Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System
Abstract
:1. Introduction
2. Materials and Methods
2.1. SESMR Experiments
2.2. Energetic Model of SESMR
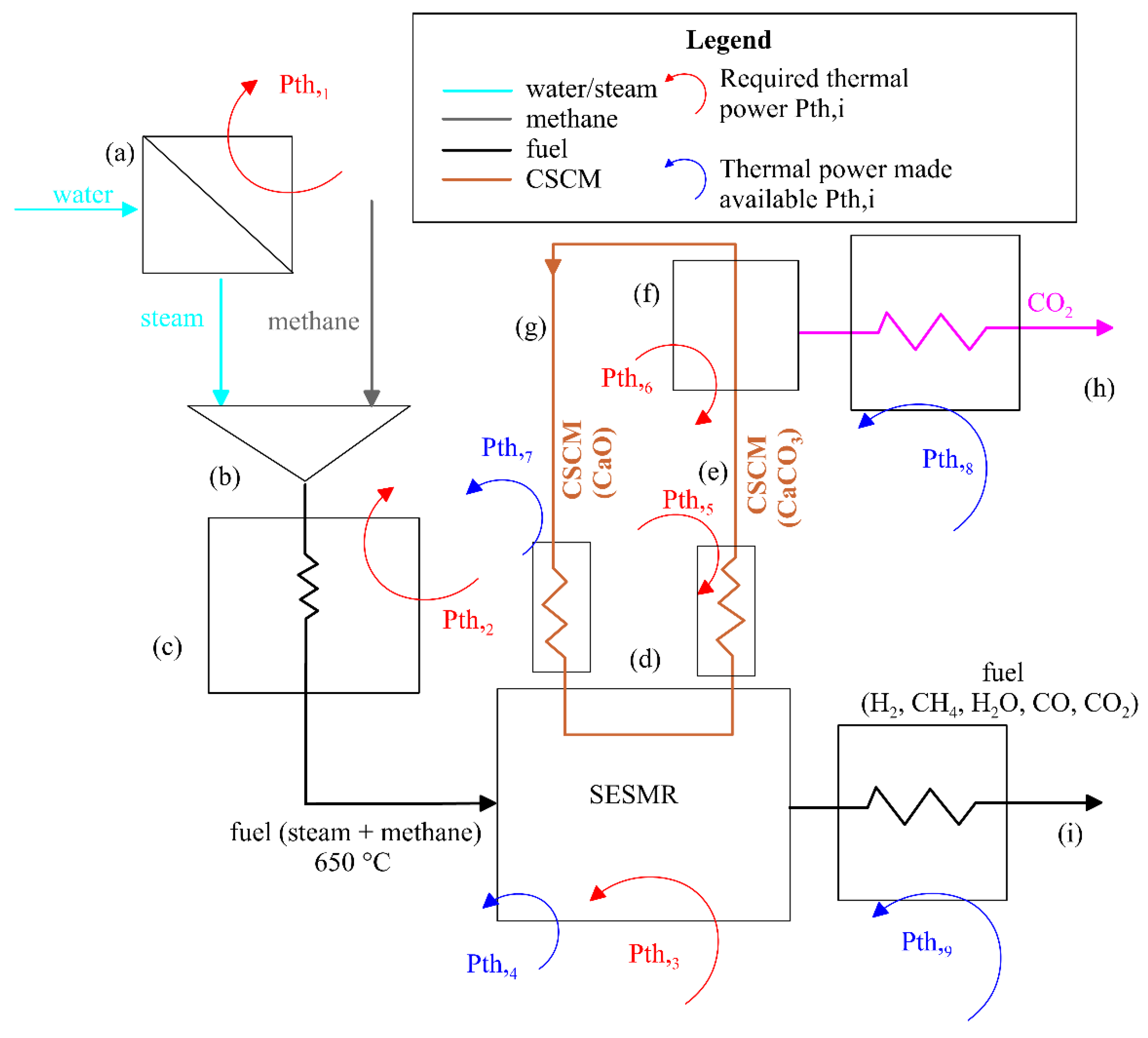
- (a)
- Subcooled water enters the evaporator, where it receives a thermal power Pth,1. Due to this thermal exchange, the water leaves the evaporator as superheated vapor (steam) at 300 °C.
