Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Electrochemical Corrosion Studies
2.3. Rusting Procedure
3. Results and Discussions
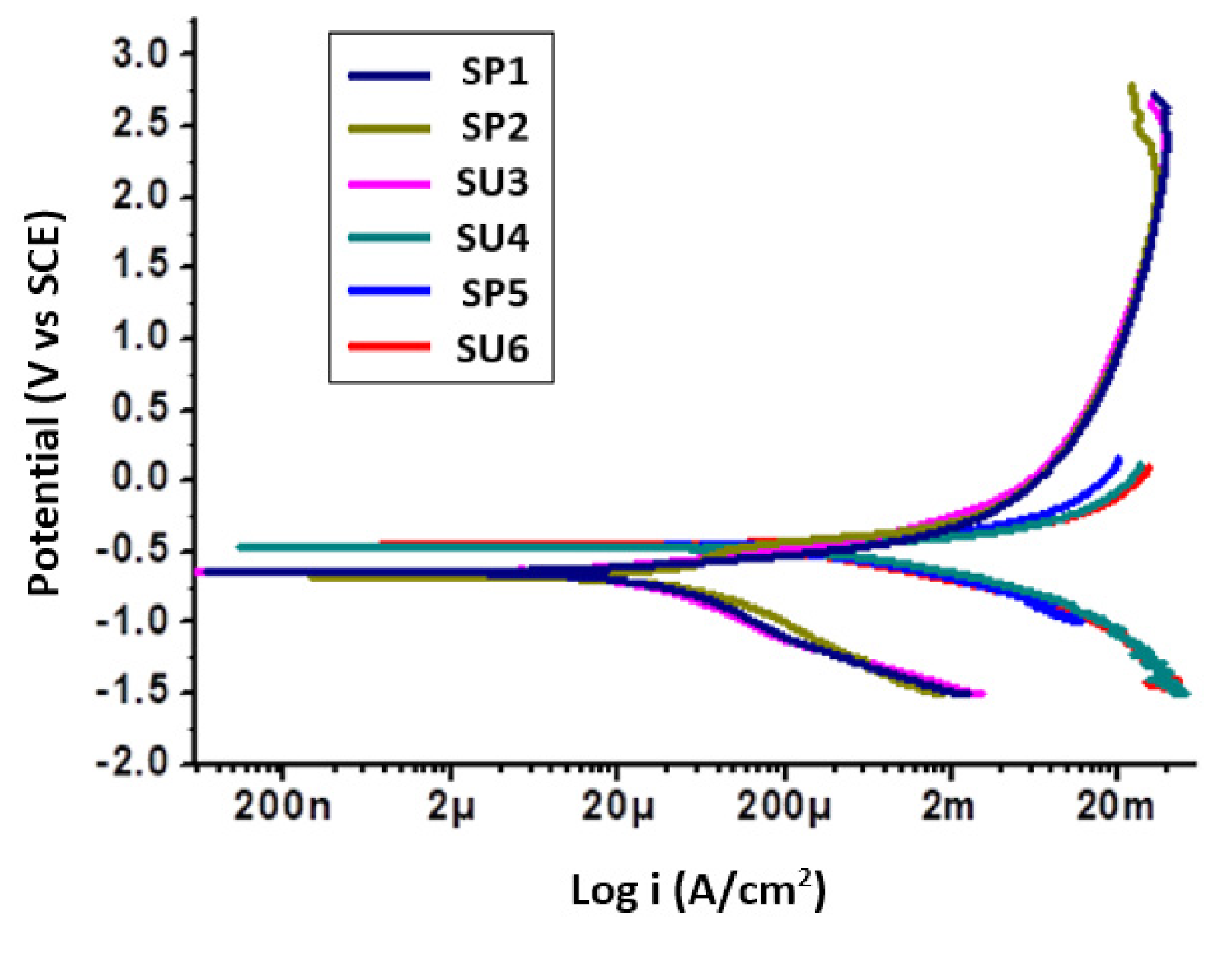
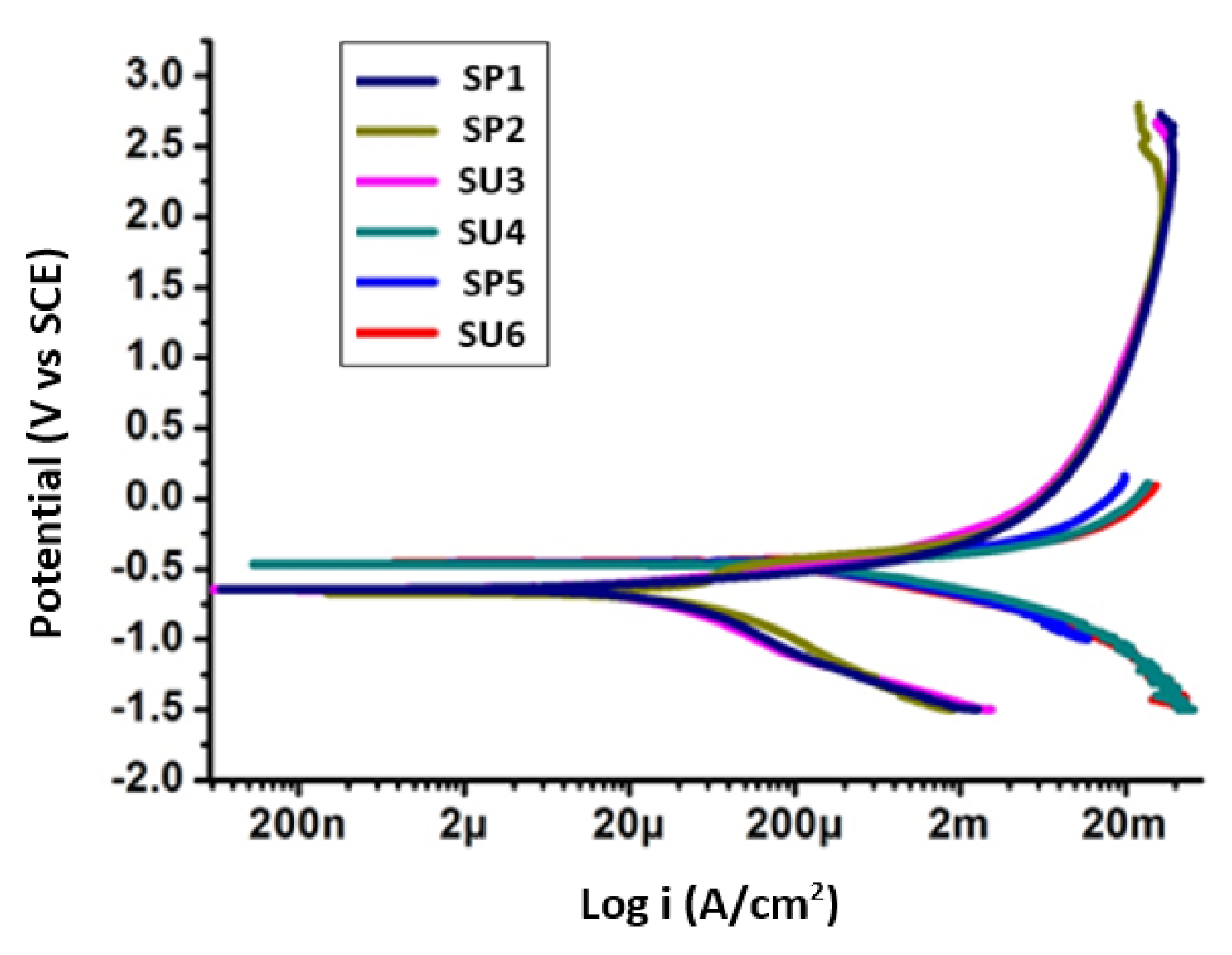
3.1. Potentiodynamic Polarization Tests
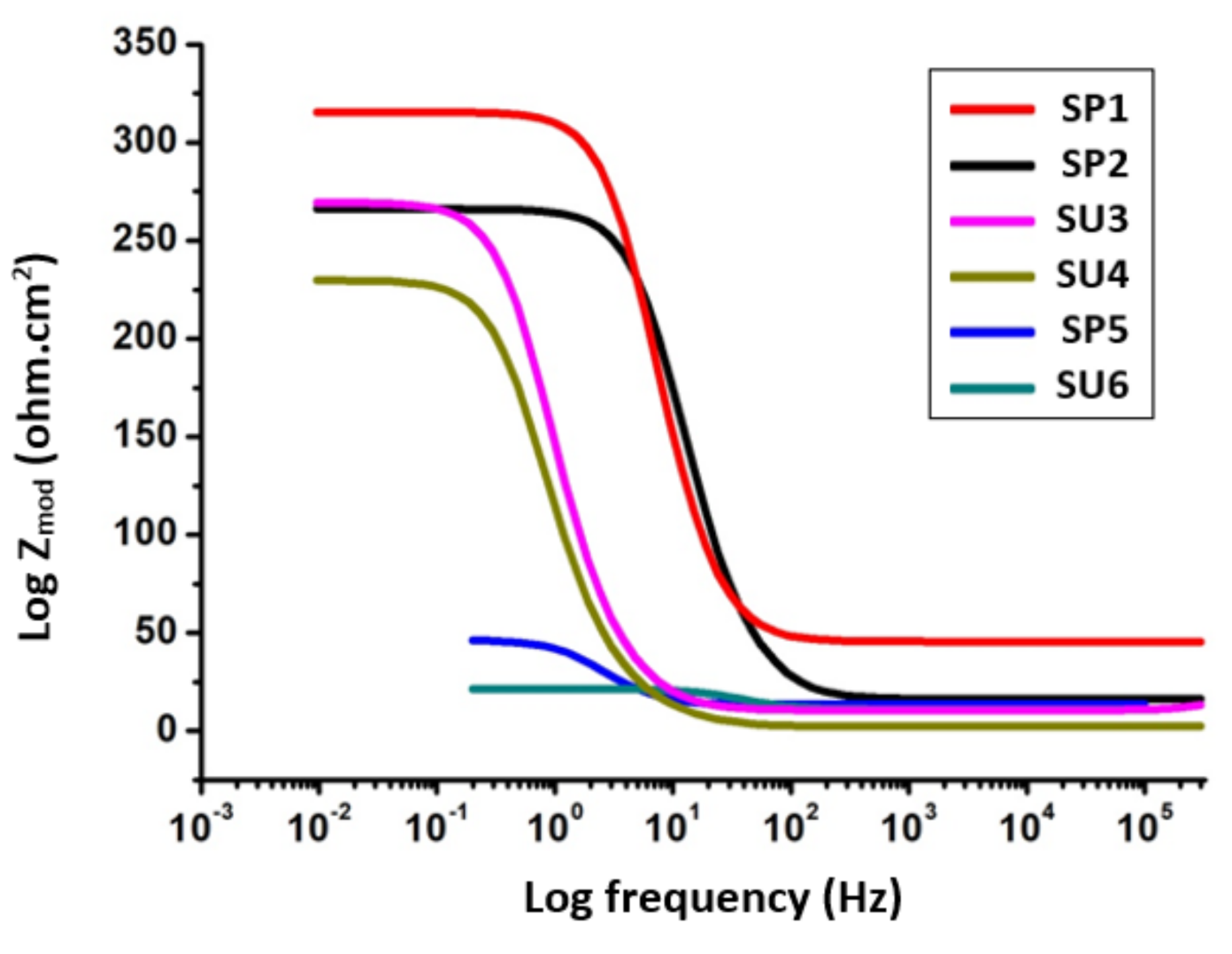
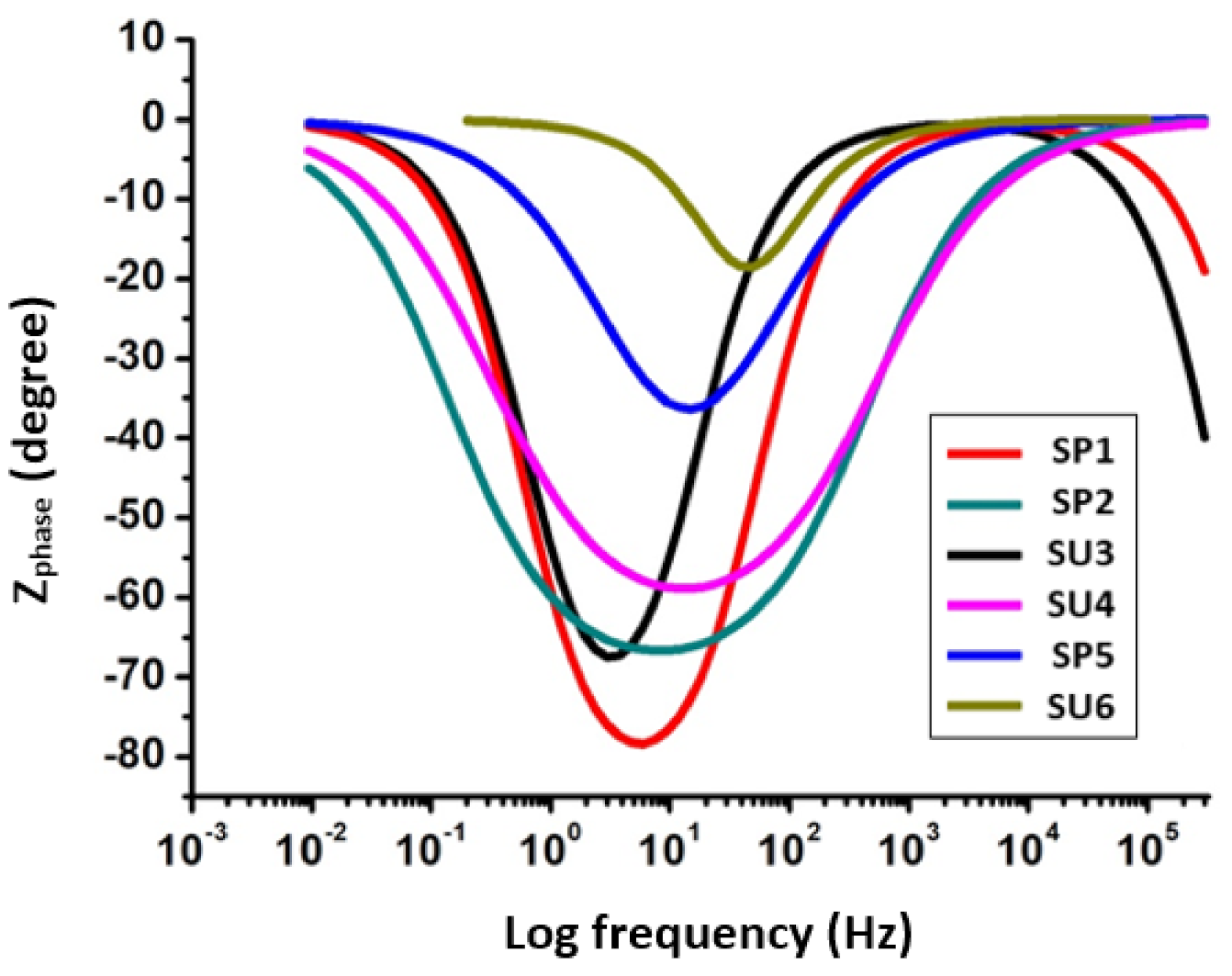
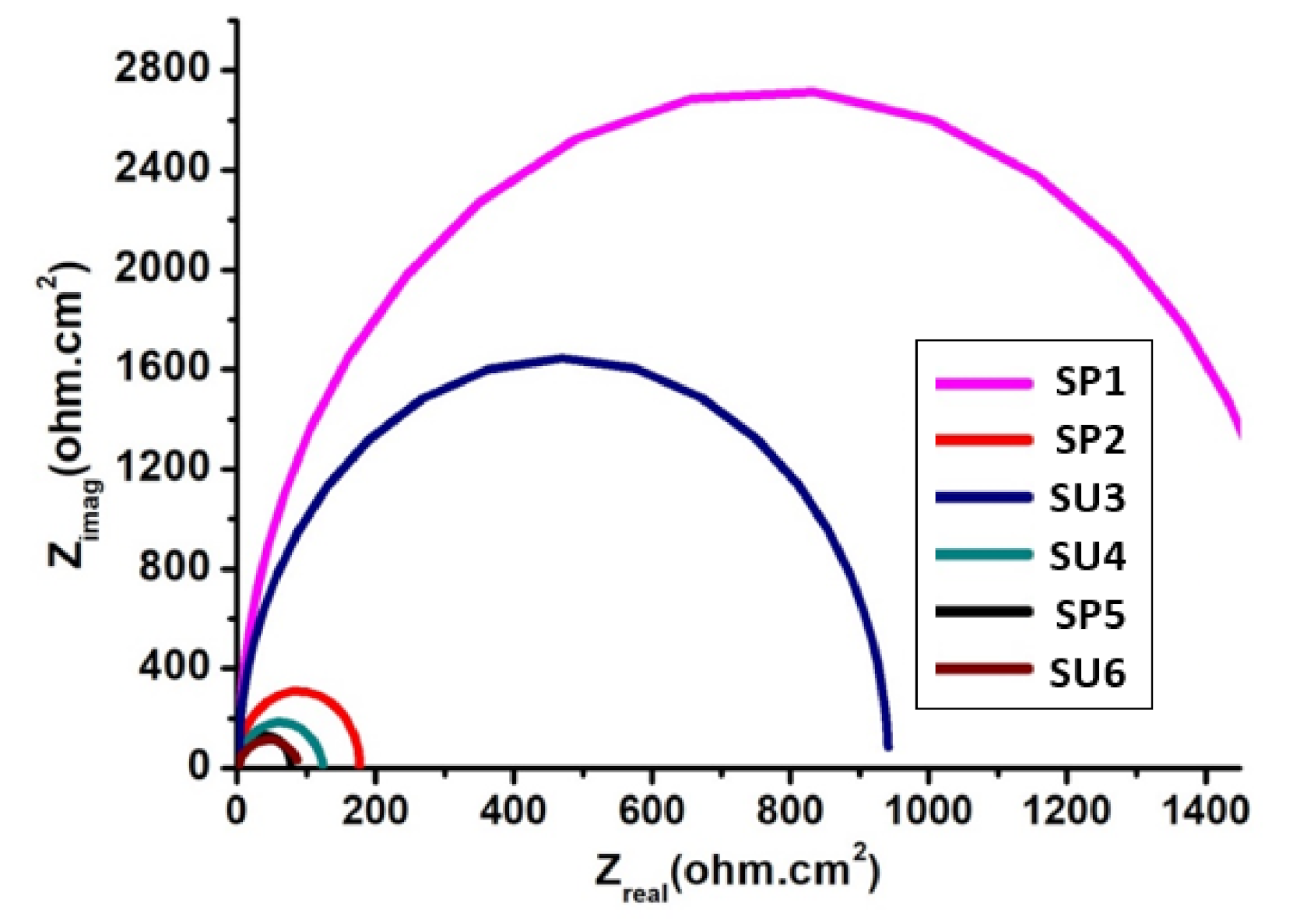
