Risk Assessment and Material Suitability Evaluation on Static Equipment of Hydrofluoric Acid Alkylation Unit
Abstract
1. Introduction
2. Risk Source Analysis of Static Equipment in HF Acid ALK Unit
2.1. Damage Driving Force
2.2. Main Damage Parts and Main Corrosion Failure Modes
2.2.1. Main Damage Parts
2.2.2. Main Corrosion Failure Modes
Corrosion Leakage of Acid-Related Heat Exchanger Tube Bundles
Local Metal Loss for Acid-Related Vessels and Pipelines
Corrosion and Leakage of Flange Sealing Surface
Leaking in Valve
Corrosion Leakage of Instruments, Meters and Their Connections
3. Risk Assessments
3.1. Failure Possibility Calculation
3.2. Failure Consequences Calculation
3.3. The Risk of the Equipment
3.4. Diffusion Model under the Largest Credible Accident
3.4.1. Largest Credible Accident
3.4.2. Leak Rate Calculation
3.4.3. Evaluation Standard
3.4.4. Diffusion Prediction Model
4. Results and Discussions
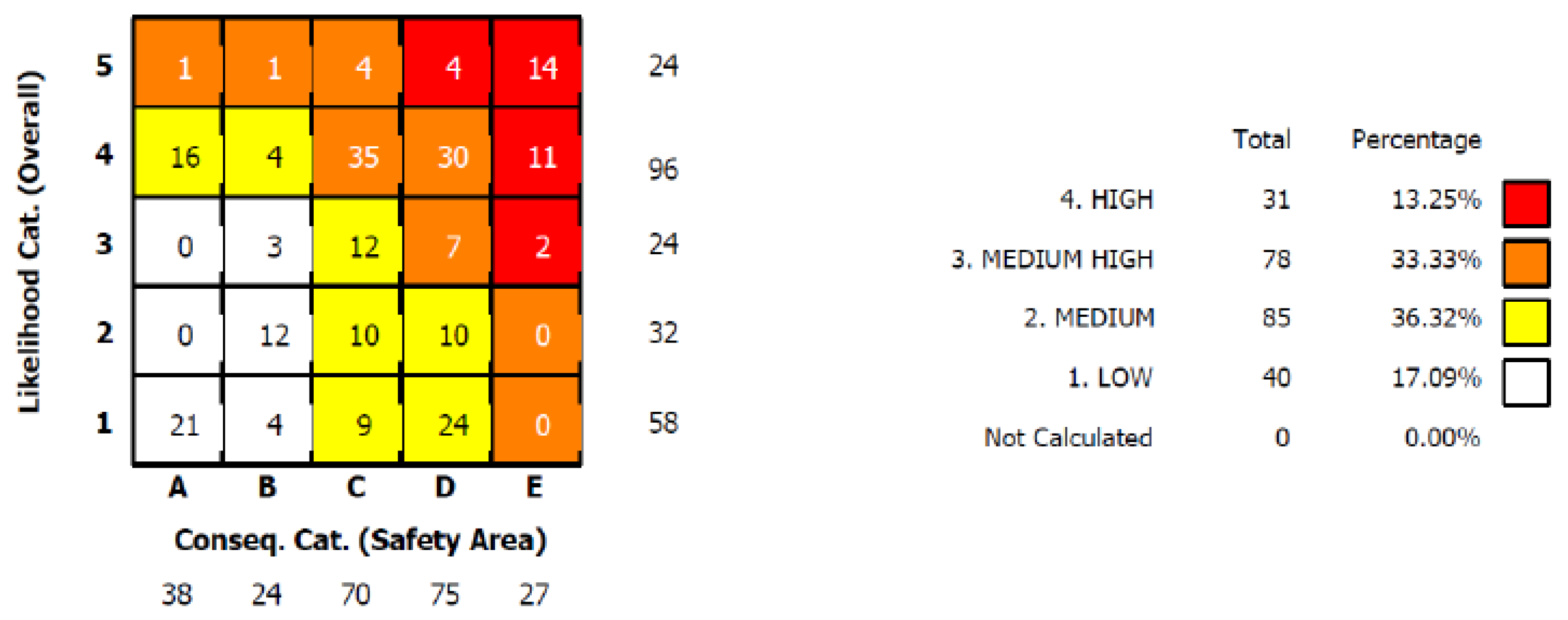
4.1. Risk Results of the Equipment
4.2. Leakage and Diffusion Results under the Largest Credible Accident
4.2.1. Leak Rate Calculation
