1. Introduction
Continuous catalytic reforming is one of the main processing techniques in the petrochemical industry. In the process of continuous catalytic reforming reaction, there are many components involved and the reaction process between each reactant is very complex. It is almost impossible to establish the kinetic equation of each component by conventional method, so lumped method must be used to model [
1]. In 1959, after Smith proposed a four-component lumped reaction kinetic model [
2], domestic and foreign universities, research institutions, major petrochemical companies, etc.—based on the actual situation and application conditions of the reforming units—researched and developed many lumped reaction kinetic models, including the 20-component lumped reaction kinetic model proposed by Krane [
3]. Compared with Smith’s four-component lumped model, this model is more detailed and closer to the actual reforming process. The 22-component lumped reaction kinetics model proposed by Kmak [
4] was compared to Krane’s 20 lumped model; this model subdivides the hydrocarbon group composition. Mobil has made great contributions to the study of lumped reaction kinetic models; Remage et al. published 13-component lumped reaction kinetic models in 1980 and 1987 [
5]. The lumped division method of the model is considered comprehensive, simple, practical, and effective. The research on the lumped reaction kinetic model evolved on this basis.
Typical lumped reaction kinetics models include Pace’s 30-component lumped reaction kinetics model [
6], Wolff’s 5-component lumped reaction kinetics model [
7], Froment’s 28-component lumped reaction kinetics model [
8], and Taskar’s 35-component lumped reaction kinetics model [
9]. Compared with foreign countries, researches on lumped reaction kinetic models in China are relatively late, mainly including the following: Based on Ramage’s 13-component lumped reaction kinetics model, Weng et al. lumped n-alkanes and isoparaffins separately, and proposed a 16-component lumped reaction kinetic model. Later, they assumed that the kinetic parameters did not change with the composition of the raw materials; 27-component lumped reaction kinetic models were thus proposed [
10,
11]. Xie et al. not only distinguished between n-alkanes and isoparaffins, but also subdivided
cycloalkanes and aromatics based on Weng et al., and proposed a 28-component lumped reaction kinetic model [
12]. Ding et al. separately lumped
based on Ramage’s 13-component lumped reaction kinetics model, and proposed a 17-component lumped reaction kinetic model [
13]. Hu considered the coking and deactivation of the catalyst based on predecessors and proposed a 17-component lumped reaction kinetic model [
14]. Hou considered all hydrocracking reactions based on Hu’s 17-component lumped reaction kinetic model and proposed a 20-component lumped reaction kinetic model [
15].
Although the research on lumped reaction kinetics has made some progress in China, there are many mature-mechanism modeling software in foreign countries [
16], such as Aspen Plus, Pro
, UniSim. It is worth mentioning that a modeling software from a country is a mechanism model established for the actual situation of the country, which leads to the problem that some of the existing models may not be applicable to the actual situation in China and cannot be modified. This is because the reforming feed composition of foreign chemical plants is relatively fixed and basically operates according to design, based on the obtained empirical parameters, factor coefficients, etc. can be used in foreign mechanism modeling software. However, for the composition of reforming feedstock changes greatly, these foreign software cannot be consistent with the actual process, which needs to be designed by ourselves. This is the case in China, where the composition of the reforming feed varies quite a lot. The reforming feed is generally obtained by reconciling the petroleum of different countries, and the proportion will also change according to the fluctuation of the price, resulting in the composition of reformation feed varying greatly. China is also quite different from foreign countries in terms of operating processes and procedures, leading to large differences in parameters, empirical coefficients, and device coefficients of related reactions. For example, the catalyst has a great influence on the catalytic reforming reaction, while the loading and regeneration cycle of catalysts in China are very different from those in foreign countries. Therefore, the use of these foreign modeling software will have problems of inapplicability.
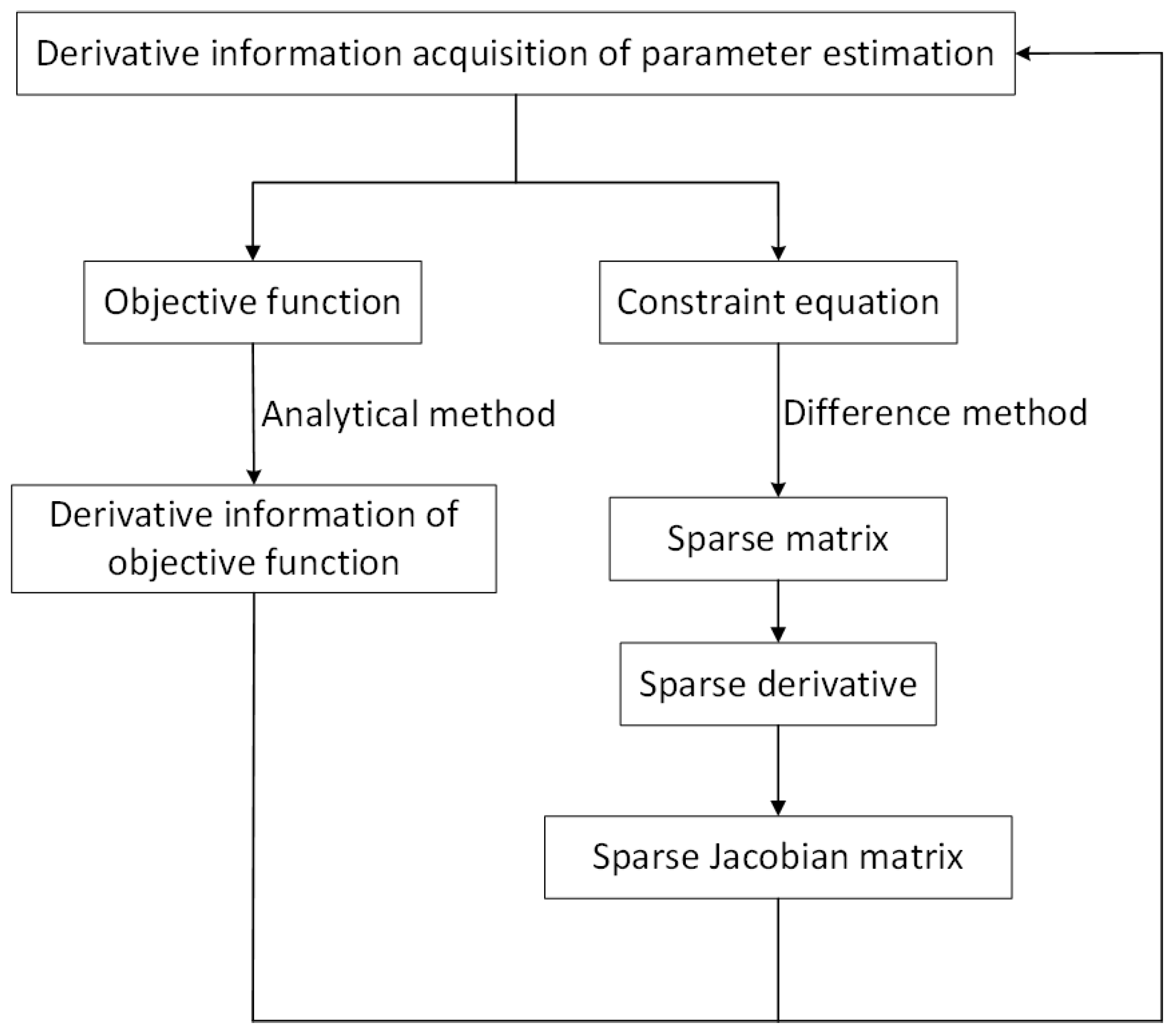
In addition, dynamic simulation includes two methods, namely, sequential method and simultaneous equations method. The advantage of the sequential method is that when it has a reliable differential–algebraic mixed equation solver, it is simpler to implement and apply, and the scale of the nonlinear programming problem obtained is smaller. The disadvantage is that there are many iteration nesting levels, which require a lot of iterative calculations, especially for large-scale problems, which consume too much time and cannot handle unstable systems. The advantages of the simultaneous equations method are that it can avoid layers of iteration and the solution of large-scale problems can speed up the solution and can deal with unstable systems; the disadvantages are that appropriate initial values and enough memory space are needed. However, in the research work, it can be found that the initial value problem is not as serious as expected, it can converge well for any given initial value in a large range. Aiming at the memory problem, with the continuous development of optimization algorithms, for example, the BFGS method with limited memory storage can solve it [
