1. Introduction
Due to the severe depletion of fuel-based monomers necessary for the production of all artificial polymers that are currently used in our modern societies, it appears important to find substitute and suitable materials for packaging and biomedical industries. Because of the low biodegradability expressed by these synthetic materials, some petroleum-based plastic wastes can persist in our environment for hundreds of years inducing thus strong economic and environmental concerns. As sustainable and low-cost alternatives, biomacromolecules isolated from biomass such as polysaccharides (cellulose, gums [
1] and chitosan) or protein [
2,
3,
4] could be transformed to form bioplastic sheets showing remarkable physico-chemical properties and recognized for their good biodegradability. Among all macromolecules, proteins are easily available from the inedible wastes of agricultural industries. Thus, plants (gluten [
5,
6,
7], soy [
8,
9,
10], sunflower, and corn) and animals (gelatin, keratin, casein [
11] and whey) are the cheaper sources of proteins used as processable precursors of bioplastics.
In fact, proteins could be considered as a multi-substituted natural polyamide holding a great number of pendant hydrogen donor and acceptor groups (hydroxyl, amino, carboxyl, and amide). Basically, a mixture made of protein chains is generally obtained and stabilized by the formation of a three-dimensional network reinforced by a great number of inter and intramolecular hydrogen bonds, hydrophobic interactions between successive side chains and covalent disulfide bonds.
Herein, the process for making bioplastic films or sheets involves generally three steps: The addition of a small polar molecule as plasticizer (glycerol [
12,
13,
14,
15,
16], urea [
17], ethylene glycol [
18,
19], diethanolamine, triethanolamine [
20], mannitol, diglycerol or lipids [
21,
22]) surrounding the macromolecules, contributing to the breaking of the intramolecular bonds; and self-reorganization of the released biopolymer chains later into a different shape for finally generating a new 3D network stabilized by new artificial intermolecular hydrogen bonds and ionic interactions. The principal consequences of a plasticizer addition are the promoted chains mobility and the decrease of glass transition temperature (Tg) in parallel to the decrease of rigidity and mechanical strength [
23,
24]. In case of disulfide bridges existence, reducing agents such as sodium disulfide could be also employed to cleave the bond between the two covalently bound cysteine residues, especially for the keratin-based materials.
The main pathways to access the protein-based plastic are casting or compression molding that requires protein melting promoted by the introduction of plasticizers into the matrix and obtained usually at temperatures located above the decomposition temperature near 200 °C.
Otherwise, chemical cross-linking is also another type of procedure for making films from denatured proteins [
17,
25,
26]. In that way, formaldehyde, glutaraldehyde, and glyoxal are compounds potent to realize the permanent connection between the two primary amine groups of lysine residues. This reaction is responsible for macromolecules closing or bridge creation between two neighboring chains. Currently, if the protein material is subject to an intensive cross-linking process, it is possible to produce a thermoset material of higher Young’s modulus and high fracture stress values, besides the bioplastic could sometimes show elongation at the elevated temperatures. These conditions are necessary for all food packaging applications. On the other hand, the tensile and viscoelastic properties of a protein bioplastic were strongly dependent of moisture content and the best protein films have to express good barrier properties against oxygen and carbon dioxide permeability. Interestingly, this kind of material often displays natural or additional antimicrobial activity [
23].
In order to improve the Young’s modulus and prevent the inherent brittleness of the protein films, graphene oxide (GO) could be loaded during the process and dispersed in the protein matrix affording the expected hardness and enhanced flexibility [
27]. This strategy was already efficient for various composite systems as described in a recent paper reporting the preparation of gelatin nanofibers incorporating GO charge. GO can be obtained in large amount by greener and chemical oxidation of graphite and processed efficiently in water as carbon molecular sheets with variation of the lateral size from 100 µm to 100 nm [
28]. Furthermore, GO can interact with its environment through its surface displaying various chemical functions (carboxylic acids, hydroxyl groups, and epoxides) able to promote the real inclusion of GO in the networks made of proteins displaying pendant reactive functions such as amino groups.
In this present work, a simple and one-step acidic wet process using formic acid was studied to produce bioplastic films from rapeseed press cake. The transformation process was first optimized by tuning experimental conditions (temperature, solid/liquid ratio, particle size, the use of catalyst). The chemical composition of the films was then determined by FT-IR spectroscopy and EDX analysis. At different stages of this study, the morphology of the generated protein membranes was observed by FE-SEM. The physico-chemical properties were also given by DSC, TGA, and tensile stress analysis. Some improvements of the film properties such as transparency have been made by the introduction of GO inside.
2. Material and Methods
2.1. Materials
Reagents grade trifluoro acetic acid (TFA), formic acid (HCOOH), sulfuric acid (H2SO4), aqueous hydrochloric acid solution (36%, HCl), and potassium permanganate (KMnO4) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France) and used as received. Rapeseed cake was provided by Olead (Pessac, France). The graphite powder was purchased from Aldrich (Saint-Quentin Fallavier, France). The water used in all experiments was a Millipore Milli-Q grade.
2.2. Grinding and Sieving of Rapeseed Cake
The rapeseed meal was supplied by Olead (Pessac, France). It was obtained by cold pressing using a screw press. It underwent grinding and sieving phases to obtain a finer powder with a particles size ranging from 300 to 600 µm.
2.3. Press Cake Defatting
Rapeseed cake defatting was performed by solid/liquid extraction using cyclohexane. Briefly, 100 g of rapeseed powder was mixed with 600 mL of cyclohexane and stirred at 50 °C for 1 h. The mixture was then centrifuged at 4500 rpm for 15 min to get a lipid-free cake after removing the upper organic layer. The defatted press cake was finally dried in an oven for 24 h at 105 °C.