- (b)
- The superheated steam is mixed with methane at ambient temperature.
- (c)
- The mixture of methane and superheated steam enters a heat exchanger, receiving the thermal power Pth,2 and reaching the state required to be fed into the SESMR reactor.
- (d)
- The feed (methane and steam) enters the reactor at the required condition (T(d) = 650 °C). From the energy point of view, two situations occur:
- (1)
- The SMR endothermic Reaction (1) requires a thermal power Pth,3 to be provided to the system. This power also takes into account the slightly exothermic contribution of WGS (2);
- (2)
- The CBN exothermic Reaction (3) makes the thermal power Pth,4 available.
- (e)
- The sorbent regeneration at 950 °C requires that a significant amount of thermal power Pth,5 is provided to heat up the CSCM at the temperature in correspondence to that with which the calcination takes place.
- (f)
- Pth,6 is the power required by the calcination (reverse of Reaction (3)), which is a highly endothermic reaction.
- (g)
- Subsequently, the regenerated CSCM is cooled down to 650 °C (SESMR condition), making the thermal power Pth,7 available. It is worth stressing that the adopted scheme may represent either a dual fluidized bed reactor with solid recirculation or a packed bed configuration. From an energy perspective, CSCM circulation can explain the thermal power required by the calcination (3), and the cooling of the regenerated CSCM makes Pth,6 available. Therefore, the difference is that in dual inter-circulating fluidized bed conditions, this representation coincides with the physics of the process, whereas, in the case of packed bed reactors, the flow charts reproduce only the thermal power exchange, as the solid is not actually circulated.
- (h)
- The absorbed CO2 is released once the CSCM regeneration is complete. To store CO2, it should be refrigerated and compressed. For the sake of simplicity, in this phase, only the refrigeration stage is considered to preliminarily assess the thermal power made available, Pth,8.
- (i)
- Depending on the application, the fuel (H2, CH4, H2O, CO, or CO2) can be subjected to a certain degree of cooling, making the thermal power Pth,9 available.
2.3. Power Contribution Assessment through the Energetic Model of SESMR
- The mass conservation was applied, as given by Equation (5), considering the species (steam and methane) involved in the mixture:where νj are stoichiometric coefficients (positive if j is a product, negative if j is a reactant) and Mj is the molecular mass of species j.
- The thermal power needed by the methane–steam mixture to reach the state required to enters the SESMR reactor is given by Equation (6), as follows:where Fj is the molar flow rate of the j substance of the mixture and cp,j(T) heat at a constant pressure of species j (cp,j) as a function of absolute temperature (T).
- The thermal power Pth,3 is associated with the overall reaction of SMR (1) and WGS (2). Thus, it is given by Equation (7):where is the heat of formation of chemical species involved in both SMR and WGS reactions. Pth,3 represents the net difference between the power needed by SMR (highly endothermic) and that provided by WGS (slightly exothermic). The energy balance return that the energy requires by the reforming is higher than that made available by the WGS; thus, Pth,3 (8) expresses a power contribution required by the system. The fuel at the inlet is constituted by a mixture of steam and methane, while that at the outlet of the reactor is a reformed gas composed of H2, CO, CO2, CH4, and H2O.
- Pth,4 (8) represents the thermal power provided by the carbonation which is an exothermic reaction. This energy contribution can be evaluated as follows:where is the absorbed CO2 molar flow rate and is the heat of reaction associated with the carbonation process.
- As above reported, the sorbent needs the thermal power Pth,5 to reach the condition required by the sorbent regeneration phase, as given by Equation (9).where is the CaCO3 molar fraction in combined sorption–catalyst material (CSCM) and is the specific heat at a constant pressure of the CSCM.