4.2.2. Prediction Results of HF Leakage and Diffusion
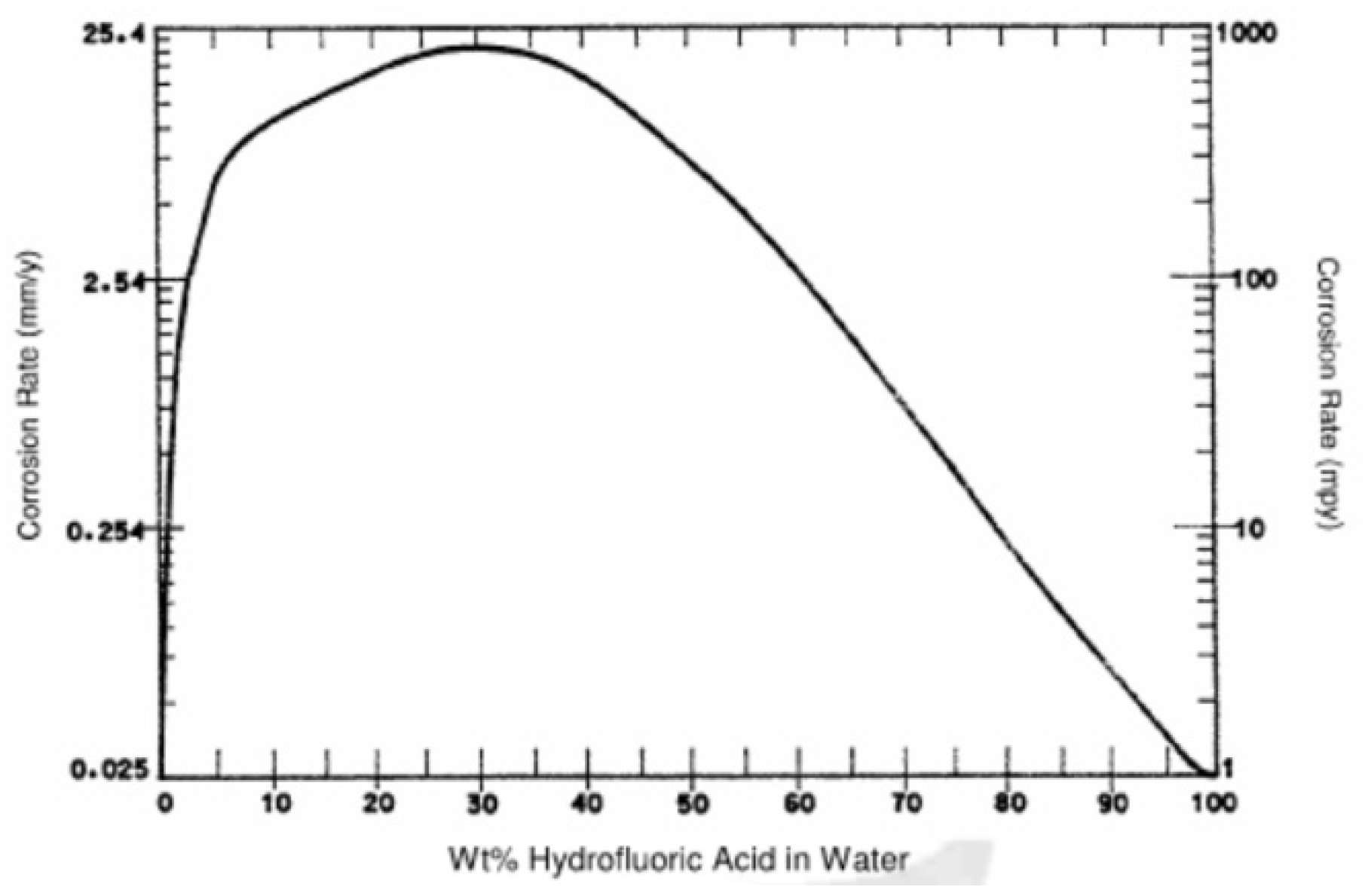
4.3. Material Suitability Evaluation
4.3.1. Current Selection Analysis
4.3.2. Materials Suitability Evaluation of Key Equipment
HF Acid Storage Equipment
Reaction and Regeneration Section
ASO Oil Treatments Section
Fractionation Column Top Section
Propane and Butane Defluorination Section
Material Suitability Evaluation of Other Equipment Materials
4.4. Management Strategy
4.4.1. Process Anti-Corrosion
4.4.2. Equipment Anti-Corrosion
- (1)
- To control the residual elements of carbon steel welds, such as chromium, nickel, copper, it is better to use hydrogen-resistant cracking steel and welding materials.
- (2)
- To prevent hydrogen embrittlement of carbon steel and stress corrosion cracking of Monel alloy, stress relief heat treatment should be performed. A stress relief heat treatment should also be conducted for the elbow of U-shaped tube heat exchanger.
- (3)
- The hardness should be tested for new vessels, new pipelines or repaired welds before being put into use. The high hardness equipment is avoid to put into service.