2.4. Press Cake Deproteinization
Rapeseed cake deproteinization was performed by solid/liquid extraction using water/ethanol mixture. Briefly, 100 g of rapeseed powder was mixed with a mixture of 750 mL of water and 250 mL of ethanol. The suspension was stirred at 50 °C for 30 min and centrifuged at 4500 rpm for 15 min. The deproteinized press cake is finally dried in an oven for 24 h at 105 °C.
2.5. Wet Treatment and Film Casting Process
In order to produce the bioplastic film, 10 g of crud, defatted, and/or deproteinized rapeseed cake was mixed with a fixed volume of formic acid (depending on the solid/liquid ratio) and 4–5 drops of a selected strong acid (sulfuric or hydrochloric acid) as catalyst. Then, the mixture was stirred for 15–60 min in a range of temperature between 40 and 80 °C. The viscous slurry was allowed to return to the room temperature and subjected to centrifugation at 4500 rpm for 30 min. The supernatant was isolated and concentrated to give a viscous liquid that was spread on a plastic film before casting. Besides, we prepared a mold cut from a thick cardboard plate that was glued uniformly on a plastic sheet. Then the viscous solution was poured into the rectangular cavity and the excess of liquid was simply removed with a metallic strip, thus the level of membrane product remains regular over the entire surface. The film was dried in the room atmosphere for 24 h to afford a film ready for further analysis. For each produced protein bioplastic film, the yield was calculated using this formula
Before starting our optimization experiments, four distinct bioplastic samples 1–4 were prepared using four different reaction conditions near to the optimized one as reported in the
Table 1.
2.6. Analysis
2.6.1. Differential Scanning Calorimetry (DSC)
DSC was conducted on a DSC NETZSCH 204 F1 (Selb, Germany) using aluminum pans. Scans were conducted under nitrogen with a heating rate at 5 °C/min in the temperature range of −10 to 450 °C.
2.6.2. Infrared Characterization (FTIR)
FT-IR (ATR) spectra of pure bioplastics and GO loaded films were recorded on FT-IR 4000 Jasco (Lisses, France) in a range of 650–4000 cm−1. The number of scans was 64.
2.6.3. Field-Emission Scanning Electron Microscopy (FE-SEM)
FE-SEM (scanning electron microscopy)-EDX (energy dispersive X-ray diffraction) analysis of all bioplastic films were performed on a Quanta FEG 250 (FEI) (Hillsboro, OR, USA) equipped with a microanalysis detector for EDX from Brucker, SEM micrographs acquired in secondary electron mode were obtained at low vacuum, 15 Kw accelerating voltage and 10 mm working distance.
2.6.4. Water Adsorption Measurements
Dried bioplastic sample were weighed on a sensitive electronic balance in order to be placed in different humidity chambers. Samples were dried by conditioning in drier apparatus for several hours before placing them in the humidity chambers for a defined period and the amount of adsorbed water was calculated based on the initial weight of dried bioplastic at the difference. The water absorption of each film with an average thickness of 0.03 mm or sample crushed into fine powder was carried out as follows: at successive times, the weights of each sample were recorded. Water absorption (
WA) of the samples was calculated using Equation (2)
where
Wo and
Wt are respectively the initial weight of the bioplastic membranes and the weight of water-exposed bioplastic at different times.
2.6.5. Mechanical Propertiesn
The tensile strength and the elongation at break were tested in conditions near to those reported in ISO 527-3, e.g., using rectangular-shaped sample (width = 1.3 cm; length = 3.5 cm) with a medium thickness of 0.4 mm cut out of wide protein film sample were evaluated under standard conditions (25 °C, 50% of relative humidity) to obtain stress from four replicates for each composition. The cross-head speed was set up at 10 mm/min and the stress-strain data were recorded until sample breakage.
2.7. Green Modified Synthesis of Graphene Oxide (GO)
Graphene oxide was produced from graphite powder (<30 µm) via a recently published and modified Hummer’s method [
28]. To 70 mL of concentrated H
2SO
4 was added 3.0 g of the graphite powder. The temperature of the resulted suspension was kept under 20 °C by means of an ice bath. Then, the black mixture was stirred during the addition of 9.0 g of KMnO
4 in small portions. The generated brown viscous solution was stirred for 30 min at 40 °C. Later, the acidic slurry was diluted with 250 mL of deionized water, followed by addition of 15 mL of hydrogen peroxide 30% aqueous solution. Thus, the mixture was stirred for 4 hours refluxing. A dark green solid was successively filtrated and washed with 36% aqueous HCl solution before being dried at the room atmosphere. The rough powder was introduced in 50 mL of deionized water and redispersed under ultrasounds. The dispersion was purified by dialysis against 1 liter of deionized water. The salt-free black slurry was diluted with 200 mL of clean water and subjected to ultrasound treatment for one day. Finally, the GO containing supernatant was recovered after centrifugation (4500 rpm, 30 min) of the previous black suspension. The liquid was concentrated under reduced pressure to afford GO as thin and shiny black sheets.
2.8. Preparation of Graphene Oxide Loaded Protein Membrane
Graphene oxide-loaded protein membrane was prepared by adding, under stirring, 0.8 g of GO to 20 g of centrifuged supernatant obtained from rapeseed cake powder of 600 µm. Then the resulted viscous dispersion is casted on a petri dish and left for one week to afford a non-adhesive membrane easily recovered from the glassware.
