Anti-Cancer Effect of Panax Ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Anti-Cancer Effect of PG and Its Metabolites on Lung Cancer
3.2. Anti-Cancer Effect of PG and Its Metabolites on Breast Cancer
3.3. Anti-Cancer Effect of PG and Its Metabolites on Colon Cancer
3.4. Anti-Cancer Effect of PG and Its Metabolites on Prostate Cancer
3.5. Anti-Cancer Effect of PG and Its Metabolites on Gastric Cancer
4. Discussion
4.1. Overview of Anti-Cancer Studies of Panax ginseng and Its Metabolites on Major 5 Cancers
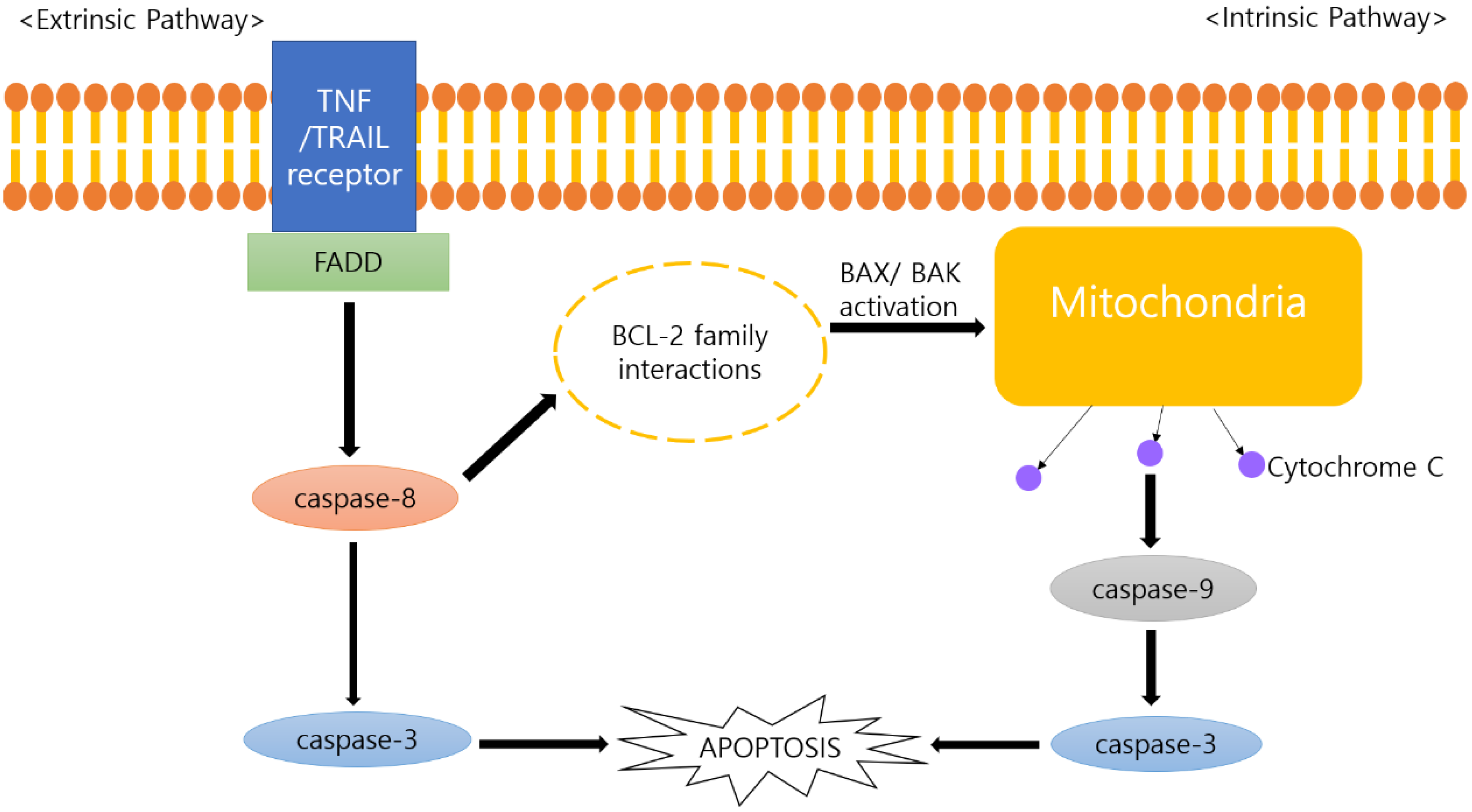
4.2. Anti-Cancer Mechanisms of Panax ginseng and Its Metabolites
4.3. Anti-Cancer Effects of Ginsenoside Rh2
4.4. Anti-Cancer Effects of Compound K
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, S.; Walsh, D. The symptoms of advanced cancer. Semin. Oncol. 1995, 22, 67. [Google Scholar]
- Walsh, D.; Donnelly, S.; Rybicki, L. The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients. Support. Care Cancer 2000, 8, 175–179. [Google Scholar] [CrossRef]
- Kwekkeboom, K.L. Cancer symptom cluster management. Semin. Oncol. Nurs. 2016, 32, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Paci, E.; Puliti, D.; Pegna, A.L.; Carrozzi, L.; Picozzi, G.; Falaschi, F.; Pistelli, F.; Aquilini, F.; Ocello, C.; Zappa, M. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017, 72, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Gan, T.; Sinner, H.F.; Walling, S.C.; Chen, Q.; Huang, B.; Tucker, T.C.; Patel, J.A.; Evers, B.M.; Bhakta, A.S. Impact of the Affordable Care Act on colorectal cancer screening, incidence, and survival in Kentucky. J. Am. Coll. Surg. 2019, 228, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Weiderpass, E. Infection and cancer: Global distribution and burden of diseases. Ann. Glob. Health 2014, 80, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori Infection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226. [Google Scholar] [PubMed]
- Chung, C.; Seo, W.; Silwal, P.; Jo, E.K. Crosstalks between inflammasome and autophagy in cancer. J. Hematol. Oncol. 2020, 13, 100. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Ginter, G.; Ptak-Belowska, A.; Dembinski, A. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J. Physiol. Pharmacol. 2014, 65, 95–106. [Google Scholar] [PubMed]
- Frascarelli, S.; Ghelardoni, S.; Ronca-Testoni, S.; Zucchi, R. Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res. Cardiol. 2003, 98, 401–405. [Google Scholar] [CrossRef]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Terauchi, Y.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar]