17,
18], which is the reason why this paper adopts the simultaneous equations method. In this paper, the simultaneous method based on finite-element collocation is used to discretize the model, and the nonlinear solver is used to simulate the system dynamically. Some output results of the dynamic simulation of the discrete model and the continuous model, such as the outlet temperature of the reactor and the molar mass of the product, are compared to prove the accuracy of the discrete model. In addition, the sensitivity analysis of the yield of each product is carried out by modifying the temperature and the ratio of hydrogen to oil—these products include
liquid yield, aromatic yield, benzene yield, toluene yield, xylene yield, trimethylbenzene yield, hydrogen yield, octane number, etc.
In order to make the continuous catalytic reforming process run better, this paper not only established a general mechanism model in line with the actual situation of our country, but also combined the more efficient and rapid dynamic simulation method with it, which would improve the yield and obtain better economic benefits.
2. Establishment of Mechanism Model for Continuous Catalytic Reforming Process
2.1. Lumped Division and Establishment of Reaction Network
The reforming feed in the continuous catalytic reforming process is determined by gas chromatography and, from the analysis of the results, it can be seen that there are many components involved in reforming reaction and the reaction process among the reactions is very complex; it is almost impossible to establish the dynamic equation of each component by the conventional method. Therefore, the lumped approach is the best choice to establish the mechanism modeling of the continuous catalytic reforming process [
1].
In this paper, according to the actual situation and application conditions of China’s continuous catalytic reforming process, the components are divided as follows:
Paraffins: Methane (), Ethane (), Propane (), N-butane orthoisomerism (), N-pentane orthoisomerism (), N-hexane orthoisomerism (), N-heptane orthoisomerism (), N-octane orthoisomerism (), N-nonane orthoisomerism (), N-decane orthoisomerism (), Undecane hydrocarbon (), Carbon dodecane ().
Cycloalkanes: Cyclopentane (), Methyl cyclopentane (), six-carbon naphthenes (), Seven carbon naphthenes (), eight-carbon naphthenes (), nine-carbon naphthenes (), ten-carbon naphthenes (), eleven-carbon naphthenes (), twelve-carbon naphthenes ().
Aromatics: Benzene (); Toluene (); Ethylbenzene (EB); o, p, m-xylene (OPMX); aromatics (), aromatics (), aromatics (), aromatics ().
Olefins: Hexene, Heptene, Octene, Nonene.
There are 34-component lumped including hydrogen. Although olefins account for a small proportion (about 1%) of the components involved in the reforming reaction, they are taken into account in order to consider the generality of the model. The lumped reaction network is shown in
Figure 1.
The reaction network in
Figure 1 includes 39 reforming reactions, including 7 dehydrogenation reactions of paraffins, 8 dehydrogenation reactions of naphthenes, 5 hydrolyzation reactions of aromatics, and 19 hydrocracking reactions of paraffins. The details are as follows:
Dehydrogenation reactions of paraffins:
Dehydrogenation reactions of naphthenes:
Hydrolyzation reactions of aromatics:
Hydrocracking reactions of paraffins:
2.2. Establishment of Reactor Model for Continuous Catalytic Reforming Process
In the continuous catalytic reforming process, the reforming reactor is the core device, so the reasonable modeling of the reforming reactor is the most critical in the whole process [
14]. For the 34-component lumped reaction network established in this paper, the Hougen–Watson rate equation is used to describe it and 39 reaction rate equations can be obtained. The equations are as follows:
Dehydrogenation reactions of paraffins:
Dehydrogenation reactions of naphthenes:
Hydrolyzation reactions of aromatics:
Hydrocracking reactions of paraffins:
where
is the reaction rate;
Y is the molar flow rate of each lumped component;
,
,
are the molar flow rates of paraffins, naphthenes, and aromatics, respectively;
is the catalyst loading;
is the feed volume flow;
is the
-th reaction equilibrium constant;
is the reaction rate constant—its expression is
where
is the reaction frequency factor;
,
is the reaction activation energy and pressure index, respectively;
is the hydrogen partial pressure in the reactants;
is the molar gas constant;
T is the reaction temperature;
is the catalyst activity factor;
is the device factor.
In this paper, the radial structure of the reactor in the continuous catalytic reforming process is used, and combined with the principle of material balance and energy balance, the model equations of the reforming reactor can be obtained:
where
is the molar flow rate of 34—component lumped;
R is the bed radius of radial reactor;
H is the reactor height;
LHSV =
Fz/
Vc is the liquid hour space velocity;
is the stoichiometric coefficient matrix of each lumped component;
is the reaction heat vector;
is the constant pressure specific heat capacity vector;
is the reaction rate vector of
the reforming reaction, so the model equations of the reforming reactor can be rewritten as follows:
where
is the reaction rate constant matrix.