3. Results and Discussion
The complete fractionation of rapeseed cake shows the presence of various elements: 13% of cellulose, 35% of water-soluble proteins, and 0.6% of lignin and other phenolic compound traces. Usually, after performing the cold-pressed for oil extraction, only 9% of hydrophobic substances identified as oil elements remain entrapped in the apparently dry solid material. The development of bioplastics from rapeseed cake was started based on possible direct transformation of the crude solid by TFA (pKa = 0.43) into a black elastic structure displaying high mechanical resistance. This work was inspired by a recent published result that demonstrated the possibility of direct transformation of vegetable wastes into bioplastics with a simple exposure to TFA [
29]. More recently, Fitzer et al. [
30] reported the production of protein films obtained after several steps including dissolution of rapeseed proteins in a sodium hydroxide solution, followed by their acidic precipitation with HCl and recovery by ultrafiltration. The resulting wet precursor was also loaded with glycerol before being casted in a Petri dish to afford adhesive and brittle protein membranes or pastes. However, in our case mainly for economic reasons, a decision was taken to replace the strong acid TFA by a greener and cheaper acid such as formic (pKa = 3.75) or acetic acid (pKa = 4.76) to perform the coagulation of the elements involved in the film. We decided to reduce the number of steps without the introduction of an alkaline dissolution step of the proteins. Finally, formic acid was chosen for its good performance to solubilize necessary rapeseed cake components for making films. In the following first part of this present study, it was important to determine the real influence of solid particles size on the bioplastic film quality and the real involvement of each cake element in order to optimize the film preparation process.
3.1. Role of Proteins and Lipids on the Formation of Bioplastic Films
In order to understand the phenomenon occurring during the transformation and identify the role of each molecules on the transformation process, hydrophobic (lipids) and hydrophilic (proteins) components of the native rapeseed cake were removed by successive solid/liquid extractions. Thus, crude, defatted and/or deproteinized cakes were treated in the same conditions to successfully produce bioplastic films. For each sample, insoluble solid elements were isolated by centrifugation from a viscous solution before realizing the bioplastic film casting on a plastic sheet equipped with a rectangular mold made of cardboard in the room atmosphere.
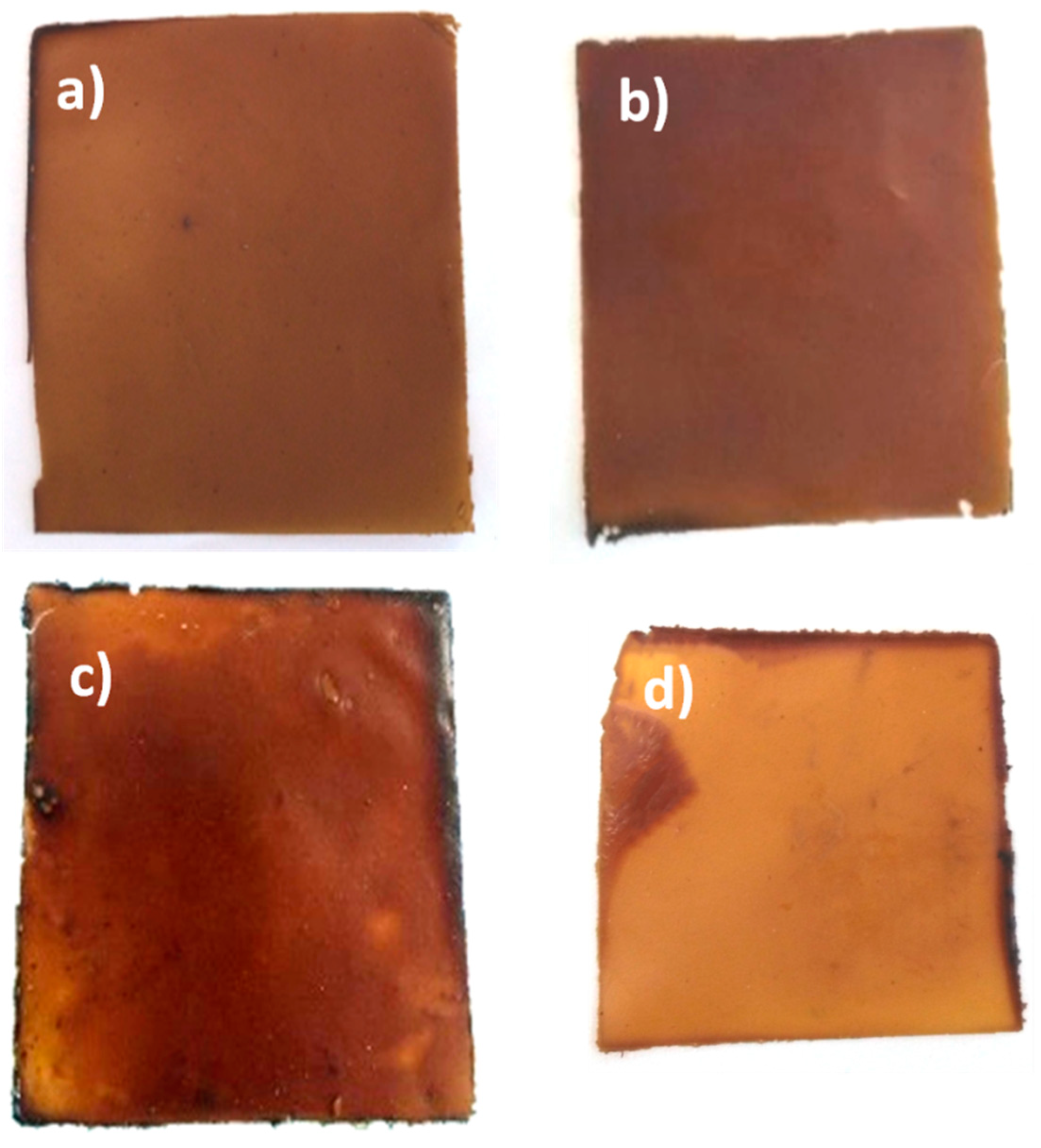
Figure 1 shows the picture of the formed films with some differences between all samples.