3.2. Electrochemical Impedance Spectroscopy (EIS)
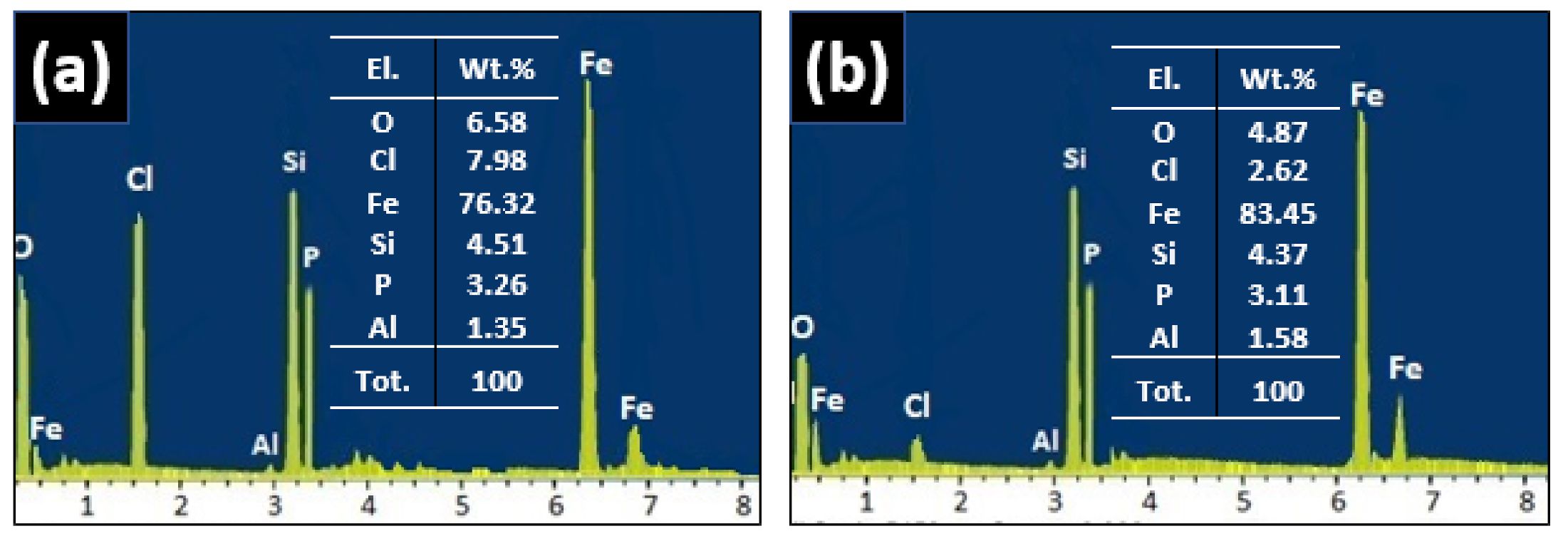
3.3. Morphology and Surface Characterization
4. Conclusions
- Anodic passivation of weathering steel comparable to potentiostatic passivation is obtained by shorting weathering steel with nobler metals (copper or graphite). However, the lower corrosion rate of the weathering steel further revealed that, besides the progressive rusting of weathering steel, other factors such as the binding force between the rust and the substrate also affect the corrosion rate significantly.
- A simulation of 18 months field exposed weathering steel in Digha is achieved by shorting weathering steel with copper and dipping it in 0.01 M KCl solutions. Meanwhile, from SEM images and EDX analysis, the morphology of the rust obtained by two weeks’ laboratory simulation correlates well with the rust obtained in 18-month field exposed samples.
- The corrosion rate of weathering steel that was anodically polarized with Cu or graphite showed a significant decrease in corrosion rate, confirming that the progressive rusting of weathering steel leads to a progressive decrease in corrosion rate. Specifically, anodic polarization using copper increased the corrosion resistance by 80 and 89% for the polished and unpolished samples, respectively; using graphite in polarization increased the corrosion resistance by only 13.6 and 20% for the polished and unpolished samples, respectively.