- The thermal power required by the calcination Pth,6 is given by Equation (10):where is the heat of formation related to the calcination stage.
- At the end of the calcination stage, the CSCM reaches a temperature equal to 950 °C (Tg,in) in case of severe calcination. Then, CSCM should be cooled down to 650 °C (Tg,out) in such a way that the reforming stage takes place again. Thus, during Step (f), the CSCM cooling makes the thermal power Pth,7 available, as given by Equation (11):where is the CaO molar fraction of CSCM.
- 8.
- Once the CO2 has been released by the sorbent (g) it must be cooled from Th,in (950 °C) to Th,out and compressed to complete the storage stage. The analysis performed here was pushed until the step that precedes the intercooled compression as the main scope was to define the main source of thermal power needed and made available by the system. In particular, the CO2 cooling provides Pth,8 to the surrounding environment, as in Equation (12):
- 9.
- In addition, once the fuel has left the reactor, it should be cooled from Ti,in (650 °C) to Ti,out (ambient temperature) to make its condition suitable for storing the hydrogen. The power Pth,9, made available by this cooling, is given by Equation (13):
3. Results
- Carbonation;
- Thermal power made available by CSCM cooling;
- Thermal power recovered through CO2 cooling;
- Thermal power recovered through reformed fuel cooling.
- The carbonation makes Pth,4 available, which reduces the thermal power needed by the reforming process (Pth,3).
- The thermal power Pth,7, provided by CSCM cooling, can be used to cover part of the remaining Pth,3 and the final stage of the fuel heating up prior to entering the reactor (Pth,2). This means that the thermal power needed to heat up the sorbent for its regeneration can be partially recovered.
- The cooling of reformed fuel exiting the reactor (Pth,9) provides thermal power needed by the first part of the fuel heating up (Pth,2) and by the steam superheating.
- The CO2 cooling (Pth,8) partially covers the water vaporization stage. A further contribution can be provided by Pth,9.
4. Conclusions
- Although the methane conversions were quite close, SESMR presented a higher hydrogen molar fraction on a dry (90 mol%) and wet basis (70 mol%), in comparison to SMR+WGS without CO2 adsorption, in agreement with the experimental results.
- SESMR allowed for achieving a higher value of the first performance index ξH2,A, defined as the ratio between hydrogen and methane chemical energy. The results show that SESMR presented an average ξH2,A equal to 115%, while that of SMR fluctuated between 105% and 95%;
- Considering the power required by the system (ηH2,B), it was observed that even though the advantages offered by SESMR were still present, they were limited to a few percentage points. This was mainly due to the higher thermal power required by the SESMR loop for sorbent regeneration.
