Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods of Transesterification in Subcritical Methanol
2.2. Transesterification in a Tubular Flow Reactor
2.3. Transesterification in a Batch Reactor
2.4. Analysis
3. Results and Discussion
3.1. Preliminary Studies
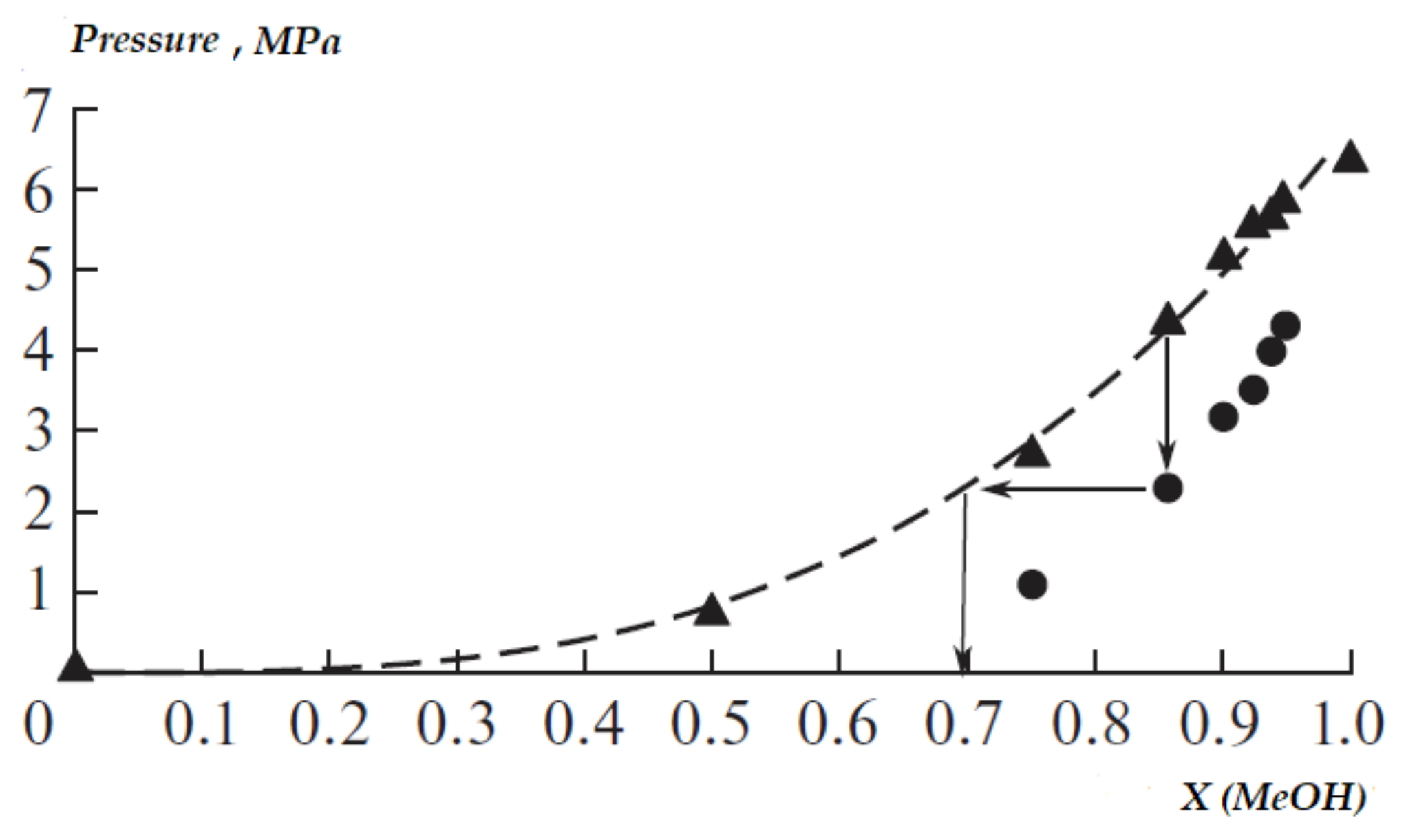
3.1.1. Mutual Solubility of the Initial Components
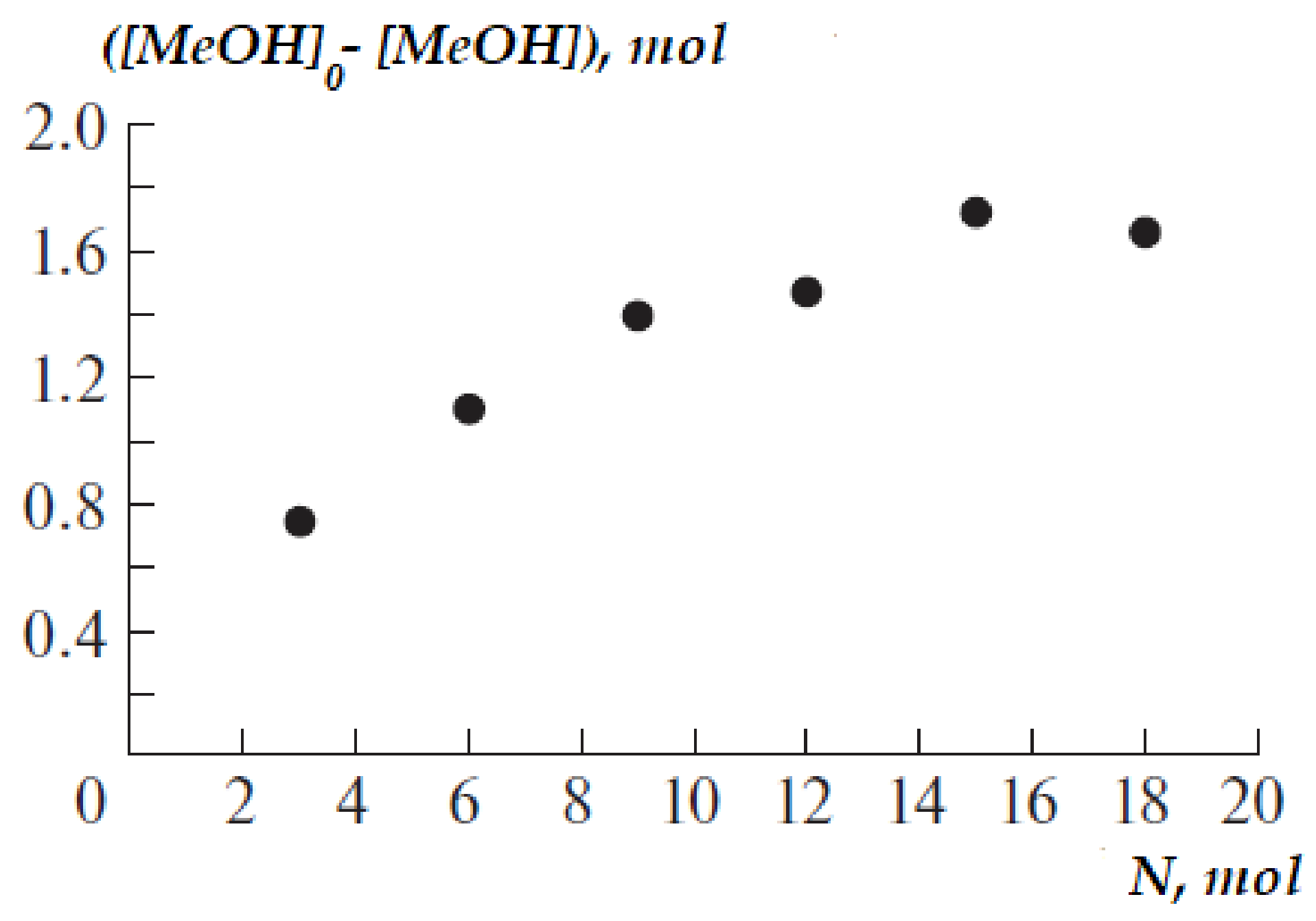
3.1.2. Effect of Molar Ratio
3.2. Effect of Reactor Design
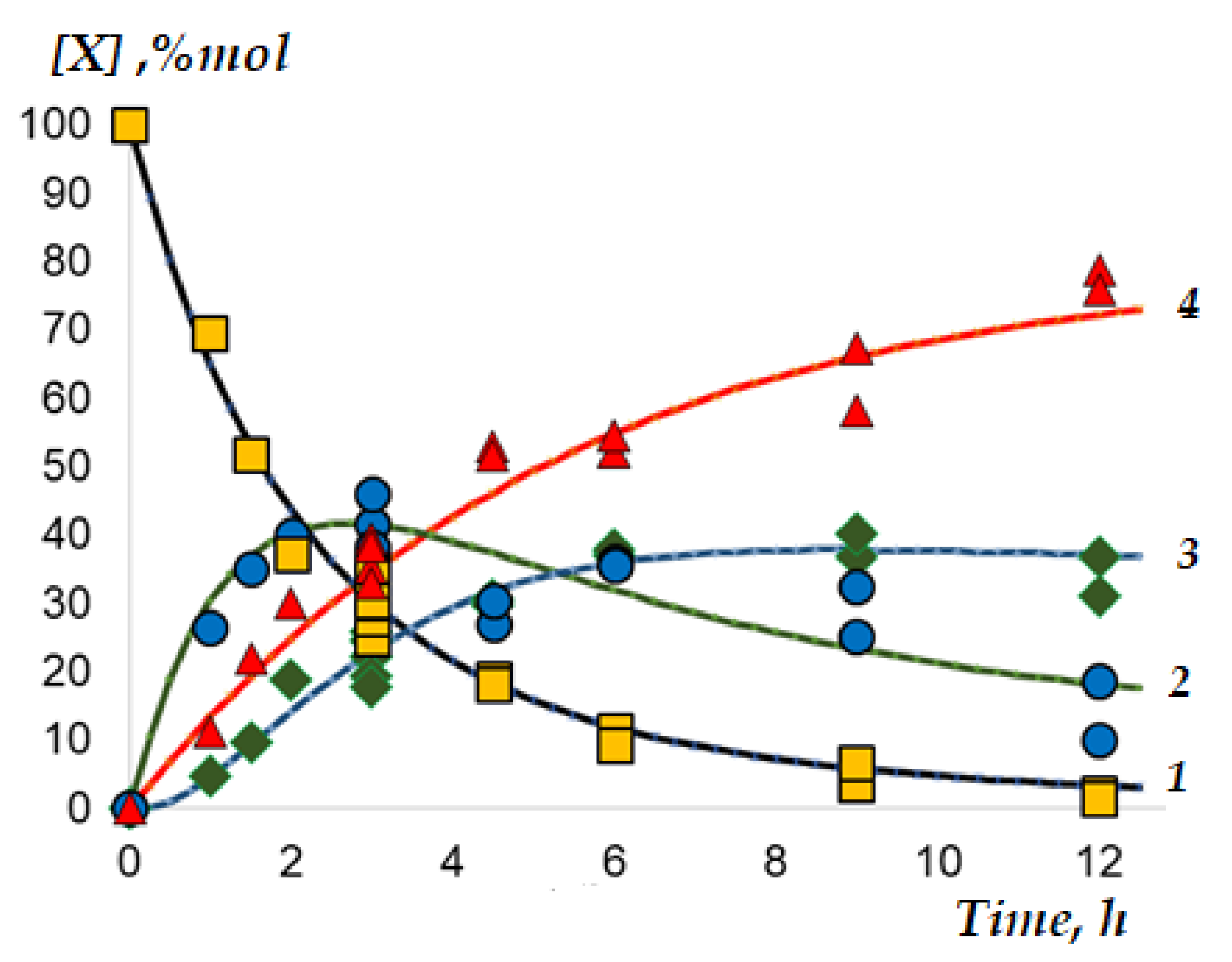
3.2.1. Transesterification in a Tubular Flow Reactor
3.2.2. Transesterification in a Batch Reactor
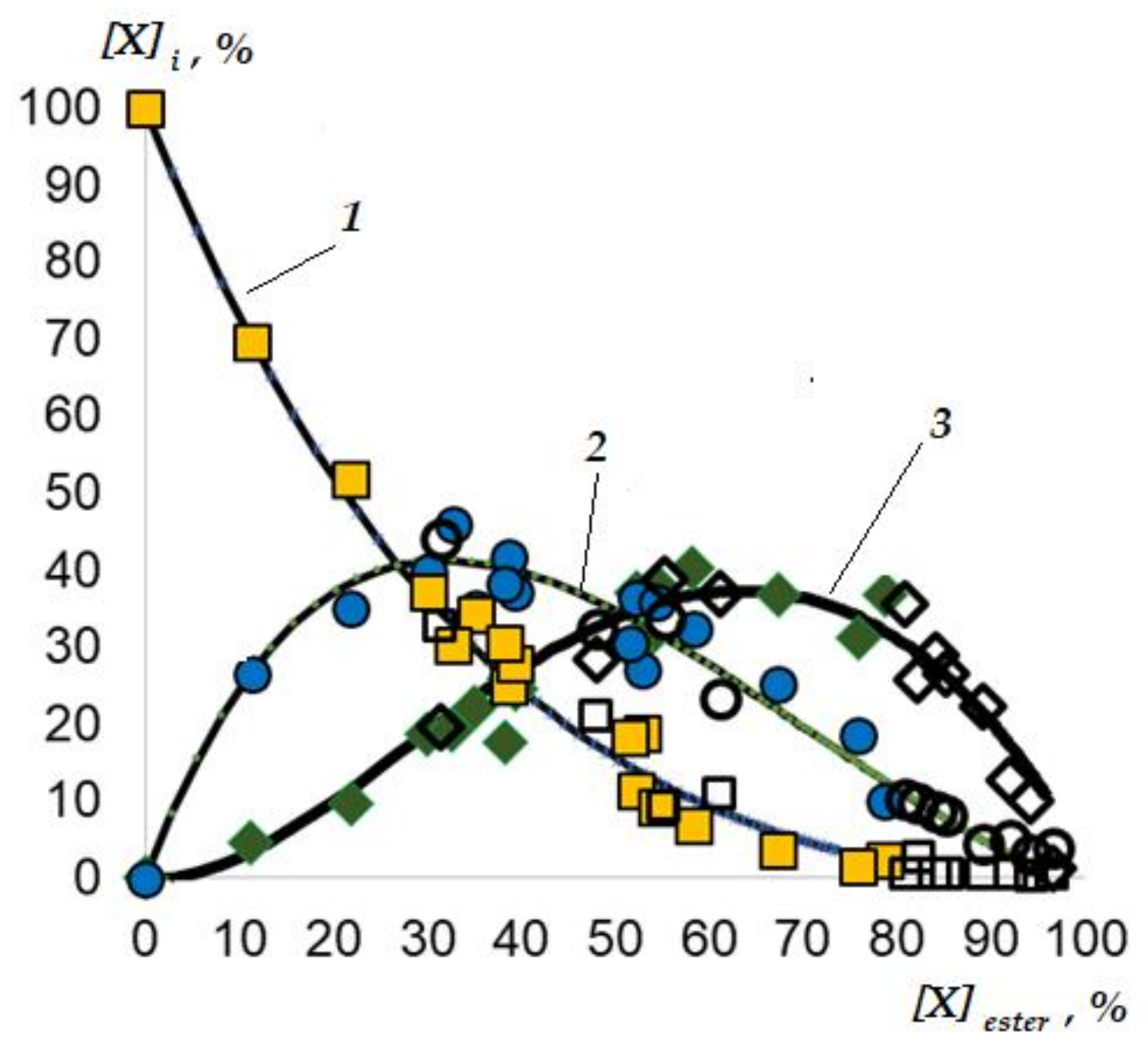
