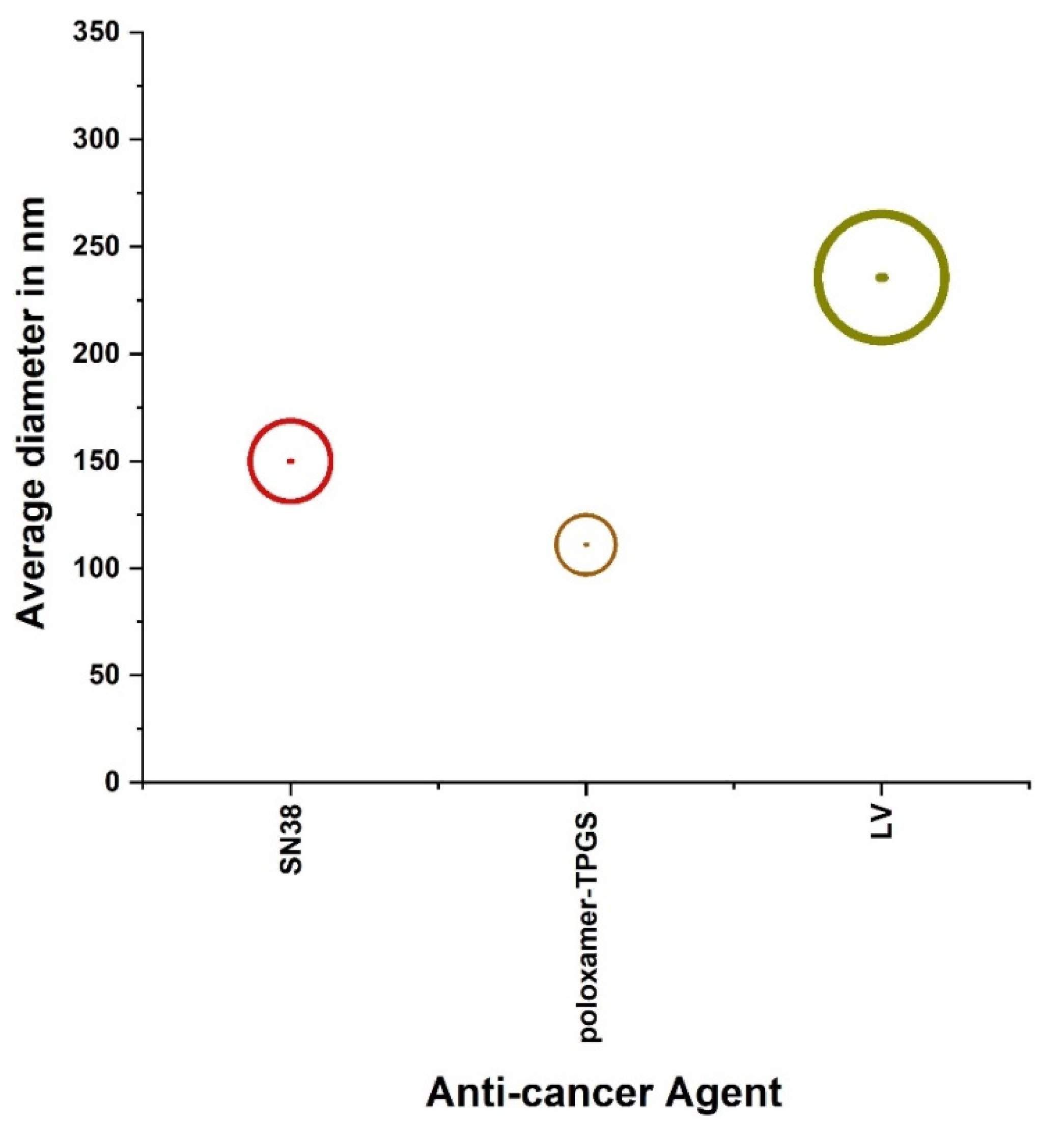
| Classification | Nano-Drug Agent | Function and Merits | Average | References |
|---|---|---|---|---|
| Diameter in nm | ||||
| Nanocarriers | Apigenin liposomes (AL) | establishing apigenin as a potential chemotherapeutic agent. | 120 | [15] |
| A redox-responsive supramolecular (RRS) | preferentially accumulate in polyamine transporter over-expressing HCT116 cells. | 240 | [16] | |
| Nanoconjugate (NC) (T22-GFP-H6-FdU) | delivering Floxuridine to CXCR4+ cells. | 14.6 ± 0.14 | [17] | |
| Hyaluronic acid modified, irinotecan and gene co-loaded LPNs (HA-I/D-LPNs) | developing ligand-modified, irinotecan and gene co-loaded lipid-polymer hybrid nanocarriers for targeted colorectal cancer combination therapy. | 182.3 ± 5.1 | [18] | |
| Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)—PHBV | exhibiting low cytotoxicity and the drug-loaded carrier acts in an efficient manner to kill HT-29 human adenocarcinoma cells. | 420 ± 10 | [19] | |
| SurMB-Joe-loaded GO | enhancing the efficacy of gene retention, cell transfection and genomic material survivability. | - | [20] | |
| 5-FU and gene co-loaded | treating cancer due to the synergistic effects | 145 | [21] | |
| LCS1 | killing cancer cells using somatic mutations in cancer cells. | - | [22] | |
| poly[2-(N,N-dimethylamino)ethyl methacrylate]-poly(ε-caprolactone) (PDMA-PCL) micellar template-based gold nano-shell | Exhibiting a profound inhibition of tumor growth compared to chemotherapy or photothermal therapy alone. | - | [23] | |
| Beta-lactoglobulin (β-LG) | solubilizing and transport of hydrophobic molecules. | 139.86 ± 13.75 | [24] | |
| PAMAM-G4-NH2, azoxymethane and capecitabine | investigating the potentiality of polyamidoamine (PAMAM) dendrimer to improve capecitabine therapeutic index and decrease its adverse side effects on healthy tissues. | - | [25] | |
| Liposomal drug loaded (LDL) | co-deliver two therapeutic entities for enhanced chemotherapeutic effect. | 200 | [28] | |
| Chitosan (CS)-based semi-interpenetrating network (semi-IPN) | enhancing entrapment and sustained release of MF for efficient treatment of CRC. | Ranging between 135 and 220 nm | [29] | |
| Three polymeric shells, namely, aminocellulose (AC), branched poly(amidoamine), and paraben-PEG | encapsulating LCS-1. Encapsulation efficiency and drug loading. | - | [30] | |
| Nanoparticle | BZI 3 nano | have the anticancer potential of the scaffold and selective, good target for drug discovery. | - | [33] |
| Silica core and xylan containing 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (TPPOH) | covalently linked to xylan. | 78.43 ± 19.92 | [34] | |
| Au-Ag@PDA | having strong near-infrared absorbance and no cytotoxicity but high photothermal conversion efficiency. | 808 | [35] | |
| nano-PECs | high potential for drug encapsulation and improved anti-rectalcell efficacy. | 10 | [36] | |
| T22-GFP-HA2-H6 | Tumor tissue selectivity over unspecific cell penetration, upon systemic administration of the material. | 30 and 45 | [37] | |