2.3. Property Calculation
The acquisition of some important physical property data is a prerequisite for model calculation, and the accuracy of these data has a great impact on the accuracy of the subsequent dynamic simulation. At the temperature of the continuous catalytic reforming reaction, all the reaction components are in a gaseous form. They can be regarded as ideal gas states and treated with the thermodynamic consistency equation [
19]. In this paper, 5 calculation methods of important physical properties are given; the methods are as follows:
(1) Enthalpy
where
is the enthalpy of ideal gas at
T;
T is the reaction temperature;
is the derived coefficient.
(2) Entropy
where
S is the entropy of an ideal gas at
T;
G is the derived coefficient.
(3) Constant pressure specific heat capacity
where
is the heat capacity of ideal gas under constant pressure.
(4) Reaction heat
At the temperature of
T, the formula is as follows:
where
represents the standard molar enthalpy of reaction;
is the stoichiometric coefficient;
represents the standard molar formation enthalpy;
represents the constant pressure specific heat capacity of each component at temperature
T.
(5) Reaction equilibrium constant calculation For a reversible reaction
, the equilibrium constant at temperature
T and a standard pressure of
is
where
is the Gibbs free energy of standard mole generation at temperature
T, also known as the standard mole free enthalpy, and the calculation formula is
where
knows that the calculation formula of standard molar entropy
is
is the standard molar entropy of the component in the standard state.
3. Simultaneous Method Based on Finite-Element Collocation
This paper belongs to the differential–algebraic optimization proposition that contains both differential equations and algebraic equations. The simultaneous method based on finite-element collocation is very suitable for solving this type of problem due to its unique advantages. This method has many advantages of the Runge–Kutta discrete method [
20,
21,
22,
23,
24,
25]. In this method, the differential state variable expression is as follows:
where
represents the value at the initial position of the
-th finite element;
represents the length of the finite element
,
; the conversion relationship between
t and
is
, and satisfies the polynomial of order
K:
; here,
z can represent space or time,
is the position of the
r-th configuration point in the finite element/the
r-th root of the Radau equation,
is the position of the
q -th configuration point in the finite element/the
q-th root of the Radau equation,