3.2.3. Reaction Kinetics in Tubular Flow and Batch Reactors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gumba, R.E.; Saallah, S. Green biodiesel production: A review on feedstock, catalyst, monolithic reactor and supercritical fluid technology. Biofuel Res. J. 2016, 11, 431–447. [Google Scholar] [CrossRef] [Green Version]
- Baskar, T.; Pravin, R.S.; Bagavathi, M. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Diasakov, M.; Loulodi, A.; Papayannakos, N. Kinetics of the Non–Catalytic Transesterification of Soybean Oil. Fuel 1998, 77, 1297–1302. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Valle, P.; Velez, A.; Hegel, P.; Mabe, G.; Brignole, E.A. Biodiesel production using supercritical alcohols with a non-edible vegetable oil in a batch reactor. J. Supercrit. Fluids 2010, 54, 61–70. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z.; Birch, J. Catalyst-Free Biodiesel Production Methods: A Comparative Technical and Environmental Evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Mujeeb, M.A.; Vedamurthy, A.B.; Shivasharana, C.T. Current strategies and prospects of biodiesel production: A review. Adv. Appl. Sci. Res. 2016, 7, 120–133. [Google Scholar]
- Kasim, N.S.; Tsai, T.-H.; Gunawan, S.; Ju, Y.H. Biodiesel production from rice bran oil and supercritical methanol. Bioresour. Technol. 