- From the EDX analysis of the rust layer, the main corrosion product of iron oxide (hematite α-Fe2O3) represents a very beneficial protective layer initiated by the mechanism of chemical interaction between the exposed steel surface and the solution intensified by the formation of micro galvanic pairs, which reduce the corrosion rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, B.; Pohlman, S.L. Metals handbook, ninth edition: Volume 13—Corrosion (ASM Handbook). In ASM Metals Handbook; ASM International: Russell Township, OH, USA, 1987; pp. 80–83. ISBN 978-0871700193. [Google Scholar]
- Abdo, H.S.; Seikh, A.H.; Mandal, B.B.; Mohammed, J.A.; Ragab, S.A.; Abdo, M.S. Microstructural Characterization and Corrosion-Resistance Behavior of Dual-Phase Steels Compared to Conventional Rebar. Crystals 2020, 10, 1068. [Google Scholar] [CrossRef]
- Shreir, L.L.; Jarman, R.A.; Bursten, G.T. Corrosion Metal—Environment Reactions. In Corrosion; Butterworth Heinemann: Oxford, UK, 1994; Volume 1, p. 22. ISBN 0-7506-1077-8. [Google Scholar]
- Abdo, H.S.; Abdus Samad, U.; Mohammed, J.A.; Ragab, S.A.; Seikh, A.H. Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals 2021, 11, 889. [Google Scholar] [CrossRef]
- Barton, K. Protection against Atmospheric Corrosion: Theories and Methods; Wiley: New York, NY, USA, 1976; ISBN 0471013498. [Google Scholar]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Uzzaman, T. Ameliorative Corrosion Resistance and Microstructure Characterization of 2205 Duplex Stainless Steel by Regulating the Parameters of Pulsed Nd:YAG Laser Beam Welding. Metals 2021, 11, 1206. [Google Scholar] [CrossRef]
- Seinfeld, J. Atmospheric Chemistry and Physics of Air Pollution; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Sundaram, M.; Mohan, P.S.; Ananth, V. Metalic corrosion. In Proceeding of the 10th International Congress on Metallic Corrosion, Madras, India, 7–11 November 1987; pp. 143–146. [Google Scholar]
- Rodríguez, J.J.S.; Hernández, F.J.S.; González, J.E.G. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environment. Corros. Sci. 2003, 45, 799–815. [Google Scholar] [CrossRef]
- Abdo, H.S.; Sarkar, A.; Gupta, M.; Sahoo, S.; Mohammed, J.A.; Ragab, S.A.; Seikh, A.H. Low-Cost High-Performance SnO2–Cu Electrodes for Use in Direct Ethanol Fuel Cells. Crystals 2021, 11, 55. [Google Scholar] [CrossRef]
- De Meybaum, B.R.; Ayllon, E.S. Characterization of Atmospheric Corrosion Products on Weathering Steels. Corrosion 1978, 36, 1139–1151. [Google Scholar] [CrossRef]
- Santana Rodríguez, J.J.; Santana Hernández, F.J.; González González, J.E. Mathematical and electro-chemical characterisation of the layer of corrosion products on carbon steel in various environments. Corros. Sci. 2002, 44, 2597–2610. [Google Scholar] [CrossRef]