First, for both defatted and deproteinized rapeseed cakes, it was possible to observe a consequent loss of matter. Furthermore, for a successively defatted and deproteinized cake, the formed film was not consistent, did not have regular shape or length, and sometimes exposed holes in its continuity. For the other two, as seen with naked eyes, there are no significant differences between the respective film quality. However, in
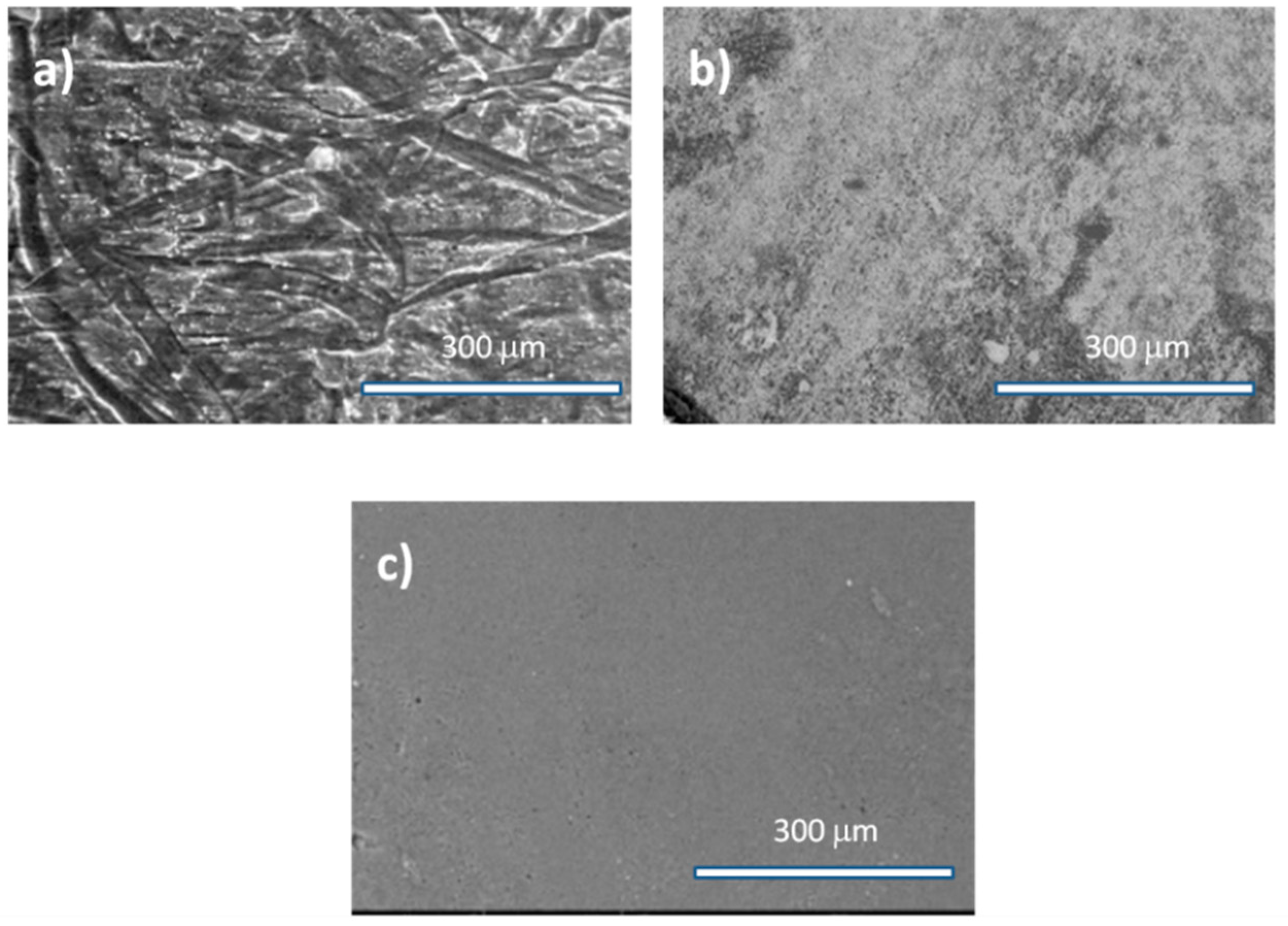
Figure 2, a more detailed microscopic electronic observation displayed variations in the film surface morphologies with regard to their origins.
For a crude cake, the surface of the film appeared to be coarser and layered compared to the defatted or deproteinized rapeseed cake due to the persistence of huge and rough particles in the film networks. For a simply defatted rapeseed cake, on the corresponding image, the surface of the film remained granular. Interestingly, the film made of deproteinized cake displayed only fine elements on its surface.
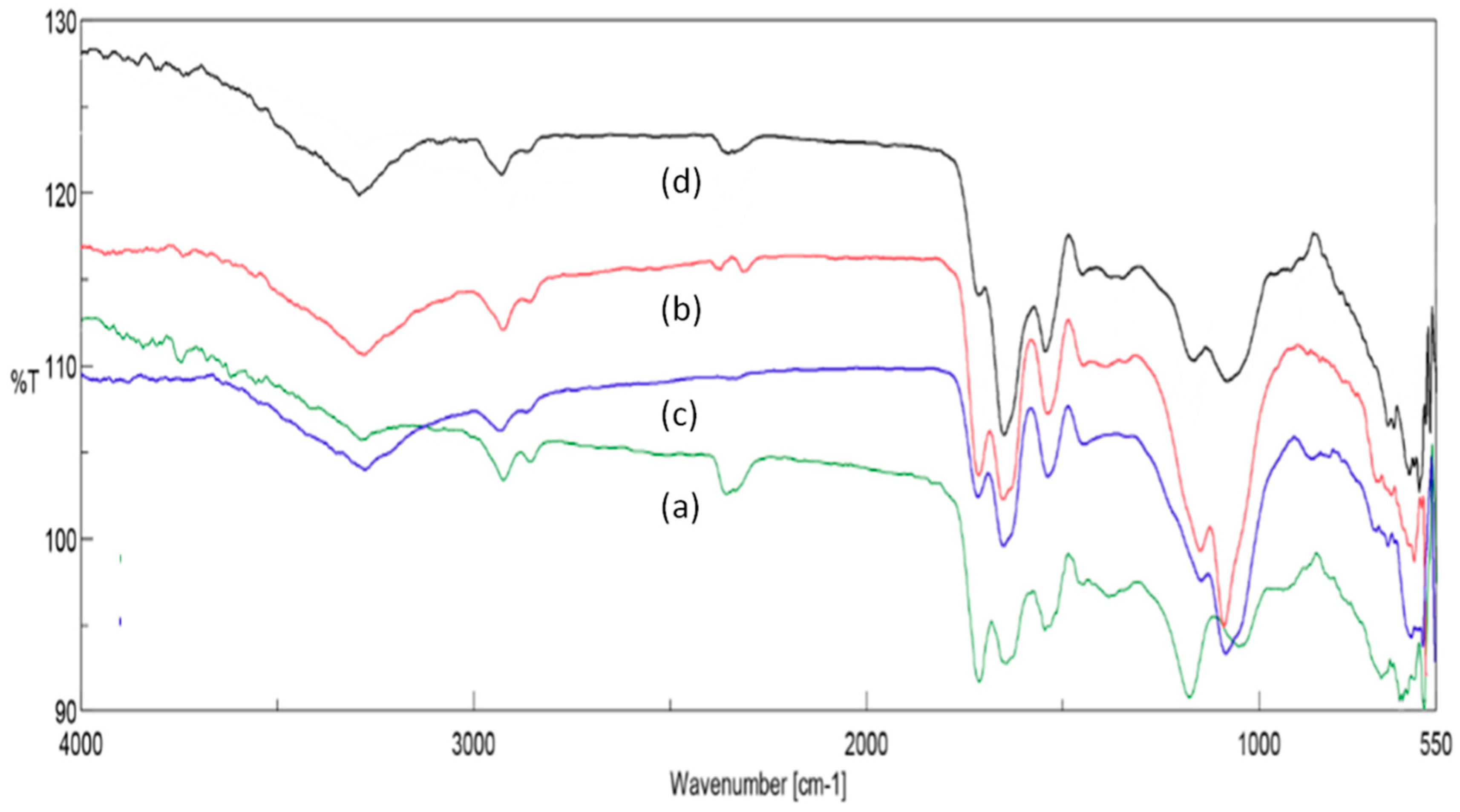
To complete our study, FT-IR analysis [
31] for each previous sample was performed, and
Figure 3 shows some slight differences.
For all samples, the common bands of microcrystalline cellulose were not recorded. Compared to the crude starting material, the bioplastic films show more intense bands located respectively at 680 and 860 cm−1 representative of aromatic compounds or benzene moieties due to polyphenol or phenylalanine residue. Bands at 1080 and 1360 cm−1 are evident of the presence of amine functions in the mixture. The peptide nature of the films was also demonstrated by the existence of the characteristic bands found at 1550 and 1648 cm−1. Other signals found at 2849, 2927, and 3300 cm−1 were observed on all spectra and correspond to methylene or simple alkyl chains, but also to hydroxyl (-OH) or primary amine groups (−NH2). Without surprise, the band of carboxylic acid found at 1700 cm−1 was observed in all samples and was evident of formic acid traces that was not eliminated from the three dimensional biopolymer networks, despite our efforts to remove it in vacuum at elevated temperature. In case of the deproteinized film, according to its spectrum, the networks appeared to be enriched in polyphenols not only because of the higher signal observed under 800 cm−1, but also by the signal amplitude increase at 1439 and 3300 cm−1.
Each film was also studied by EDX. Results showed that the most representative elements were C, O, and S. The presence of many other inorganic elements such as calcium, potassium, or phosphorus was confirmed. Some points need to be clarified. For example, for a deproteinized rapeseed cake, the amount of sulfur atom decreased drastically. It is possible to imagine that the liquid extracted protein sample fraction is rich in sulfur-based amino acid residues such as cysteine or methionine. On the other hand, when hydrochloric acid was used as catalyst instead of sulfuric acid there was no variation in the signal expressed by the sulfur element.