2009, 100, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Kanna, R. Biodiesel Production from Waste Cooking Oil Using Co-solvent Technique. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 1573–1574. [Google Scholar] [CrossRef]
- Aghel, B.; Mohadesi, M.; Sahraei, S. Effect of Different Cosolvents on Transesterification of Waste Cooking Oil in a Microreactor. Chem. Eng. Technol. 2018, 41, 598–605. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, H.; Liang, B. Solubility of Multicomponent Systems in the Biodiesel Production by Transesterification of Jatropha curcas L. Oil with Methanol. J. Chem. Eng. Data 2006, 51, 1130–1135. [Google Scholar]
- Glisic, S.B.; Skala, D.U. Phase transition at subcritical and supercritical conditions of triglycerides methanolysis. J. Supercrit. Fluids 2010, 54, 71–80. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Z.; Li, L.; Fang, T. A review of multi-phase equilibrium studies on biodiesel production with supercritical methanol. RSC Adv. 2014, 4, 23447–23455. [Google Scholar] [CrossRef]
- Griend, L.V.; Feldman, M.; Peterson, C.L. Properties of rape oil and its methyl ester relevant to combustion modelling. ASAE 1988, Iss .fiche88–6507, p.17. Available online: https://agris.fao.org/agris-search/search.do?recordID=US9100316 (accessed on 16 August 2021).