- Wang, J.H.; Wei, F.I.; Chang, Y.S.; Shih, H.C. The corrosion mechanisms of carbon steel and weathering steel in SO2 polluted atmospheres. Mater. Chem. Phys. 1997, 47, 1–8. [Google Scholar] [CrossRef]
- Misawa, T.; Asami, K.; Hashimoto, K.; Shimodaira, S. The mechanism of atmospheric rusting and the protective amorphous rust on low alloy steel. Corros. Sci. 1974, 14, 279–289. [Google Scholar] [CrossRef]
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Feliu, S.; Morcillo, M.; Feliu, S. The prediction of atmospheric corrosion from meteorological and pollution parameters-II. Long-term forecasts. Corros. Sci. 1993, 34, 415–422. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Luqman, M.; Ragab, S.A.; Almotairy, S.M. Influence of Chloride Ions on Electrochemical Corrosion Behavior of Dual-Phase Steel over Conventional Rebar in Pore Solution. Appl. Sci. 2020, 10, 4568. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Samosir, R.; Simanjuntak, S.L. The influence of concentration and pH on corrotion rate in stainless steels—316 solution HNO3 medium. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 237, p. 012047. [Google Scholar] [CrossRef] [Green Version]
- Alodan, M.A. Modeling of pH Distribution over Corrosion Sites. J. King Saud Univ. Eng. Sci. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Rozali, A.A.; Masdek, N.R.N.; Murad, M.C.; Salleh, Z.; Hyie, K.M. The Effect of pH Value on the Corrosion Behaviour of Ti-6Al-4V and 316L SS Alloys under Physiological Environment. Chem. Eng. Trans. 2018, 63, 769–774. [Google Scholar] [CrossRef]
- Pettersson, J.; Asteman, H.; Svensson, J.E.; Johansson, L.G. KCl Induced Corrosion of a 304-type Austenitic Stainless Steel at 600 °C.; The Role of Potassium. Oxid. Met. 2005, 64, 23–41. [Google Scholar] [CrossRef]
- Jonsson, T.; Froitzheim, J.; Pettersson, J.; Svensson, J.E.; Johansson, L.G.; Halvarsson, M. The Influence of KCl on the Corrosion of an Austenitic Stainless Steel (304L) in Oxidizing Humid Conditions at 600 °C: A Microstructural Study. Oxid. Met. 2009, 72, 213–239. [Google Scholar] [CrossRef]
- Sim, J.H.; Kim, Y.S.; Cho, I.J. Corrosion behavior induced by LiCl-KCl in type 304 and 316 stainless steel and copper at low temperature. Nucl. Eng. Technol. 2017, 49, 769–775. [Google Scholar] [CrossRef]
- Larsson, E.; Gruber, H.; Hellström, K.; Jonsson, T.; Liske, J.; Svensson, J.E. A Comparative Study of the Initial Corrosion of KCl and PbCl2 on a Low-Alloyed Steel. Oxid. Met. 2017, 87, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Malede, Y.C.; Montgomery, M.; Dahl, K.V.; Hald, J. Effect of microstructure on KCl corrosion attack of modified AISI 310 steel. Mater. High Temp. 2018, 35, 243–254. [Google Scholar] [CrossRef]