refers to the first derivative at the configuration point
q in the
-th finite element, and
is the time corresponding to the
r-th configuration point. The idea of the simultaneous method based on finite-element collocation is to uniformly discretize the entire time domain
into
finite elements, and the entire time domain is divided into
The length
of each finite element can be expressed as follows:
The expressions of differential state variables, algebraic state variables, and control variables are as follows:
where
represents the value of the algebraic variable at the
q-th configuration point at the
-th finite element, and
represents the value of the control variable at the
q-th configuration point at the
-th finite element.
represents the Lagrange function of the
q-th configuration point at the
-th finite element, and the expression is as follows:
The configuration point of each finite element is
K; the position corresponding to each configuration point is the root
of the orthogonal polynomial of the Radau configuration point; and the time corresponding to each configuration point is
, where
represents the initial time at the
-th configuration point of the
-th finite element.
When
, then
As the differential state variable has continuity, it means that the final value of the current finite element differential state variable is equal to the initial value of the next finite element differential state variable. The expression is as follows:
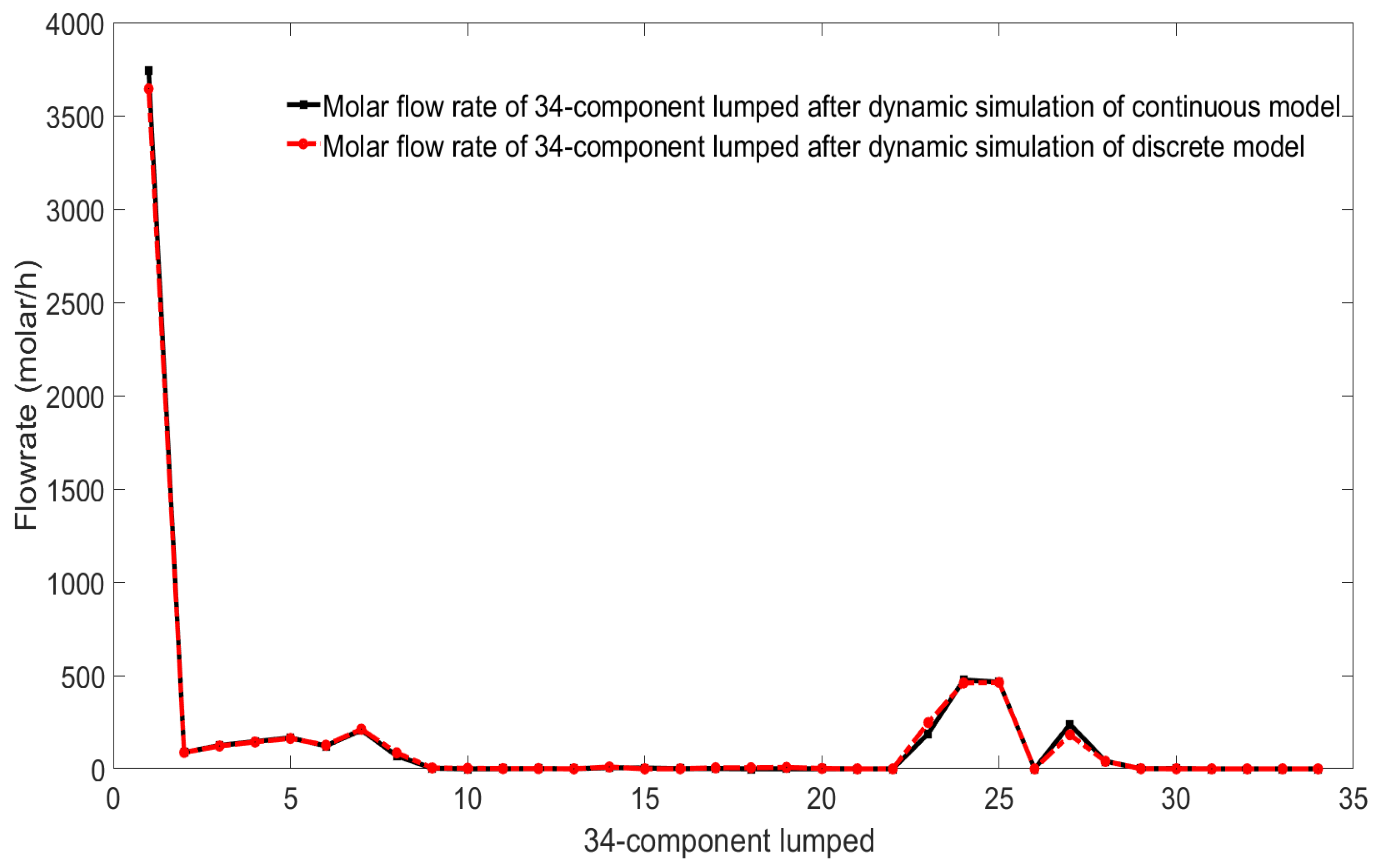
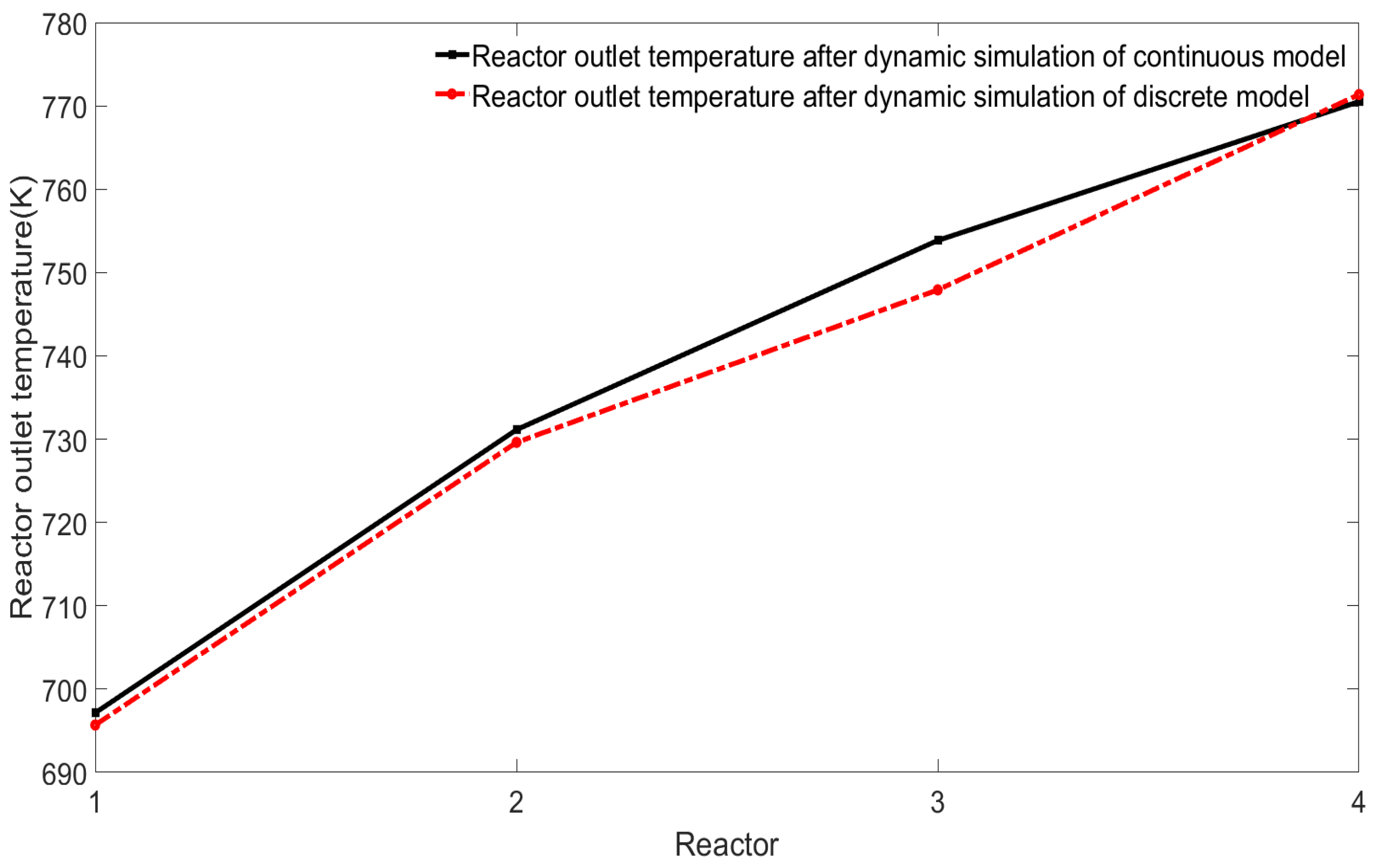
After the mechanism model of the continuous catalytic reforming process is discretized by a simultaneous method based on finite-element collocation, many discrete variables and algebraic equations will be obtained, so that the nonlinear solver can be used to dynamically simulate the system. Since this method has many advantages of the Runge–Kutta discrete method, and the Runge–Kutta discrete method has high discrete accuracy, the dynamic simulation of the discrete model and the dynamic simulation of its own continuous model will have a very high consistency. Comparison and verification will be conducted in the following model verification phase.
5. Dynamic Simulation in Different Situations
5.1. Dynamic Simulation under Different Input Parameters
After the mechanism model is established, the lumped components at the outlet of each reactor and the changes in the internal temperature of the reactor can be observed, and the influence of these parameters on the reactor can be observed by modifying the values of some parameters.