5. Conclusions
- (1)
- The overall risk of the static equipment in HF acid ALK unit is relatively high. The proportion of total risk to high risk and medium high risk is 46.58%. The HF acid has a high corrosion with a medium HF acid concentration. The flange sealing surfaces are easy to corrode due to HF acid condensation or aggregation.
- (2)
- According to the principle of maximum credible event, when the wind speed is 2 m/s and the atmospheric stability is D level and E level, the allowable exposure concentration range radius can reach 2753 m and 2650 m respectively at 20 min after the accident. The nearest sensitive point, Dongfengli Community of Beijing Yanshan Petrochemical Company, is only about 2500 m away from the accident site. Thus, the residents of Dongfengli community must evacuate within 20 min.
- (3)
- The overall failure risk is high for vessels and pipelines with carbon steel. If a large number of carbon steel materials are upgraded to Monel, the cost is high. A regular replacement method can be used to maintain the reliability of the equipment. The management strategies of process anti-corrosion and equipment anti-corrosion of the ALK unit were proposed. It is meaningful for reducing the number of shutdowns of ALK units and maintaining safe and stable operation of the unit.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Philippa, M.; John, W. Extending the internal examination intervals for pressure vessels using a Risk Based Inspection (RBI) approach. Weld. Cut. 2010, 9, 160–166. [Google Scholar]
- Choi, S.-C. The Implementation of Risk-Based Inspection for the Refinery Plant. Corros. Sci. Technol. 2012, 11, 43–47. [Google Scholar] [CrossRef][Green Version]
- Wu, W.-F.; Lin, S.-R.; You, J.-S. Risk-based inspection and maintenance in process plants and their practices in Taiwan. J. Chin. Inst. Eng. 2016, 39, 392–403. [Google Scholar] [CrossRef]
- Akanni, J.; Alonge, O. Effective implementation of Risk Based Inspection (RBI) approach in asset integrity man-agement of oil and gas facilities. In Proceedings of the ASME 2015 Pressure Vessels and Piping Conference, Paris, France, 19–23 July 2015. [Google Scholar]
- Whittle, D.K.; Lorenzo, D.K.; Kirkman, J.Q. Results of alkylation unit hazard, operability study are analyzed and summarized. Oil Gas J. 1989, 87. Available online: https://www.osti.gov/biblio/5718837 (accessed on 19 August 2021).
- Gary, B.; Teddy, B.; Kristin, B.-B.; Dauber, J.; Frederick, J.; Hemingway, D.; Lefton, J.; Lidston, J.; Lewis, T.; Lippin, T.; et al. A Risk too Great. Hydrofluoric Acid in U.S. Refineries. Available online: http://assets.usw.org/resources/hse/pdf/A-Risk-Too-Great.pdf (accessed on 12 March 2013).
- Muralidhar, R.; Jersey, G.; Krambeck, F.; Sundaresan, S. A two-phase release model for quantifying risk reduction for modified HF alkylation catalysts. J. Hazard. Mater. 1995, 44, 141–183. [Google Scholar] [CrossRef]
- Juliana, P.; Gómez, S.; Ruiz, I. Application of HAZOP, LOPA and SIL to an Alkylation Unit in a Refinery: A Case Study. Chem. Eng. Trans. 2020, 82, 343–348. [Google Scholar]
- Wang, R.; Wanling, Z.; Weihua, W. Risk Assessment (RBI) and Inspection and Verification on Catalytic Cracking Unit. Sci. Technol. Eng. 2011, 11, 7091. [Google Scholar]
- Yan, T.; Yang, P.; Chen, W. Application of RBI technology in delayed coking unit of refinery. Chem. Mach. 2011, 38, 226–227, 235. [Google Scholar]
- Wang, B. Application of RBI in ethylene plant. Chem. Mach. 2012, 39, 512–514, 535. [Google Scholar]
- Shi, K.; Jiang, H.; Zhao, W.; Lu, D. Risk analysis of pipeline system of natural gas installation based on RBI. Petrochem. Safety Environ. Prot. Technol. 2014, 31, 37–39. [Google Scholar]
- Liang, J.; Li, Z.; Li, Y.; Li, H. Application of risk-based inspection (RBI) in pressure-bearing special equipment at LNG receiving station. Pet. Chem. Equip. 2018, 43, 75–79. [Google Scholar]