Due to the negligible influence of the initial crude mixture composition on the produced film quality, according to the
Table 2, it appears to be economic to start from a native rapeseed cake to get a strong and stable film from a larger volume of the liquid precursor. In fact, using the crude press-cake gives the highest yield (28%) compared to defatted (22%) and/or deproteinized (9%) cake.
Indeed, with cake made of particle of 600 µm of average diameter, higher yields of 28% were obtained from 100 g of the material subjected to the current acidic treatment. These results corroborate with the previous films observation made in the above paragraph and their related quality.
3.2. Process Optimization
The conversion process of crude press cake was then optimized by tuning experimental conditions (particles size, time, temperature, and solid/liquid ratio). In order to study the impact of particle size on the performances of the transformation process, particles of 300 and 600 µm were used in the same reaction conditions: 10 g of a selected rapeseed cake; 100 mL of formic acid with 4–5 drops of H
2SO
4; stirred at 60 °C for 30 min. Result showed that the conversion was increased from 28% to 51% by reducing the particle size to 300 µm with additional grinding and sieving, it was easy to double the amount of the generated films without alteration of the composition. Further mechanical treatment performed on the rapeseed cake results in the release of larger number of compounds becoming more accessible to the acid. The intact composition of the films was confirmed by the FT-IR spectroscopy and EDX analysis, except an unexpected improvement of the sulfur content. As displayed on the
Figure 4, the resulted bioplastic film was more transparent and in regards to the SEM observation, its morphology was more homogeneous compared to the film created from a native rapeseed cake of 600 µm. On the corresponding FT-IR spectrum of bioplastic film made of 300 µm rapeseed cake, there was no new apparent peak.
Variation of the reaction time has also additional effects on the yield and the external aspect of the bioplastic films. These results were reported in
Table 3. From a rapeseed cake of 600 µm, by increasing the reaction time from 15 to 60 min, the yield was finally lowered to 25% when the dispersion was heated for 60 min, the usual process afforded us a slightly colored film without apparent degradation, but a loss of transparency was observed. Obviously, a short period of 15 min was enough to create a resistant film compared to those obtained from extended time reactions.