- The final step of the study demonstrated that in SESMR, a high fraction of the required thermal power could be recovered from energy contributions provided by the carbonation process, sorbent, CO2, and fuel cooling. This allowed for reducing the power demand of the system and improving the plant energy performance. Indeed, considering the contributions of recovered thermal power in the energy performance (ηH2,C), an average value of 79% was observed for SESMR, while for SMR+WGS a maximum value of 72% was observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| CBN | Carbonation |
| CSCM | Combined catalyst sorption material |
| SESMR | Sorption-enhanced steam–methane reforming |
| SMR | Steam–methane reforming |
| WGS | Water–gas Shift |
| Symbols | |
| Specific heat at constant pressure of species j [kJ/(kmolK)]/[kJ/kgK] | |
| F | Molar flow rate of species j [kmol/s] |
| h | Specific enthalpy [kJ/kg] |
| M | Molecular masses [kg/kmol] |
| ṁ | Mass flow rate [kg/s] |
| Pth | Thermal power [W] |
| T | Temperature [K] |
| Subscripts | |
| (a) | Vaporization phase |
| (b) | Steam/methane mixing phase |
| (c) | Fuel heating phase |
| (d) | Sorption-enhanced reforming phase |
| (e) | Sorbent heat up for regeneration |
| (f) | Calcination phase |
| (g) | Sorbent cooling phase |
| (h) | CO2 cooling phase |
| (i) | Fuel cooling phase |
| j | Generic species |
| in | Inlet |
| out | Outlet |
| Greek symbols | |
| Standard heat of formation of i-substance [kJ/kmol] | |
| ηH2,B | H2 production efficiency (without energy recovery) |
| ηH2,C | H2 production efficiency (with energy recovery) |
| ν | Stochiometric coefficient [kmol] |
| ξH2,A | H2 vs. CH4 chemical energy |
Appendix A
| j | Phase | aj (cal mol−1 K−1) | bj (cal mol−1 K−2) | cj (cal mol−1 K−3) | dj (cal mol−1 K3) | Validity (K) | Ref. |
|---|---|---|---|---|---|---|---|
| CaCO3 | (c) | 19.68 | 0.01189 | 0 | −307,600 | 273–1033 | [59] |
| CaO | (c) | 10.00 | 0.00484 | 0 | −108,000 | 273–1173 | [59] |
| CO2 | (g) | 10.34 | 0.00274 | 0 | −195,500 | 273–1200 | [59] |
| CH4 | (g) | 5.34 | 0.01150 | 0 | −130,300 | 273–1373 | [59] |
| CO | (g) | 6.60 | 0.00120 | 0 | 0 | 273–2500 | [59] |
| H2 | (g) | 6.62 | 0.00081 | 0 | 0 | 273–2500 | [59] |
| H2O | (g) | 8.22 | 0.00015 | 0.00000134 | 0 | 300–2500 | [59] |
| Ni | (cα) | 4.26 | 0.0064 | 0 | 0 | 273–626 | [59] |
| Ni | (cβ) | 6.99 | 0.000905 | 0 | 0 | 626–1725 | [59] |
| NiO | (c) | 11.3 | 0.00215 | 0 | 0 | 273–1273 | [59] |
| O2 | (g) | 8.27 | 0.000258 | 0 | −187,700 | 300–5000 | [59] |
| Ca12Al14O33 | (cα) | 301.96 | 0.0655 | 0 | −5,530,000 | 298–1300 | [60] |
| Ca12Al14O33 | (cβ) | 228.52 | 0.09844 | 0 | 0 | 1300–1700 | [60] |
References
- UNFCCC. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 17 March 2021).
- Normile, D. Can China, the world’s biggest coal consumer, become carbon neutral by 2060? Science 2020. [Google Scholar] [CrossRef]
- Claeys, G.; Tagliapietra, S.; Zachmann, G. How to Make the European Green Deal Work. Bruegel Policy Contribution; Bruegel: Brussels, Belgium, 2019; p. 21. [Google Scholar]
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 17 March 2021).
- European Commission. Recovery Plan for European. Available online: https://ec.europa.eu/info/strategy/recovery-plan-europe_en (accessed on 17 March 2021).
- USA Formally Rejoins the Paris Climate Accord. The New York Times. Available online: https://www.nytimes.com/2021/02/19/world/us-rejoins-paris-climate-accord.html (accessed on 17 March 2021).