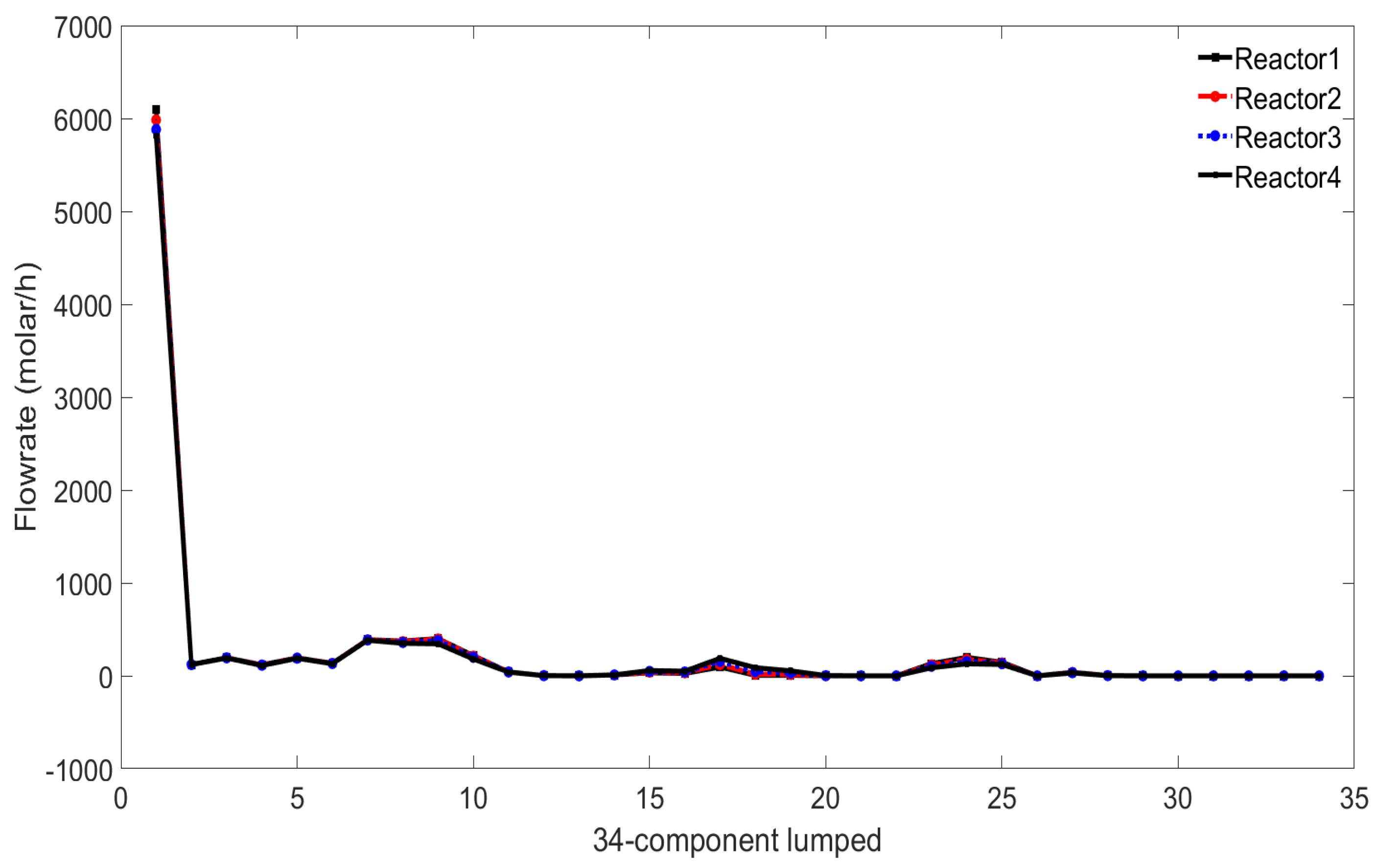
First, observe the change of the lumped components at the outlet of each reactor. The consumption of each lumped component can be seen from
Figure 7.
As it is a 34-component lumped reaction, in order to make the results look simple, the lumped is simplified into five parts: H (hydrogen), P (paraffins), N (naphthenes), A (aromatics), C5 (carbon 5 and below). The actual molar masses of these five lumps can be obtained from the factory as 3743.435, 282.211, 14.228, 1411.125, and 646.046.
First of all, the effect of catalyst loading on each lumped component can be observed by proportionally increasing the catalyst loading. The results are shown in
Table 3.
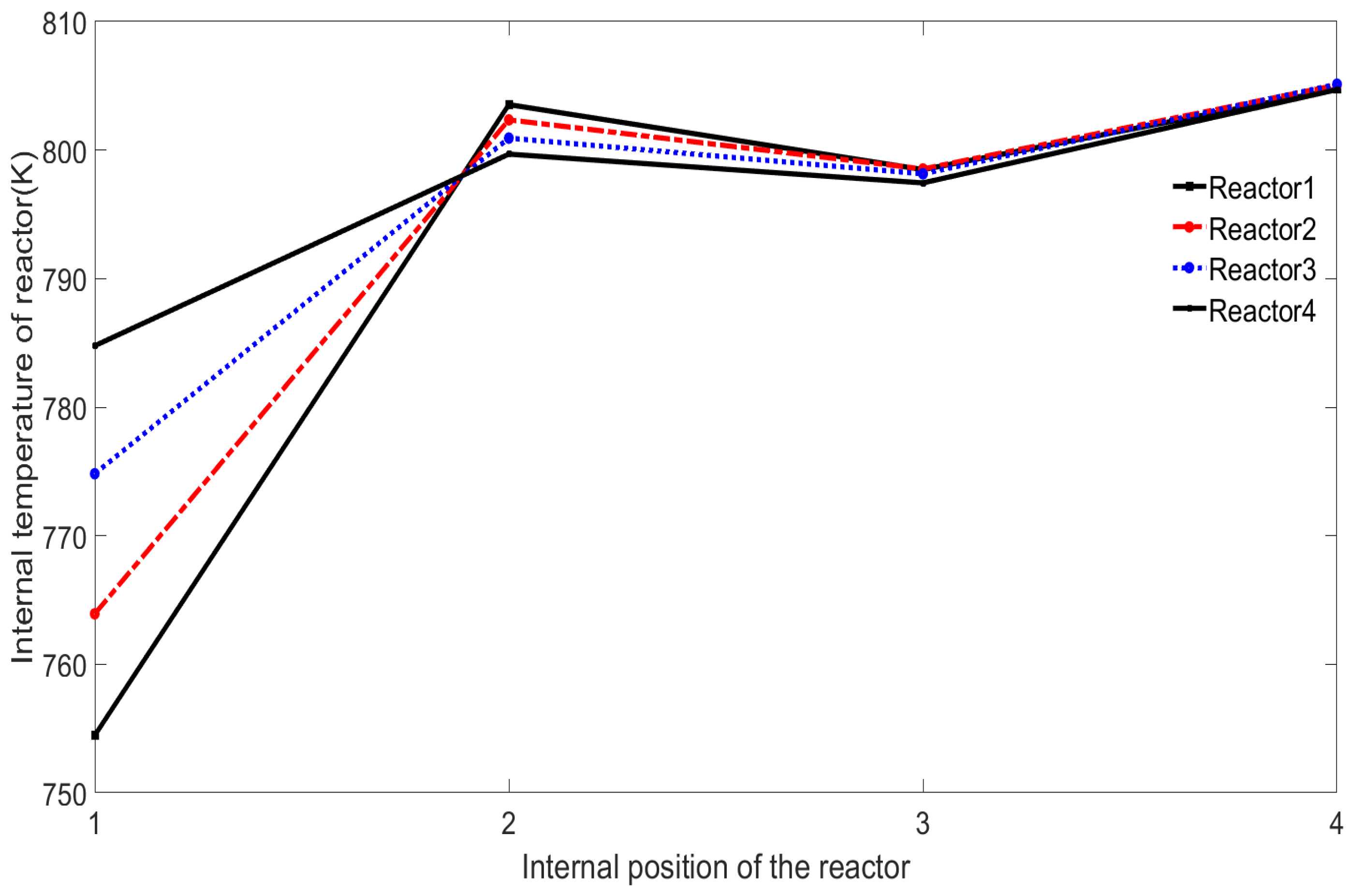
Secondly, observe the changes in the internal temperature of each reactor. As shown in
Figure 8, the inside of each reactor is again divided into four parts for observation.