As reported in
Table 4, the temperature rise could cause negative effect on the appearance of the films in parallel to a yield decrease. Up to 80 °C, the liquid precursor gave only opaque and dark brown colored bioplastic films. At 60 °C, the yields of the produced material always remained low (28%) as for other products; there was no modification in the FT-IR spectrum which indicates the cause of this darker shade.
In order to measure the impact of the solid/ liquid ratio (w/w), this parameter was varied in the range 1/5, 1/10, and 1/20 and keeping all optimized reaction conditions, e.g., a mixture of 10 g of 600 µm rapeseed cake stirred in a defined volume of formic acid with 4–5 drops of sulfuric acid at 40 °C for 15 min. Curiously, even the higher diluted material dispersion can enhance the yield to 43%, it will never become the perfect pathway to access bioplastic from rapeseed cake. However, it seems to be important to consider the recycling of the solvent to reduce the cost of the process. The best ratio was 1/5 w/w.
3.3. Films Analysis and Characterization
3.3.1. Water Vapor Adsorption
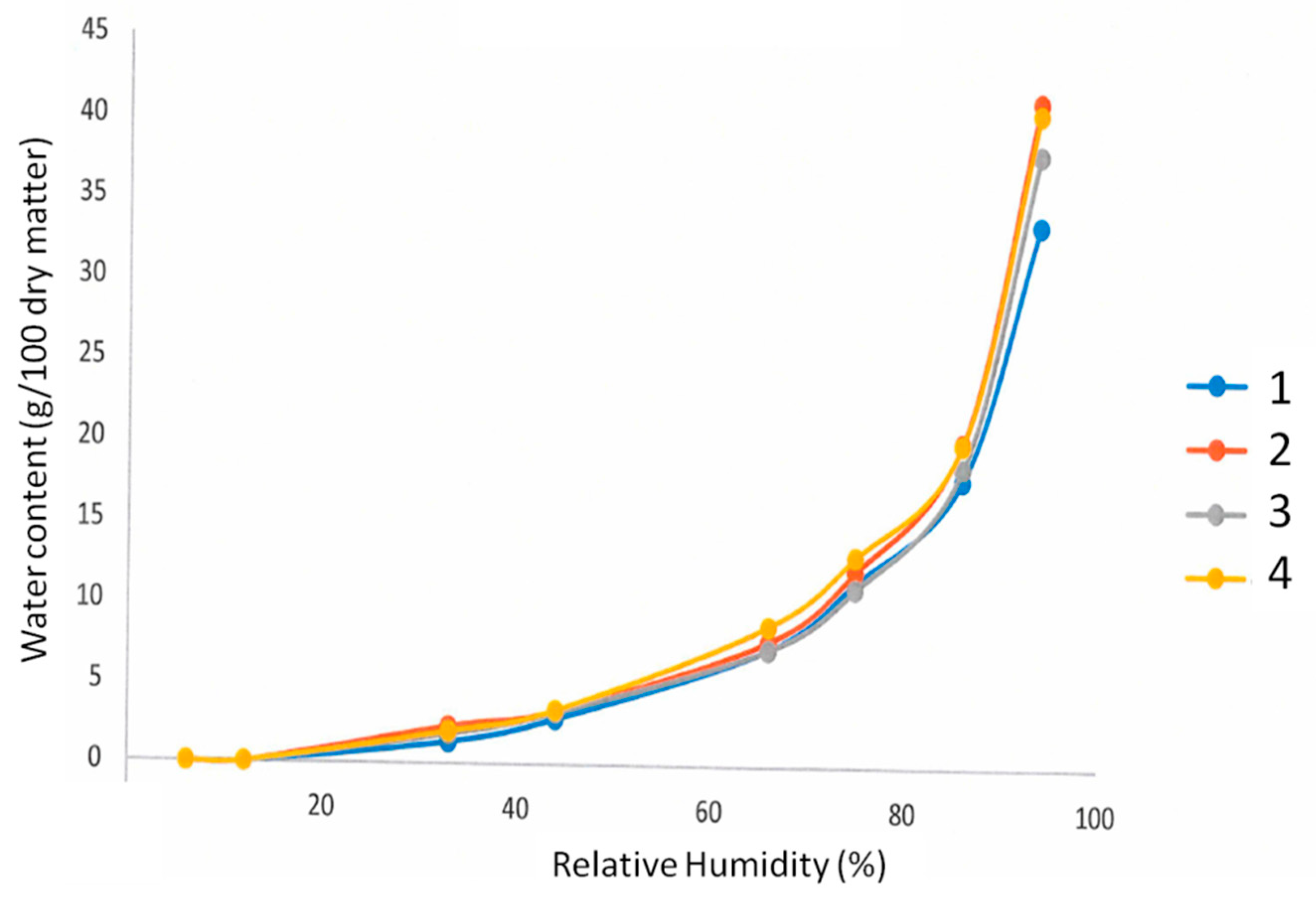
In
Figure 5, the samples 1–4 showed similar curve patterns and according to the IUPAC classification, they are of type III with a slight slop of the sorption curve under 70% of the strong. The sorption curves are representative of water vapor-sensitive polymer such as cellulose or polysaccharides [
32]. Due to the evident modification of protein conformation source of polar residue exposure, the material absorbs rapidly water for humidity level superior to 80%. All bioplastic films are hygroscopic materials independent of the process employed for producing them. Obviously, according to FE-SEM images of
Figure 1 and
Figure 5, the bioplastic films were not porous and there are only a few interactions between vapor and the materials.