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.C.; Litynski, J.T.; Brickett, L.A.; Morreale, B.D. Toward transformational carbon capture systems. AIChE J. 2016, 62, 2–10. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Meyer, L.; Van Leo, Y.; Brinkman, J.-P.; Van Kesteren, S.; Leprince-Ringuet, L.; Van Noëmie Boxmeer, F. Climate Change 2013-The Physical Science Basis; IPCC: Geneva, Switzerland, 2014; ISBN 9781107415324. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. ISBN 978-92-9169-143-2. [Google Scholar]
- Di Giuliano, A.; Gallucci, K. Sorption enhanced steam methane reforming based on nickel and calcium looping: A review. Chem. Eng. Process.-Process Intensif. 2018, 130, 240–252. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Wallace, J.S.; Ward, C.A. Hydrogen as a fuel. Int. J. Hydrogen Energy 1983, 8, 255–268. [Google Scholar] [CrossRef]
- Vezirolu, T.N.; Barbir, F. Hydrogen: The wonder fuel. Int. J. Hydrogen Energy 1992, 17, 391–404. [Google Scholar] [CrossRef]
- Da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Alazemi, J.; Andrews, J. Automotive hydrogen fuelling stations: An international review. Renew. Sustain. Energy Rev. 2015, 48, 483–499. [Google Scholar] [CrossRef]
- Toyota Mirai. Toyota Motor Company Hydrogen Fuel Cell Car. Available online: https://ssl.toyota.com/mirai/fcv.html (accessed on 18 September 2017).
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic Steam Reforming. Catal. Sci. Technol. 1984, 5, 1–117. [Google Scholar]
- Rostrup-Nielsen, T. Manufacture of hydrogen. In Proceedings of the Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2005; Volume 106, pp. 293–296. [Google Scholar]
- Harrison, D.P. Sorption-Enhanced Hydrogen Production: A Review. Ind. Eng. Chem. Res. 2008, 47, 6486–6501. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Carvill, B.T.; Hufton, J.R.; Anand, M.; Sircar, S. Sorption-enhanced reaction process. AIChE J. 1996, 42, 2765–2772. [Google Scholar] [CrossRef]
- Meyer, J.; Mastin, J.; Bjørnebøle, T.-K.; Ryberg, T.; Eldrup, N. Techno-economical study of the Zero Emission Gas power concept. Energy Procedia 2011, 4, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Martavaltzi, C.S.; Pampaka, E.P.; Korkakaki, E.S.; Lemonidou, A.A. Hydrogen Production via Steam Reforming of Methane with Simultaneous CO2. Chem. Eng. J. 2010, 33, 2589–2595. [Google Scholar] [CrossRef]
- Shokrollahi Yancheshmeh, M.; Radfarnia, H.R.; Iliuta, M.C. High temperature CO2 sorbents and their application for hydrogen production by sorption enhanced steam reforming process. Chem. Eng. J. 2016, 283, 420–444. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Pefkos, T.D.; Lemonidou, A.A. Operational Window of Sorption Enhanced Steam Reforming of Methane over CaO−Ca12Al14O33. Ind. Eng. Chem. Res. 2011, 50, 539–545. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Lemonidou, A.A. Hydrogen production via sorption enhanced reforming of methane: Development of a novel hybrid material-reforming catalyst and CO2 sorbent. Chem. Eng. Sci. 2010, 65, 4134–4140. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review-calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Diglio, G.; Hanak, D.P.; Bareschino, P.; Pepe, F.; Montagnaro, F.; Manovic, V. Modelling of sorption-enhanced steam methane reforming in a fixed bed reactor network integrated with fuel cell. Appl. Energy 2018, 210, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Diglio, G.; Hanak, D.P.; Bareschino, P.; Mancusi, E.; Pepe, F.; Montagnaro, F.; Manovic, V. Techno-economic analysis of sorption-enhanced steam methane reforming in a fixed bed reactor network integrated with fuel cell. J. Power Sources 2017, 364, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.; Mastin, J.; Sanz, C. Sustainable Hydrogen Production from Biogas Using Sorption- Enhanced Reforming. Energy Procedia 2014, 63, 6800–6814. [Google Scholar] [CrossRef] [Green Version]