- Stempniewicz, A.; Ceranowicz, P.; Warzecha, Z. Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis. Int. J. Mol. Sci. 2019, 20, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembiński, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med Sci. Monit. 2012, 18, Br181–Br187. [Google Scholar] [CrossRef]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous Ghrelin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, M.; Kanemaru, A.; Fukushima, T.; Yamamoto, K.; Tanaka, H.; Haruyama, Y.; Itoh, H.; Matsumoto, N.; Kangawa, K.; Nakazato, M.; et al. Ghrelin administration suppresses inflammation-associated colorectal carcinogenesis in mice. Cancer Sci. 2015, 106, 1130–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J.; Kim, D.H.; Zhong, X.; Yum, H.W.; Kim, S.J.; Chun, K.S.; Na, H.K.; Surh, Y.J. Preventive effects of Korean red ginseng on experimentally induced colitis and colon carcinogenesis. J. Tradit. Complement. Med. 2020, 10, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Debatin, K.-M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kent, L.N.; Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 2019, 19, 326–338. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Tabassum, N.; Uddin, M.R.; Park, S.U. Ginseng: A miracle sources of herbal and pharmacological uses. Orient. Pharm. Exp. Med. 2016, 16, 243–250. [Google Scholar] [CrossRef]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Rajabian, A.; Rameshrad, M.; Hosseinzadeh, H. Therapeutic potential of Panax ginseng and its constituents, ginsenosides and gintonin, in neurological and neurodegenerative disorders: A patent review. Expert Opin. Ther. Pat. 2019, 29, 55–72. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Baraya, Y.S.; Wong, K.K.; Yaacob, N.S. The Immunomodulatory Potential of Selected Bioactive Plant-Based Compounds in Breast Cancer: A Review. Anti Cancer Agents Med. Chem. 2017, 17, 770–783. [Google Scholar] [CrossRef]
- Xiao, H.; Xue, Q.; Zhang, Q.; Li, C.; Liu, X.; Liu, J.; Li, H.; Yang, J. How Ginsenosides Trigger Apoptosis in Human Lung Adenocarcinoma Cells. Am. J. Chin. Med. 2019, 47, 1737–1754. [Google Scholar] [CrossRef] [PubMed]
- Vayghan, H.J.; Ghadimi, S.S.; Nourazarian, A.R. Preventive and therapeutic roles of ginseng-focus on colon cancer. Asian Pac. J. Cancer Prev. 2014, 15, 585–588. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, M.K.; Lee, M.; Kwon, B.S.; Suh, D.H.; Song, Y.S. Effect of Red Ginseng on Genotoxicity and Health-Related Quality of Life after Adjuvant Chemotherapy in Patients with Epithelial Ovarian Cancer: A Randomized, Double Blind, Placebo-Controlled Trial. Nutrients 2017, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.-W.; Wang, C.-Z.; Du, G.-J.; Zhang, Z.-Y.; Calway, T.; Yuan, C.-S. Metabolism of ginseng and its interactions with drugs. Curr. Drug Metab. 2011, 12, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, J.; Du, F.; Gao, X.; Ma, X.; Huang, Y.; Xu, F.; Niu, W.; Wang, F.; Mao, Y. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takino, Y. Studies on the pharmacodynamics of ginsenoside-Rg1,-Rb1 and-Rb2 in rats. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1994, 114, 550–564. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Sung, J.-H.; Matsumiya, S.; Uchiyama, M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996, 62, 453–457. [Google Scholar] [CrossRef]