3.3.2. Thermal Analysis
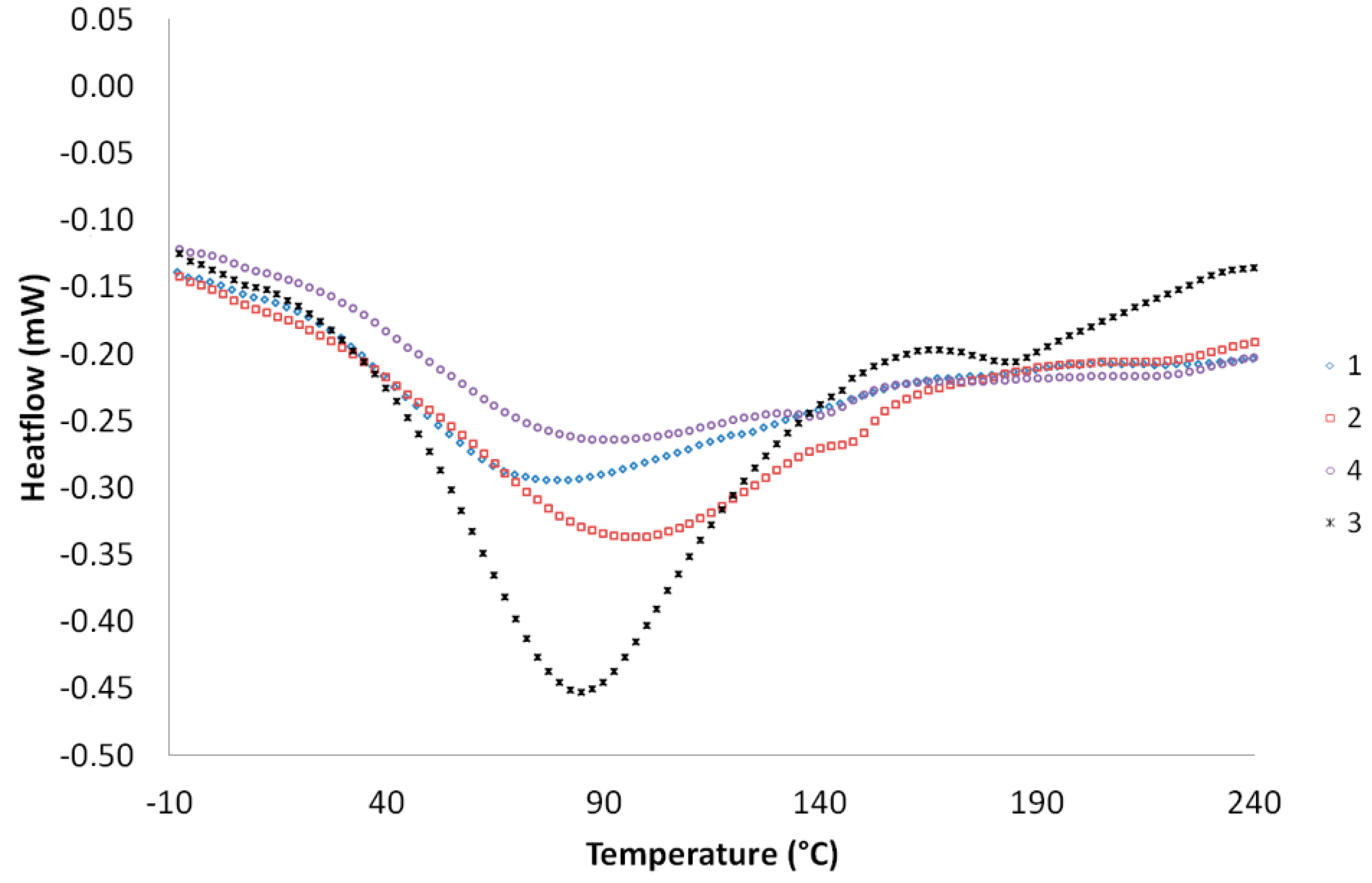
As displayed on the
Figure 6, the results of the heat-induced denaturation of the bioplastic films 1–4 were well measured. Under heating, the protein-based films are subjected to breaking and rearrangement of intra or inter-molecular interactions. Generally, this kind of material absorbs energy and this phenomenon leads to the formation of an endothermic peak on the DSC thermograms that correspond to a specific denaturation temperature (
Td) of the bioplastic films. For the sample 2 and 3, there was no influence of the dilution on the final thermal stability of each film with value of
Td recorded around 94 °C. In fact, these values appeared to be inferior to those generally encountered in the literature. Both thermograms show also a small peak located at 145 °C, maybe due to the presence of an unknown impurity inside the film. On the other hand, compared to 2 and 3, the samples 1 and 4 expressed lower values of
Td of 75 and 85 °C respectively. After all, for the film 1, the particle size played a role in the thermal stability of the bioplastic film and the material became more sensitive to the temperature variation. As reported in the previous paragraph, when the rapeseed cake transformation was carried out at 60 °C, the bioplastic film molecular organization was modified compared to 2 and 3, and the denaturation seems to be easier. In summary, in regards to the process, for a constant amount of formic acid included in the plastic and the lower values of
Td, in our case the acid could be recognized also as a plasticizer able to lower the glass transition of the rapeseed proteins; it is responsible for polymer chains lubrication and flexibility increase at moderate temperatures.
3.3.3. Mechanical Properties
If we consider that formic acid could act as a plasticizer, it can interact with hydroxyl groups and carboxylic acid groups of the proteins. Nevertheless, we have to bear in mind that it is also able to salify a slight amount of primary amine functions. However, Jogi et al., [
33] showed that proteins extracted from rapeseed cakes are poor in histidine. The major proteins found in the rapeseed cake are cruciferin and napin. Cruciferin is referred to as a globulin of 300 kDa able to gelate a solution containing it. Thus, it is possible to imagine the disruption of further inter and intramolecular hydrogen bonds between more flexible protein chains, but cruciferin has the ability to precipitate at lower pH. The phenomenon is also accelerated by temperature decrease during the casting of supernatant returning at room temperature. All phenomenon are exclusively driven by hydrophobic interactions caused by constitutive amino acid residues such as leucine. Furthermore, our process did not permit to remove the phytic acid found in large excess in the rapeseed meal recognized as responsible for protein complexation. Inside the bioplastic films, the current charges and polar interactions between side chains elements of the film prevent segment rotation and its relative molecular mobility. The consequence is an increase of modulus (E) and tensile strength (s) as reported in the
Table 5.