| Composition in Weight% | Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | Ni | Cu | YS (MPa) | UTS (MPa) |
| 0.09 | 0.41 | 0.38 | 0.11 | 0.01 | 0.45 | 0.27 | 0.35 | 320 | 460 |
| Sample Code | Condition | Connected in Solution W.R.T. | Electrolytes |
|---|---|---|---|
| SP1 | Polished | Cu | 0.01 M KCl |
| SP2 | Polished | Graphite | 0.01 M KCl |
| SU3 | Unpolished | Cu | 0.01 M KCl |
| SU4 | Unpolished | Graphite | 0.01 M KCl |
| SP5 | Polished sample potentiodynamically polarized | 0.01 M KCl | |
| SU6 | Unpolished Weather exposed sample potentiodynamically polarized | 0.01 M KCl | |
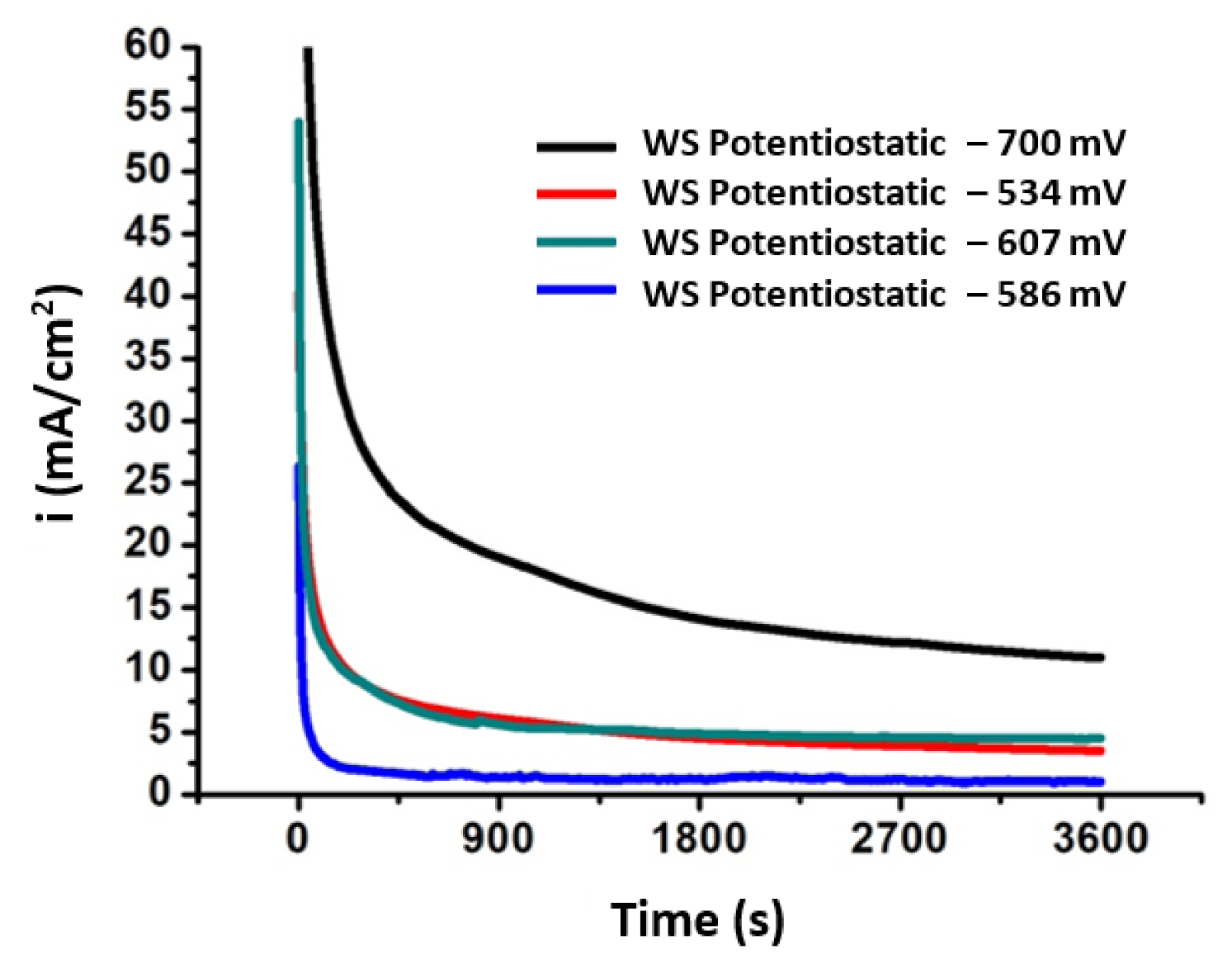
| Sample Code | Electrolyte | Stabilized Voltage (mV) vs. SCE | Stabilized Current (mA/cm2) | Stabilized Voltage (mV) vs. SCE in Current Transient Curve * | Stabilized Current (mA/cm2) in Current Transient Curve |
|---|---|---|---|---|---|
| SP1 | 0.01 M KCl | −607 | 44 | −607 | 3.5 |
| SP2 | 0.01 M KCl | −700 | 8 | −700 | 4.2 |
| SU3 | 0.01 M KCl | −586 | 6 | −586 | 3.5 |
| SU4 | 0.01 M KCl | −534 | 43 | −534 | 3.7 |
| Sample Code | Icorr (µA/cm2) | Icorr μm/yr | Ecorr (mV) vs. SCE |
|---|---|---|---|
| SP1 | 15.88 | 19.50 | −659.00 |
| SP2 | 16.89 | 20.20 | −456.20 |
| SU3 | 16.30 | 21.50 | −576.20 |
| SU4 | 22.33 | 23.20 | −588.20 |
| SP5 | 26.26 | 30.66 | −329.50 |
| SU6 | 30.52 | 35.63 | −512.90 |
| Sample Code | Icorr (µA/cm2) | Ecorr (mV) vs. SCE |
|---|---|---|
| SP1 | 70.69 | −910 |
| SP2 | 109.7 | −800 |
| SU3 | 102.5 | −1000 |
| SU4 | 112.9 | −788.2 |
| Sample Code | Ru (ohm) | Y0 | α | Wd | Rp (ohm) |
|---|---|---|---|---|---|
| SP1 | 3.5 × 10−3 | 1.125 × 10−6 | 523 × 10−3 | 12.61 × 10−3 | 529.62 |
| SP2 | 4.467 × 10−3 | 1.329 × 10−9 | 1 | 44.38 × 10−3 | 336.25 |
| SU3 | 5.6 × 10−3 | 7.284 × 10−9 | 859.5 × 10−3 | 69.81 × 10−3 | 502.00 |
| SU4 | 44.82 × 10−6 | 1.533 × 10−9 | 1 | 6.588 × 10−3 | 317.20 |
| SP5 | 12.02 × 10−6 | 16.63 × 10−9 | 853.2 × 10−3 | 249 × 10−3 | 278.80 |
| SU6 | 235.8 × 10−12 | 24.16 × 10−10 | 845.5 × 10−3 | 65.98 × 10−3 | 68.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, H.S.; Seikh, A.H.; Fouly, A.; Hashmi, F.H. Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique. Processes 2021, 9, 1469. https://doi.org/10.3390/pr9081469
Abdo HS, Seikh AH, Fouly A, Hashmi FH. Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique. Processes. 2021; 9(8):1469. https://doi.org/10.3390/pr9081469
Chicago/Turabian StyleAbdo, Hany S., Asiful H. Seikh, Ahmed Fouly, and Faraz H. Hashmi. 2021. "Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique" Processes 9, no. 8: 1469. https://doi.org/10.3390/pr9081469
APA StyleAbdo, H. S., Seikh, A. H., Fouly, A., & Hashmi, F. H. (2021). Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique. Processes, 9(8), 1469. https://doi.org/10.3390/pr9081469