- Bae, E.-A.; Choo, M.-K.; Park, E.-K.; Park, S.-Y.; Shin, H.-Y.; Kim, D.-H. Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol. Pharm. Bull. 2002, 25, 743–747. [Google Scholar] [CrossRef] [Green Version]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.S.; Roh, H.-S.; Baek, K.-H.; Lee, S.; Kim, S.; So, H.M.; Moon, E.; Pang, C.; Jang, T.S.; Kim, K.H. Bioactivity-guided isolation of ginsenosides from Korean Red Ginseng with cytotoxic activity against human lung adenocarcinoma cells. J. Ginseng Res. 2018, 42, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-S.; Kim, J.M.; Jo, E.; Cho, C.-K.; Lee, S.-Y.; Kang, H.S.; Lee, M.-G.; Yang, P.-Y.; Jang, I.-S. Modified Panax ginseng extract regulates autophagy by AMPK signaling in A549 human lung cancer cells. Oncol. Rep. 2017, 37, 3287–3296. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.-H.; Kwon, Y.-K.; Cho, C.-K.; Lee, Y.-W.; Sung, J.-S.; Joo, J.-C.; Lee, K.-B.; Yoo, H.-S.; Jang, I.-S. Modified Panax ginseng extract inhibits uPAR-mediated α 5 β1-integrin signaling by modulating caveolin-1 to induce early apoptosis in lung cancer cells. Am. J. Chin. Med. 2016, 44, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Jang, S.-I.; Kim, Y.-R.; Yang, K.E.; Yoon, S.J.; Lee, Z.-W.; An, H.J.; Jang, I.-S.; Choi, J.-S.; Yoo, H.-S. Anti-proliferative effects of ginsenosides extracted from mountain ginseng on lung cancer. Chin. J. Integr. Med. 2016, 22, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-L.; Liu, H.-J.; Liu, Z.-C.; Liu, N.; Liu, R.; Kang, Y.-R.; Ji, J.-G.; Zhang, C.; Hua, B.-J.; Kang, S.-J. Adjuvant effects of fermented red ginseng extract on advanced non-small cell lung cancer patients treated with chemotherapy. Chin. J. Integr. Med. 2017, 23, 331–337. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, T.; Ma, C.; Song, W.; Zhang, J.; Yu, Z. Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells. J. Thorac. Dis. 2015, 7, 400. [Google Scholar]
- Jin, X.; Yang, Q.; Cai, N. Preparation of ginsenoside compound-K mixed micelles with improved retention and antitumor efficacy. Int. J. Nanomed. 2018, 13, 3827. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, Z.; Hou, J.; Jin, X.; Ke, Z.; Liu, D.; Du, M.; Jia, X.; Lv, H. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int. J. Nanomed. 2017, 12, 7653. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Patino, C.; Bossowski, J.P.; De Donatis, G.M.; Mondragon, L.; Villa, E.; Aira, L.E.; Chiche, J.; Mhaidly, R.; Lebeaupin, C.; Marchetti, S.; et al. Low-Protein Diet Induces IRE1alpha-Dependent Anticancer Immunosurveillance. Cell Metab. 2018, 27, 828–842. [Google Scholar] [CrossRef] [Green Version]
- Ge, G.; Yan, Y.; Cai, H. Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol. Pharm. Bull. 2017, 40, 2117–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, H.; Lu, Z.; Yu, X.; Lv, C.; Tian, Y.; Sui, D. Pseudo-Ginsenoside Rh2 induces A549 cells apoptosis via the Ras/Raf/ERK/p53 pathway. Exp. Ther. Med. 2018, 15, 4916–4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.K.; Kim, K.M.; Choi, K.D.; Im, W.T. Production of the Rare Ginsenoside Rh2-MIX (20(S)-Rh2, 20(R)-Rh2, Rk2, and Rh3) by Enzymatic Conversion Combined with Acid Treatment and Evaluation of Its Anti-Cancer Activity. J. Microbiol. Biotechnol. 