- Liu, W.; Sun, J.; Ru, W. Risk assessment of pressure vessel for methanol gasification unit based on RBI technology. Nondestruct. Test. 2019, 36, 23–26. [Google Scholar]
- American Institute of Chemical Engineers. Guidelines for Use of Vapor Cloud Dispersion Models; American Institute of Chemical Engineers: New York, NY, USA, 1996. [Google Scholar]
- NACE International. NACE Publication 5A171 (2001 Edition), Materials for Storing and Handling Commercial Grades of Aqueous Hydrofluoric Acid and Anhydrous Hydrogen Fluoride; NANCE: Huston, TX, USA, 2007. [Google Scholar]






| Pollutants | Concentration mg/m3 | Degree of Harm to Human Body |
|---|---|---|
| HF | 400 | lethal concentration |
| 26 | acute poisoning concentration | |
| 2 | allowable exposure concentration |
| Predictive Time/Min Predictive Project | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|
| Maximum landing concentration/mg/m3 | 331,985 | 57,054 | 20,367 | 9807 | 5563 | 3500 |
| Corresponding distance of maximum landing concentration/m | 598 | 1196 | 1795 | 2394 | 2992 | 3591 |
| Radius of allowable exposure concentration range/m | 723 | 1410 | 2085 | 2753 | 3416 | 4075 |
| Radius of acute poisoning concentration /m | 706 | 1377 | 2036 | 2688 | 3333 | 3974 |
| Radius of lethal concentration /m | 687 | 1338 | 1975 | 2603 | 3224 | 3838 |
| Predictive Time/Min Predictive Project | 5 | 10 | 15 | 20 | 25 | 30 |
|---|---|---|---|---|---|---|
| Maximum landing concentration/mg/m3 | 1,539,023 | 273,878 | 99,794 | 48,750 | 27,967 | 17,761 |
| Corresponding distance of maximum landing concentration/m | 599 | 1198 | 1798 | 2397 | 2997 | 3596 |
| Radius of allowable exposure concentration range/m | 683 | 1345 | 2000 | 2650 | 3297 | 3942 |
| Radius of acute poisoning concentration/m | 674 | 1326 | 1972 | 2613 | 3250 | 3885 |
| Radius of lethal concentration/m | 663 | 1304 | 1938 | 2566 | 3191 | 3813 |
| Material | Concentration/% Temperature/°C | 1–5 | 6–15 | 16–25 | 26–35 | 36–45 | 46–55 | 56–65 | 66–75 | 76–85 | 86–95 | 96–100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon steel | 20 | / | / | 9.0 | / | / | 8.0 | 3.8 | / | / | / | 0.03 |
| 40 | 6.2 | 6.0 | 18 | 20 | 16 | 7.1 | 1.6 | 1.1 | 0.48 | 0.21 | / | |
| Monel alloy | 20 | 0.0 | / | 0.10 | / | 0.02 | / | 0.0 | / | / | 0.04 | 0.05 |
| 21 | / | 0.0 | / | 0.10 | / | 0.03 | / | 0.0 | / | 0.04 | 0.05 | |
| 40 | 0.18 | / | / | 0.32 | / | 0.46 | / | / | / | / | / | |
| 52 | 0.25 | 0.27 | 0.38 | / | / | 0.48 | / | 0.71 | / | / | 0.05 | |
| 60 | / | 0.16 | 0.25 | 0.23 | 0.30 | 0.22 | 0.06 | 0.16 | / | / | / | |
| 70 | / | / | / | / | / | / | / | 0.46 | / | / | / | |
| 79 | 0.42 | 0.42 | 0.06 | / | / | 0.01 | / | / | / | / | / | |
| 90 | / | / | / | / | / | / | 0.16 | / | / | / | / | |
| 111 | / | / | / | / | / | 0.28 | / | / | / | / | / | |
| 117 | / | / | 0.25 | / | / | / | / | / | / | / | / | |
| Boiling point | / | / | / | 0.02 | 0.24 | / | / | / | / | / | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lu, D.; Li, X.; Liang, L. Risk Assessment and Material Suitability Evaluation on Static Equipment of Hydrofluoric Acid Alkylation Unit. Processes 2021, 9, 1464. https://doi.org/10.3390/pr9081464
Wang W, Lu D, Li X, Liang L. Risk Assessment and Material Suitability Evaluation on Static Equipment of Hydrofluoric Acid Alkylation Unit. Processes. 2021; 9(8):1464. https://doi.org/10.3390/pr9081464
Chicago/Turabian StyleWang, Weihua, Duhui Lu, Xiang Li, and Lin Liang. 2021. "Risk Assessment and Material Suitability Evaluation on Static Equipment of Hydrofluoric Acid Alkylation Unit" Processes 9, no. 8: 1464. https://doi.org/10.3390/pr9081464
APA StyleWang, W., Lu, D., Li, X., & Liang, L. (2021). Risk Assessment and Material Suitability Evaluation on Static Equipment of Hydrofluoric Acid Alkylation Unit. Processes, 9(8), 1464. https://doi.org/10.3390/pr9081464