- DIN EN 14103. Fat and Oil Derivatives-Fatty Acid Methyl Esters (FAME)-Determination of Ester and Linolenic Acid Methyl Ester Contents; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- EN 14105:2020. Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Free and Total Glycerol and Mono-, Di-, Triglyceride Contents; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- Shimoyama, Y.; Iwai, Y.; Jin, B.S.; Hirayama, T.; Arai, Y. Measurement and correlation of vapor–liquid equilibria for methanol + methyl laurate and methanol + methyl myristate systems near critical temperature of methanol. Fluid Phase Equilib. 2007, 257, 217–222. [Google Scholar] [CrossRef]
- Glisic, S.B.; Montoya, O.; Orlovic, A.; Skala, D. Vapor–liquid equilibria of triglycerides–methanol mixtures and their influence on the biodiesel synthesis under supercritical conditions of methanol. J. Serb. Chem. Soc. 2007, 72, 13–27. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nabetani, H.; Nakajima, M. Non-catalytic alcoholics process for production of biodiesel fuel by using bubble column reactor. In Proceedings of the Tunisia-Japan Symposium: R&D of Energy and Material Sciences for Sustainable Society, Gammarth, Tunisia, 28–30 November 2014; Volume 596, p. 012017. [Google Scholar] [CrossRef] [Green Version]
- Sapunov, V.N.; Kustov, A.V.; Zilin, V.F.; Staroverov, D.V. Subcritical methanol biodiesel production in a tubular reactor. Chem. Ind. Today 2012, 12, 13–20. [Google Scholar]
- Sapunov, V.N.; Kustov, A.V.; Hanikyn, V.L.; Staroverov, D.V. Subcritical methanol biodiesel production in a batch reactor. Chem. Ind. Today 2012, 7, 7–14. [Google Scholar]
- Ascher, U.; Petzold, M. Computer Methods for Ordinary Differential Equations and Differential Algebraic Equations; Society for Industrial and Applied Mathematics 3600 University City Science Center Philadelphia: Philadelphia, PA, USA, 1998; p. 314. [Google Scholar]
- Bogomolov, B.B.; Boldyrev, V.S.; Zubarev, F.V.; Meshalkin, V.P.; Men’shikov, V.V. Intelligent logical information algorithm for choosing energy and resource-efficient chemical technologies. Theor. Found. Chem. Eng. 2019, 53, 709–718. [Google Scholar] [CrossRef]
- Meshalkin, V.P.; Panchenko, S.V.; Dli, M.I.; Panchenko, D.S. Analysis of the Thermophysical Processes and Operating Modes of Electrothermic Reactor Using a Computer Model. Theor. Found. Chem. Eng. 2018, 52, 166–174. [Google Scholar] [CrossRef]
| Oil Phase Outlet, %w | The Composition of the Glycerol Phase, %w | ||||
|---|---|---|---|---|---|
| Glycerol | FAME | MG * | DG ** | TG *** | |
| 75.1 | 92.3 | 2.1 | 0.9 | 3.0 | 1.2 |
| 80.9 | 95.7 | 0.2 | 2.6 | <0.5 | 1.5 |
| 70.1 | 86.6 | 0.5 | 6.9 | <0.5 | 3.3 |
| 80.7 | 89.9 | 1.2 | 1.1 | <0.5 | 7.8 |
| 82.3 | 88.9 | 0.1 | 6.3 | <0.5 | 4.7 |