It can be seen from
Table 3 that with the proportional increase in the catalyst loading, the contents of these five components all show a downward trend.
Secondly, by increasing the input temperature of the reactor proportionally, the influence of the input temperature of the reactor on each lumped component can be observed. The results are shown in
Table 4.
It can be seen from
Table 4 that as the reactor temperature increases proportionally, most of these five components show a downward trend. However, when the temperature is too high—for example, when the reactor temperature ratio increases to 1.75 times—A and C5 no longer decrease but increase, which means that the temperature is too high and some reversible reactions may occur, which is obviously not the desired result, so the temperature needs to be controlled below a reasonable range.
5.2. Sensitivity Analysis
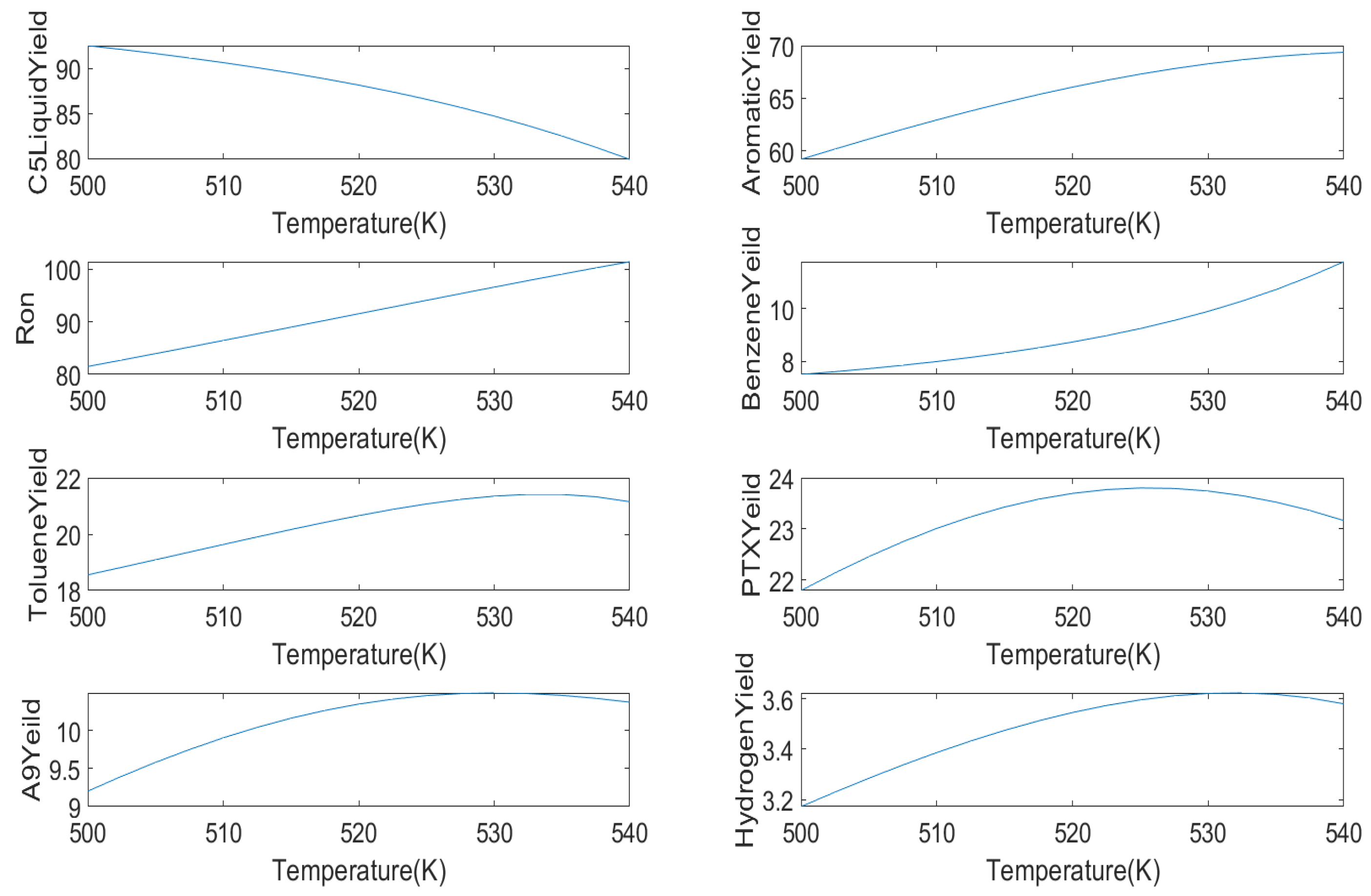
In this paper, the sensitivity of the yield of each product was analyzed by modifying the temperature and hydrogen oil ratio. As the liquid yield, aromatics yield, and octane number are more important than for other products, these three products are mainly analyzed in this paper.
The first is the influence of temperature on
liquid yield, aromatic yield, benzene yield, toluene yield, mixed xylene yield, C9 aromatics yield, hydrogen yield, and octane number, as shown in
Figure 9.
In industry, the reaction temperature is generally set to 480∼530 °C; this article sets the reaction temperature to 500∼540 °C on this basis. With the increase in the reaction temperature, the yield of aromatics and octane number also increase. When the temperature reaches 530 °C, the yield of aromatics tends to be flat. At this time, the yield of aromatics is between 72 and 74 (wt%). This is because the cycloalkane dehydrogenation reaction is a strong endothermic reaction, and higher temperature will increase the aromatics yield. However, when the temperature is too high, the alkane hydrocracking reaction will exceed the cycloalkane dehydrogenation reaction rate, so the aromatics yield will decrease. The liquid yield of decreases with the increase in the reaction temperature, which is approximately a linear relationship. When the reaction temperature is higher than 510 °C, a lower liquid yield will be obtained.