Unfortunately, all films 1–4 were easily breakable. Without surprise, the more resistant sample was the number 3. This effect could be explained by the higher concentration of the supernatant and a possible high stability of phytates and protein precipitates, especially when the reaction medium was heated at a temperature never exceeding 40 °C. This result corroborates with the higher value of Td reported in the thermal analysis. In summary, the films obtained by casting often express lower resistance and elongation at break, comparable to gelatin-based material.
3.4. Improvement of Film Stability by GO Loading
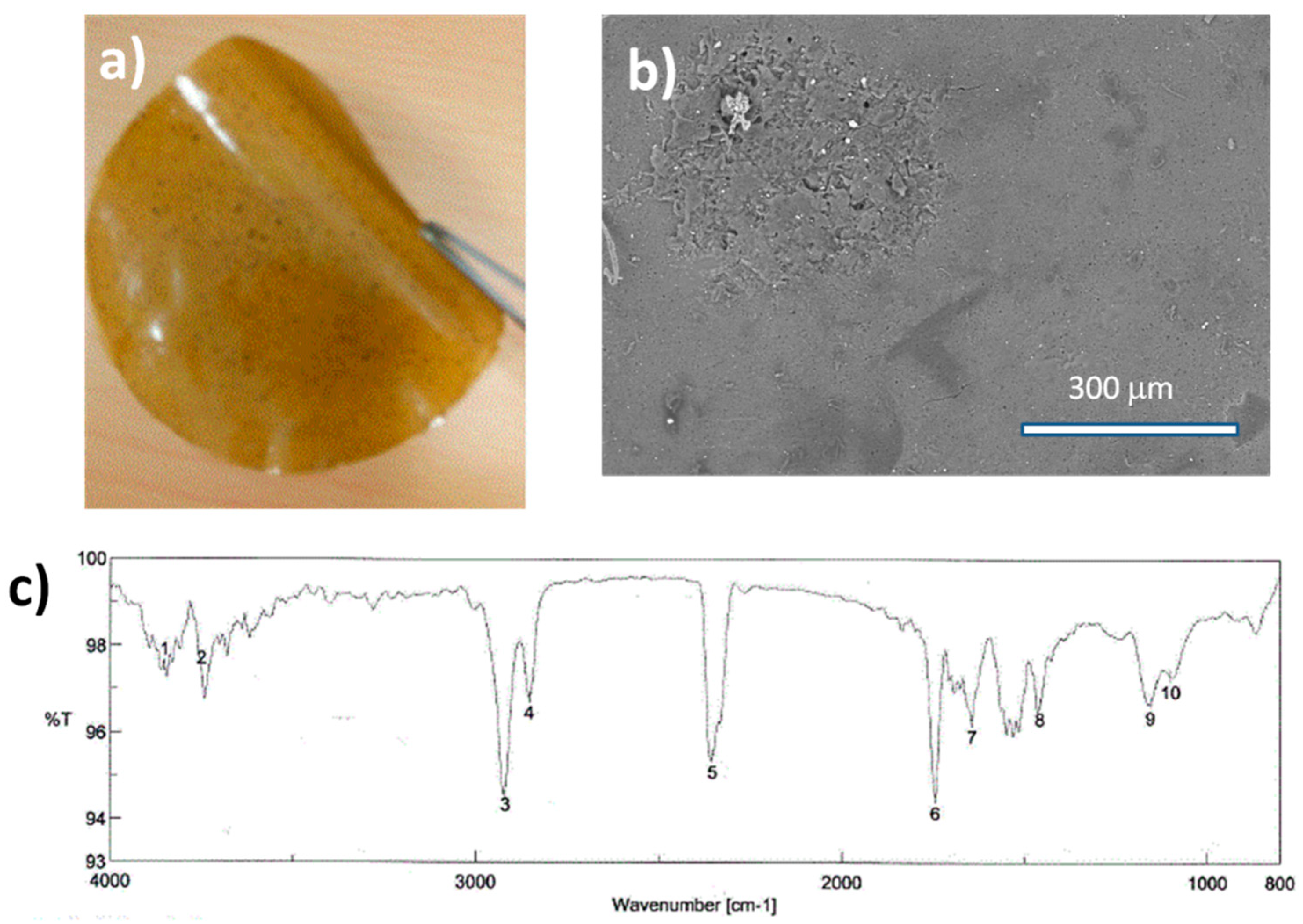
All reported films were hygroscopic materials. When introduced in water, these films are not stable and subjected to degradation or solubilization. According to the FT-IR analysis, for all samples formic acid traces are always present in the bioplastic films. Even if these films are dried in vacuum at room temperature in presence of solid sodium hydroxide (NaOH), it was impossible to remove the acid completely. When heated at 110 °C, all traces of the acid disappeared, but the film became rigid and breakable. According to some recent works, the introduction of GO into a biopolymer matrix could attribute interesting behaviors to the material. GO could be added in small mass percentage directly in the viscous liquid precursor just after the centrifugation, prior to casting in a simple glass Petri dish. At the end, a yellow transparent film was easily detached from the glass surface. In the
Figure 7, the GO loading inside the biopolymer networks did not alternate the morphology of the film surface.
As depicted in the
Figure 7a, the resulted plastic sheet could be bent by hand without cracking, however up to three months later the composite sheet became a little bit brittle. On the FT-IR spectrum, all typical signals are apparent on the spectrum, except the peak of the primary amine that evolved into two different peaks located at 1156 and 1457 cm
−1 characteristic of secondary amine. Usually, primary amine functions can react with the high reactive epoxide functions found on the surface of GO, thus proteins become covalently bound to the carbon based-sheets. Between 3500 and 4000 cm
−1, two vibration bands are clearly visible, representative of -OH, -NH
2, and –NH groups.