- Broda, M.; Manovic, V.; Imtiaz, Q.; Kierzkowska, A.M.; Anthony, E.J.; Müller, C.R. High-Purity Hydrogen via the Sorption-Enhanced Steam Methane Reforming Reaction over a Synthetic CaO-Based Sorbent and a Ni Catalyst. Environ. Sci. Technol. 2013, 47, 6007–6014. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Gallucci, K.; Kazi, S.S.; Giancaterino, F.; Di Carlo, A.; Courson, C.; Meyer, J.; Di Felice, L. Development of Ni- and CaO-based mono- and bi-functional catalyst and sorbent materials for Sorption Enhanced Steam Methane Reforming: Performance over 200 cycles and attrition tests. Fuel Process. Technol. 2019, 195, 106160. [Google Scholar] [CrossRef]
- García-Lario, A.L.; Aznar, M.; Martinez, I.; Grasa, G.S.; Murillo, R. Experimental study of the application of a NiO/NiAl2O catalyst and a CaO-based synthetic sorbent on the Sorption Enhanced Reforming process. Int. J. Hydrogen Energy 2015, 40, 219–232. [Google Scholar] [CrossRef]
- Satrio, J.A.; Shanks, B.H.; Wheelock, T.D. Development of a novel combined catalyst and sorbent for hydrocarbon reforming. Ind. Eng. Chem. Res. 2005, 44, 3901–3911. [Google Scholar] [CrossRef] [Green Version]
- Satrio, J.A.; Shanks, B.H.; Wheelock, T.D. A combined catalyst and sorbent for enhancing hydrogen production from coal or biomass. Energy Fuels 2007, 21, 322–326. [Google Scholar] [CrossRef] [Green Version]
- Cesário, M.R.; Barros, B.S.; Zimmermann, Y.; Courson, C.; Melo, D.M.A.; Kiennemann, A. CO2 Sorption Enhanced Steam Reforming of Methane Using Ni/CaO · Ca12Al14O33 Catalysts. Adv. Chem. Lett. 2013, 1, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zhou, Z.; Zhao, C.; Cheng, Z. Ni/CaO-Al2O3 bifunctional catalysts for sorption-enhanced steam methane reforming. AIChE J. 2014, 60, 3547–3556. [Google Scholar] [CrossRef]
- Radfarnia, H.R.; Iliuta, M.C. Development of Al-stabilized CaO–nickel hybrid sorbent–catalyst for sorption-enhanced steam methane reforming. Chem. Eng. Sci. 2014, 109, 212–219. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Z.; Zhao, C.; Cheng, Z. Catalytic performance of Ni/CaO-Ca5Al6O14 bifunctional catalyst extrudate in sorption-enhanced steam methane reforming. Catal. Today 2016, 259, 347–353. [Google Scholar] [CrossRef]
- Rout, K.R.; Jakobsen, H.A. A numerical study of pellets having both catalytic- and capture properties for SE-SMR process: Kinetic- and product layer diffusion controlled regimes. Fuel Process. Technol. 2013, 106, 231–246. [Google Scholar] [CrossRef]
- Kim, J.-N.; Ko, C.H.; Yi, K.B. Sorption enhanced hydrogen production using one-body CaO–Ca12Al14O33–Ni composite as catalytic absorbent. Int. J. Hydrogen Energy 2013, 38, 6072–6078. [Google Scholar] [CrossRef]
- Chanburanasiri, N.; Ribeiro, A.M.; Rodrigues, A.E.; Arpornwichanop, A.; Laosiripojana, N.; Praserthdam, P.; Assabumrungrat, S. Hydrogen Production via Sorption Enhanced Steam Methane Reforming Process Using Ni/CaO Multifunctional Catalyst. Ind. Eng. Chem. Res. 2011, 50, 13662–13671. [Google Scholar] [CrossRef]
- Aloisi, I.; Jand, N.; Stendardo, S.; Foscolo, P.U. Hydrogen by sorption enhanced methane reforming: A grain model to study the behavior of bi-functional sorbent-catalyst particles. Chem. Eng. Sci. 2016, 149, 22–34. [Google Scholar] [CrossRef]