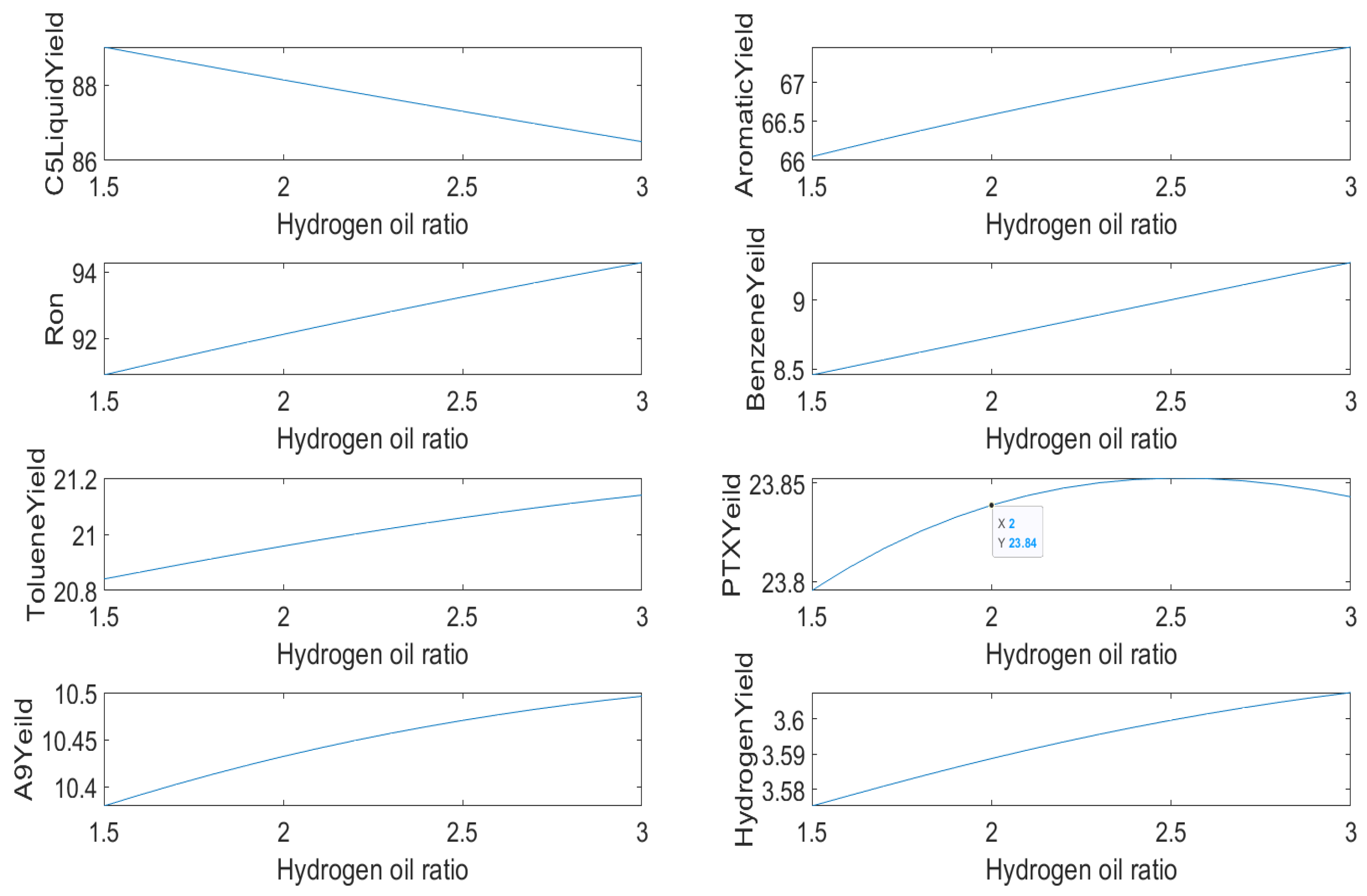
The second is the influence of hydrogen–oil ratio on
liquid yield, aromatic yield, benzene yield, toluene yield, mixed xylene yield, C9 aromatics yield, hydrogen yield, and octane number, as shown in
Figure 10.
With the increase in hydrogen–oil ratio, the catalyst activity can be improved, which is conducive to improve the yield of aromatics and octane number. At the same time, the increase in hydrogen–oil ratio will also accelerate the reaction rate of alkane hydrocracking and aromatics hydrocracking, and reduce the yield of liquid.
From the sensitivity analysis, we can discover the influence of temperature and hydrogen–oil ratio on the yield of each product. These results can also be compared with the actual situation to verify the accuracy of the model, and it can also pave the way for subsequent optimization solutions.
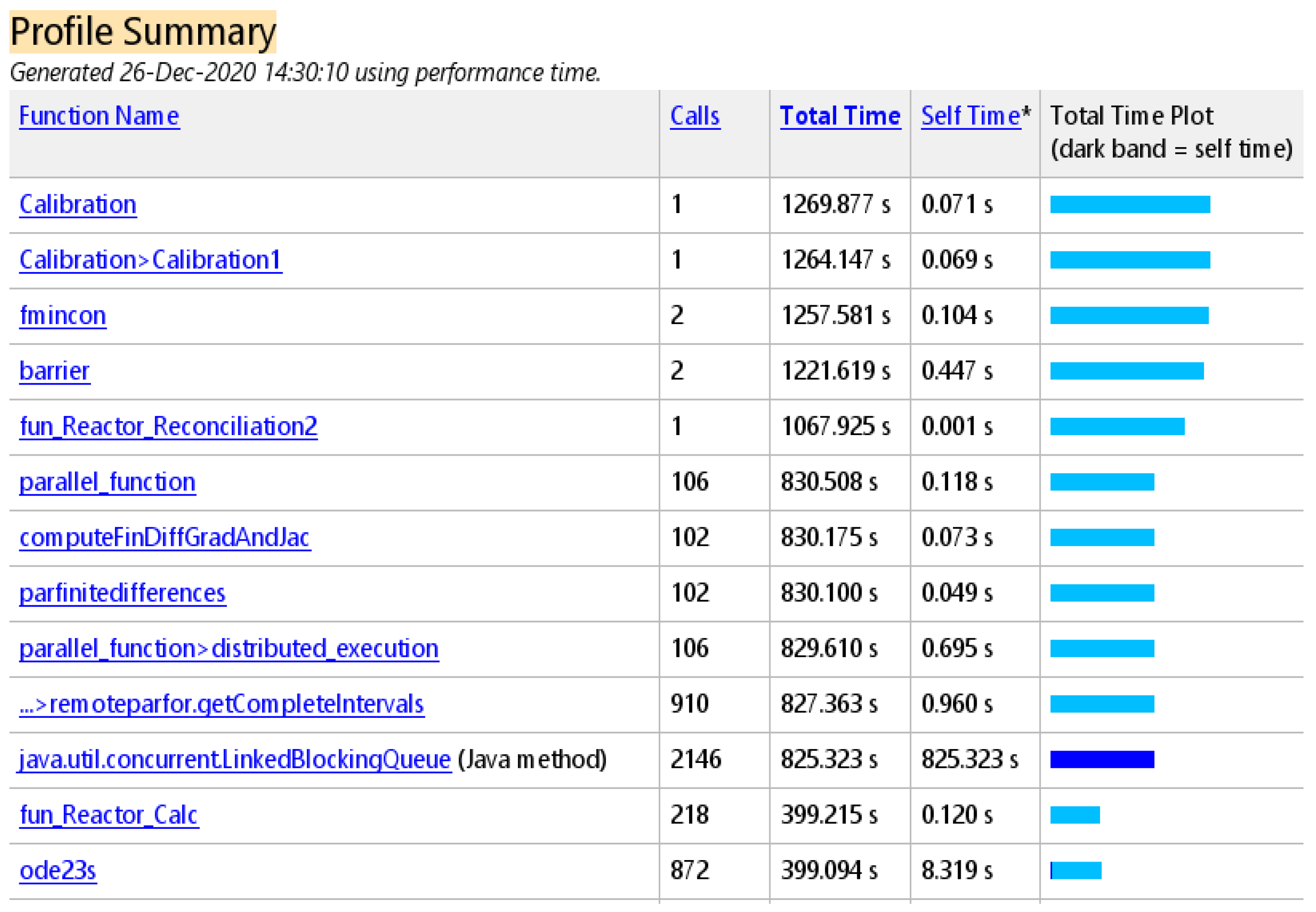
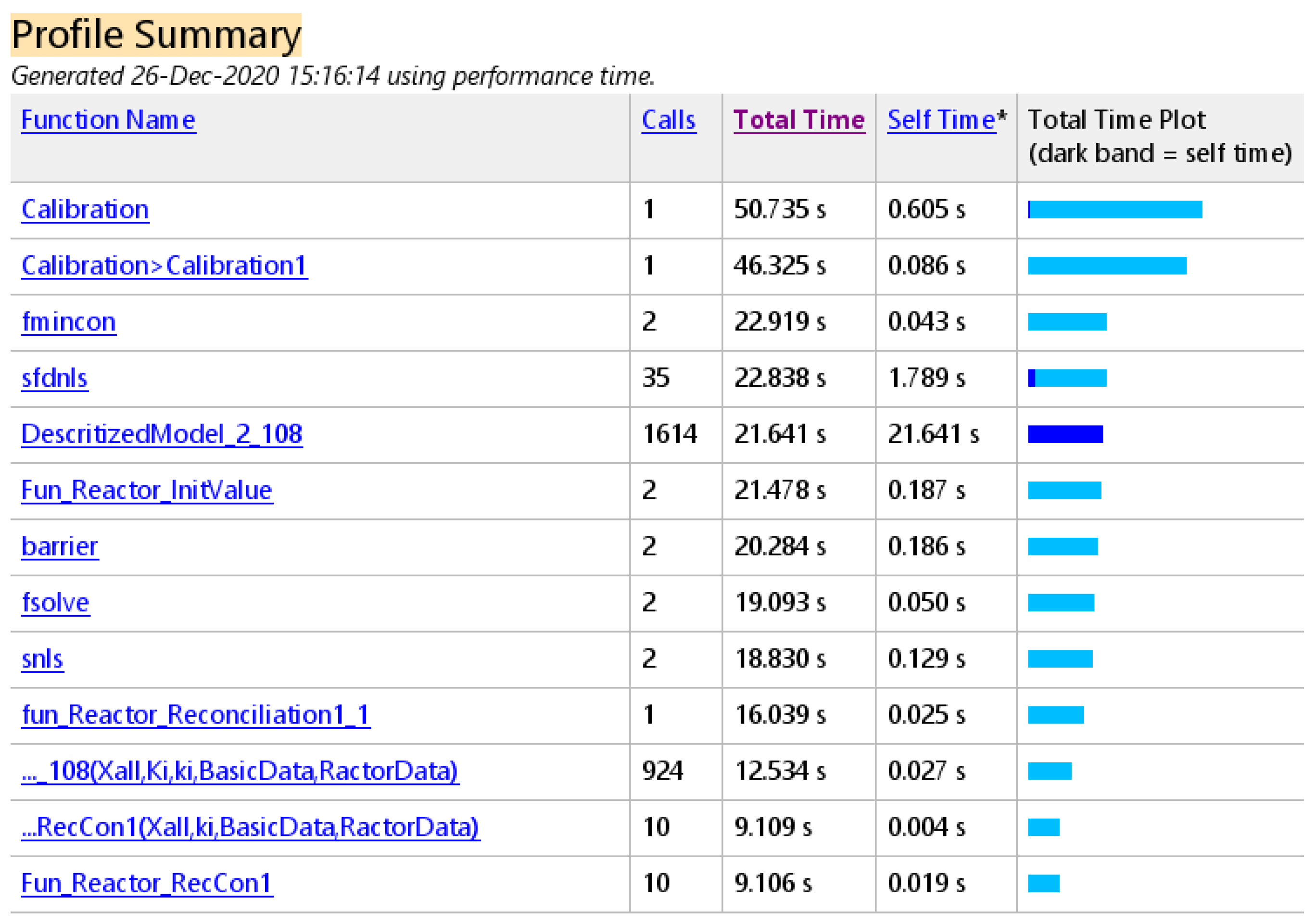
6. Conclusions
Based on the basic principles of reforming kinetics, thermodynamics, material balance, energy balance, etc., a 34-lumped mechanism model described by differential algebra is established in this paper. The differential equations are discretized through the simultaneous equations method of finite-element collocation to transform them into large-scale nonlinear programming problems, so as to solve and simulate the mechanism model consisting of 144 differential equations and several algebraic equations, and adopt the internal point algorithm for parameter estimation and model verification. In order to avoid the problem of too long a derivative solution time and too large memory in the solution process, methods such as sparse derivative and limited storage BFGS are used. In the model verification, taking the MATLAB platform as an example, the sequential method and the simultaneous equations method were used on the MATLAB platform to dynamically simulate the mechanism model established in this paper. It can be seen from the results that the time taken by the sequential method is about 25 times the time taken by the simultaneous equations method. Finally, based on the model verification, the catalyst loading amount and reactor input temperature are to observe its impact on each reactor, and then observe the impact on each component through the proportional increase in catalyst loading and reactor input temperature. The above process is to conduct dynamic simulation and sensitivity analysis of the whole process by modifying different input parameters. In addition, sensitivity analysis can verify the accuracy of the model and pave the way for subsequent optimization solutions. The results show that the mechanism model given in this paper is in line with the actual situation in our country and the solution method proposed can quickly and accurately simulate the continuous catalytic reforming process.