| PROM1-NP | It is mo re effective as a radiosensitizer than irinotecan and non-in vivo NP. | 17.2 ± 0.2 | [38] | |
| HA | Determining optimal tumor growth delay and subsequent treatment efficacy. | 115 | [40] | |
| (17-AAG) and gold nanoparticle (GNP) | Inhibiting cell viability, and increased apoptosis occurrence by upregulating caspase-3 expression. | - | [41] | |
| Ag-LNPs | Synthesizing anticancer AgLNPs using probiotic bacteria which it can be more investigated through in vivo tests in order to be used for different biomedical applications. | 233 | [42] | |
| PNPs | Offering a new approach to reduce toxicity of cancer therapy and has the potential to improve outcomes for patients with lung metastasis. | 100 | [43] | |
| ie-rBcm-ga/Plga NPs | better antitumor efficacy than free GA in spite of the similar effects of cytotoxicity and apoptosis to GA in vitro. | 153 ± 3.83 | [44] | |
| PNP-Ce6 | having great potential in enhancing the therapeutic efficacy of drug-resistant colorectal cancer. | 98.32 ± 3.65 | [45] | |
| SNPs | Apoptotic cell death induced by TPPOH-X SNPs-PDT and the interest of autophagy inhibition to increase anticancer efficacy. | 80 | [46] | |
| apt-ANP | Maximum retention of apt-ANP in the rectalas compared to free drug and aptamer-free apigenin-loaded nanoparticle (ANP). | 226 | [48] | |
| LC-HRMS/MS | treating human rectalcarcinoma with a low dose leading to decreasing side effects and allowing uninterrupted therapy. | 254.77 | [66] | |
| PLGA-PTX | Retaining preferential cytotoxicity toward various colorectal cancer cells while effectively sparing healthy cells. | 147.5 ± 9.5 | [67] | |
| Anti-cancer agents | SN38 | Folate-decorated functional micelles with disulfide bonds could be an effective chemotherapeutic agent for rectalcancer treatment. | 150 | [49] |
| poloxamer-TPGS | Increasing drug encapsulation effi- ciency for exosomes, improved dissolution and strongly enhanced cytotoxicity of aspirin to cancer cells. | 111 | [50] | |
| liposomal venoms (LV) | Exhibiting better efficacy and act more vigorously as an anti-cancer agent on the colorectal cancer cell line. | 235.8 ± 12.5 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.; Alqahtani, M.S. The Potential Role of Nanoparticles as an Anticancer Therapy in the Treatment of Rectal Cancer. Processes 2021, 9, 2172. https://doi.org/10.3390/pr9122172
Abbas M, Alqahtani MS. The Potential Role of Nanoparticles as an Anticancer Therapy in the Treatment of Rectal Cancer. Processes. 2021; 9(12):2172. https://doi.org/10.3390/pr9122172
Chicago/Turabian StyleAbbas, Mohamed, and Mohammed S. Alqahtani. 2021. "The Potential Role of Nanoparticles as an Anticancer Therapy in the Treatment of Rectal Cancer" Processes 9, no. 12: 2172. https://doi.org/10.3390/pr9122172
APA StyleAbbas, M., & Alqahtani, M. S. (2021). The Potential Role of Nanoparticles as an Anticancer Therapy in the Treatment of Rectal Cancer. Processes, 9(12), 2172. https://doi.org/10.3390/pr9122172