- Aloisi, I.; Di Giuliano, A.; Di Carlo, A.; Foscolo, P.U.; Courson, C.; Gallucci, K. Sorption enhanced catalytic Steam Methane Reforming: Experimental data and simulations describing the behaviour of bi-functional particles. Chem. Eng. J. 2016, 314, 570–582. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Aloisi, I.; Jand, N.; Foscolo, P.U.; Courson, C.; Gallucci, K. Sorption enhanced steam methane reforming: Experimental data and simulations describing the behaviour of bi-functional particles. In Proceedings of the WHEC 2016-21st World Hydrogen Energy Conference 2016, Zaragoza, Spain, 13–16 June 2016; Spanish Hydrogen Association (AeH2): Zaragoza, Spain, 2016; Volume 1, pp. 111–113. [Google Scholar]
- Martínez, I.; Romano, M.C.; Chiesa, P.; Grasa, G.; Murillo, R. Hydrogen production through sorption enhanced steam reforming of natural gas: Thermodynamic plant assessment. Int. J. Hydrogen Energy 2013, 38, 15180–15199. [Google Scholar] [CrossRef]
- Ochoa-Fernandez, E.; Haugen, G.; Zhao, T.; Ronning, M.; Aartun, I.; Borresen, B.; Rytter, E.; Rønnekleiv, M.; Chen, D. Process design simulation of H2 production by sorption enhanced steam methane reforming: Evaluation of potential CO2 acceptors. Green Chem. 2007, 9, 654–662. [Google Scholar] [CrossRef]
- Romano, M.C.; Cassotti, E.N.; Chiesa, P.; Meyer, J.; Mastin, J. Application of the sorption enhanced-steam reforming process in combined cycle-based power plants. Energy Procedia 2011, 4, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Carapellucci, R.; Giordano, L. dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Lopez Ortiz, A.; Harrison, D.P. Hydrogen production using sorption-enhanced reaction. Ind. Eng. Chem. Res. 2001, 40, 5102–5109. [Google Scholar] [CrossRef]
- Brun-Tsekhovoi, A.R.; Zadorin, A.N.; Katsobashvili, Y.R.; Kourdyumov, S.S. The process of catalytic steam reforming of hydrocarbons in the presence of carbon dioxide acceptor. In Hydrocarbon Energy Progress VII, Proceedings of the 7th World Hydrogen Energy Conference, Moscow, Russia, 25–29 September 1988; Pergamon Press: Oxford, UK; p. 885.
- Han, C.; Harrison, D.P. Simultaneous shift reaction and carbon dioxide separation for the direct production of hydrogen. Chem. Eng. Sci. 1994, 49, 5875–5883. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Lopez Ortiz, A.; Kaytakoglu, S.; Harrison, D.P. Hydrogen from methane in a single-step process. Chem. Eng. Sci. 1999, 54, 3543–3552. [Google Scholar] [CrossRef]
- Antzara, A.; Heracleous, E.; Lemonidou, A.A. Energy efficient sorption enhanced-chemical looping methane reforming process for high-purity H2 production: Experimental proof-of-concept. Appl. Energy 2016, 180, 457–471. [Google Scholar] [CrossRef]
- Wang, S.; Xu, S.; Liu, S.; Hu, B. Prediction of sorption-enhanced reforming process on hydrotalcite sorbent in a fluidized bed reactor. Energy Convers. Manag. 2019, 180, 924–930. [Google Scholar] [CrossRef]
- Vicente, J.; Montero, C.; Ereña, J.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Coke deactivation of Ni and Co catalysts in ethanol steam reforming at mild temperatures in a fluidized bed reactor. Int. J. Hydrogen Energy 2014, 39, 12586–12596. [Google Scholar] [CrossRef]
- Fernández, J.R.; Martínez, I.; Abanades, J.C.; Romano, M.C. Conceptual design of a Ca–Cu chemical looping process for hydrogen production in integrated steelworks. Int. J. Hydrogen Energy 2017, 42, 11023–11037. [Google Scholar] [CrossRef]
- Noorman, S.; van Sint Annaland, M.; Kuipers, H. Packed Bed Reactor Technology for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2007, 46, 4212–4220. [Google Scholar] [CrossRef] [Green Version]