| № | Q, L h−1 | n | Residence Time, h | The Composition of the Oil Phase, (%w) | |||
|---|---|---|---|---|---|---|---|
| FAME | MG | DG | TG | ||||
| 1 | 12 | 1 | 1 | 11.1 | 1.9 | 19.1 | 71.9 |
| 2 | 8 | 1 | 1.5 | 16.9 | 3.1 | 19.9 | 42 |
| 3 | 12 | 2 | 2 | 31.9 | 8.3 | 31.1 | 41.3 |
| 4 | 12 | 3 | 3 | 39.9 | 11.1 | 31.5 | 27 |
| 5 | 8 | 2 | 3 | 30.4 | 8 | 22.2 | 31.1 |
| 6 | 8 | 2 | 3 | 32.8 | 8.6 | 22.7 | 24.3 |
| 7 | 4 | 1 | 3 | 31.3 | 7.7 | 32.3 | 30.3 |
| 8 | 4 | 1 | 3 | 40.4 | 7.8 | 29.6 | 33.7 |
| 9 | 8 | 3 | 4.5 | 46 | 11.1 | 17.2 | 17.2 |
| 10 | 8 | 3 | 4.5 | 40.2 | 9.7 | 17.4 | 14.8 |
| 11 | 4 | 2 | 6 | 56.8 | 16.8 | 28.9 | 12.9 |
| 12 | 4 | 2 * | 6 | 51.7 | 15 | 24.9 | 9.2 |
| 13 | 4 | 3 * | 9 | 61.6 | 14.1 | 16.9 | 3.4 |
| 14 | 4 | 3 | 9 | 62.9 | 18.2 | 25.7 | 7.6 |
| 15 | 1 | 1 | 12 | 68.5 | 13.4 | 6.4 | 2.1 |
| 16 | 4 | 4 * | 12 | 66.3 | 11.4 | 11.9 | 1.3 |
| № | Residence Time, h * | T, °C | N | The Composition of the Oil Phase, %w | |||
|---|---|---|---|---|---|---|---|
| FAME | MG | DG | TG | ||||
| 1 | 4 | 220 | 9 | 52.1 | 15.3 | 23.3 | 9.3 |
| 2 | 8 | 220 | 9 | 81.6 | 11.8 | 6.1 | 0.6 |
| 3 | 12 | 220 | 9 | 78.0 | 14.3 | 7.2 | 0.5 |
| 4 | 16 | 220 | 9 | 82.9 | 10.8 | 5.8 | 0.5 |
| 5 | 8 + 8 ** | 220 | 9 | 90.9 | 5.2 | 3.5 | 0.5 |
| 6 | 8 + 8 + 8 ** | 220 | 9 | 96.2 | 0.5 | 2.8 | 0.5 |
| 7 | 0.5 | 230 | 6 | 45.5 | 11.3 | 22.5 | 20.8 |
| 8 | 0.5 | 230 | 9 | 58.1 | 14.7 | 16.3 | 10.9 |
| 9 | 0.5 | 230 | 15 | 29.4 | 7.7 | 30.2 | 32.2 |
| 10 | 8 | 230 | 6 | 79.9 | 10.4 | 6.9 | 2.8 |
| 11 | 8 + 8 | 230 | 6 | 87.2 | 9.1 | 3.2 | 0.5 |
| 12 | 8 + 8 + 8 ** | 230 | 6 | 93.2 | 4.1 | 2.3 | 0.4 |
| , mol L−1 h−1 | ||||||
| 7.10 ± 0.04 | 6.10 ± 0.04 | 3.90 ± 0.02 | <0.01 | 0.41 ± 0.03 | 0.40 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshalkin, V.; Sapunov, V.; Kozlovskiy, R.; Kozlovskiy, I.; Staroverov, D.; Luganskiy, A.; Voronov, M. Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type. Processes 2021, 9, 1488. https://doi.org/10.3390/pr9091488
Meshalkin V, Sapunov V, Kozlovskiy R, Kozlovskiy I, Staroverov D, Luganskiy A, Voronov M. Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type. Processes. 2021; 9(9):1488. https://doi.org/10.3390/pr9091488
Chicago/Turabian StyleMeshalkin, Valeriy, Valentin Sapunov, Roman Kozlovskiy, Ivan Kozlovskiy, Dmitry Staroverov, Artur Luganskiy, and Mikhail Voronov. 2021. "Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type" Processes 9, no. 9: 1488. https://doi.org/10.3390/pr9091488
APA StyleMeshalkin, V., Sapunov, V., Kozlovskiy, R., Kozlovskiy, I., Staroverov, D., Luganskiy, A., & Voronov, M. (2021). Experimentally Calculated Study of the Effectiveness on the Process of Non-Catalytic Synthesis of Biodiesel in Reactors of Various Type. Processes, 9(9), 1488. https://doi.org/10.3390/pr9091488