- Di Giuliano, A.; Giancaterino, F.; Courson, C.; Foscolo, P.U.; Gallucci, K. Development of a Ni-CaO-mayenite combined sorbent-catalyst material for multicycle sorption enhanced steam methane reforming. Fuel 2018, 234, 687–699. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Pellegrino, E. Numerical integration strategies of PFR dynamic models with axial dispersion and variable superficial velocity: The case of CO2 capture by a solid sorbent. Heliyon 2019, 5, e02040. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Girr, J.; Massacesi, R.; Gallucci, K.; Courson, C. Sorption enhanced steam methane reforming by Ni–CaO materials supported on mayenite. Int. J. Hydrogen Energy 2017, 42, 13661–13680. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Giancaterino, F.; Gallucci, K.; Foscolo, P.U.; Courson, C. Catalytic and sorbent materials based on mayenite for sorption enhanced steam methane reforming with different packed-bed configurations. Int. J. Hydrogen Energy 2018, 43, 21279–21289. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Gallucci, K.; Giancaterino, F.; Courson, C.; Foscolo, P.U. Multicycle sorption enhanced steam methane reforming with different sorbent regeneration conditions: Experimental and modelling study. Chem. Eng. J. 2019, 377, 119874. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Gallucci, K.; Foscolo, P.U.; Courson, C. Effect of Ni precursor salts on Ni-mayenite catalysts for steam methane reforming and on Ni-CaO-mayenite materials for sorption enhanced steam methane reforming. Int. J. Hydrogen Energy 2019, 44, 6461–6480. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Gallucci, K.; Di Carlo, A.; Stendardo, S.; Courson, C.; Foscolo, P.U. Sorption enhanced steam methane reforming by mayenite combined systems: Overview of experimental results from uropean research project. Can. J. Chem. Eng. 2020, 98, 1907–1923. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Courson, C.; Gallucci, K.; Kiennemann, A. Ni-CaO combined sorbent-catalyst materials usage for sorption enhanced steam methane reforming. In Proceedings of the WHEC 2016-21st World Hydrogen Energy Conference 2016, Zaragoza, Spain, 13–16 June 2016; Spanish Hydrogen Association (AeH2): Zaragoza, Spain, 2016; pp. 68–70. [Google Scholar]
- Di Giuliano, A.; Gallucci, K.; Foscolo, P.U. Determination of Kinetic and Diffusion Parameters Needed to Predict the Behavior of CaO-Based CO2 Sorbent and Sorbent-Catalyst Materials. Ind. Eng. Chem. Res. 2020, 59, 6840–6854. [Google Scholar] [CrossRef]
- Carapellucci, R. A unified approach to assess performance of different techniques for recovering exhaust heat from gas turbines. Energy Convers. Manag. 2009, 50, 1218–1226. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatigati, F.; Di Giuliano, A.; Carapellucci, R.; Gallucci, K.; Cipollone, R. Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System. Processes 2021, 9, 1440. https://doi.org/10.3390/pr9081440
Fatigati F, Di Giuliano A, Carapellucci R, Gallucci K, Cipollone R. Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System. Processes. 2021; 9(8):1440. https://doi.org/10.3390/pr9081440
Chicago/Turabian StyleFatigati, Fabio, Andrea Di Giuliano, Roberto Carapellucci, Katia Gallucci, and Roberto Cipollone. 2021. "Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System" Processes 9, no. 8: 1440. https://doi.org/10.3390/pr9081440
APA StyleFatigati, F., Di Giuliano, A., Carapellucci, R., Gallucci, K., & Cipollone, R. (2021). Experimental Characterization and Energy Performance Assessment of a Sorption-Enhanced Steam–Methane Reforming System. Processes, 9(8), 1440. https://doi.org/10.3390/pr9081440