2017, 27, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.H.; Park, J.Y.; Lee, D.; Kwak, J.Y.; Park, E.H.; Kim, K.H.; Park, H.J.; Kim, H.Y.; Jang, H.J.; Ham, J.; et al. Inhibitory effects of ginseng sapogenins on the proliferation of triple negative breast cancer MDA-MB-231 cells. Bioorg. Med. Chem. Lett. 2014, 24, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Fujita, M.; Itokawa, H.; Tanaka, O.; Ishii, T. Studies on the Constituents of Japanese and Chinese Crude Drugs. XI. Panaxadiol, A Sapogenin of Ginseng Roots. Chem. Pharm. Bull. 1963, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Jeon, S.B.; Lee, Y.; Lee, H.; Kim, J.; Kwon, B.R.; Yu, K.Y.; Cha, J.D.; Hwang, S.M.; Choi, K.M.; et al. Fermented red ginseng extract inhibits cancer cell proliferation and viability. J. Med. Food 2015, 18, 421–428. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, H.J.; Jang, J.H.; Kim, D.H.; Surh, Y.J. The standardized Korean Red Ginseng extract and its ingredient ginsenoside Rg3 inhibit manifestation of breast cancer stem cell-like properties through modulation of self-renewal signaling. J. Ginseng Res. 2019, 43, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.W.; Son, Y.M.; Gu, M.J.; Kim, G.; Lee, I.K.; Kye, Y.C.; Kim, H.W.; Song, K.D.; Chu, H.; Park, B.C.; et al. A Bacterial Metabolite, Compound K, Induces Programmed Necrosis in MCF-7 Cells via GSK3beta. J. Microbiol. Biotechnol. 2015, 25, 1170–1176. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y. Effects of ginsenoside compound K combined with cisplatin on the proliferation, apoptosis and epithelial mesenchymal transition in MCF-7 cells of human breast cancer. Pharm. Biol. 2016, 54, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, S.; Jeong, D.; Kim, S.J. Ginsenoside Rh2 epigenetically regulates cell-mediated immune pathway to inhibit proliferation of MCF-7 breast cancer cells. J. Ginseng Res. 2018, 42, 455–462. [Google Scholar] [CrossRef]
- Hou, J.G.; Jeon, B.M.; Yun, Y.J.; Cui, C.H.; Kim, S.C. Ginsenoside Rh2 Ameliorates Doxorubicin-Induced Senescence Bystander Effect in Breast Carcinoma Cell MDA-MB-231 and Normal Epithelial Cell MCF-10A. Int. J. Mol. Sci. 2019, 20, 1244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Gong, J.; Zhang, H.; Kong, D. Induction of apoptosis and reversal of permeability glycoprotein-mediated multidrug resistance of MCF-7/ADM by ginsenoside Rh2. Int. J. Clin. Exp. Pathol. 2015, 8, 4444–4456. [Google Scholar] [PubMed]
- Ren, G.; Shi, Z.; Teng, C.; Yao, Y. Antiproliferative Activity of Combined Biochanin A and Ginsenoside Rh(2) on MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, X.; Zhang, H.D.; Zhao, L.; Yao, Y.F.; Zhao, J.H.; Tang, J.H. Ginsenoside Rh2 differentially mediates microRNA expression to prevent chemoresistance of breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 1105–1109. [Google Scholar] [CrossRef] [Green Version]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwon, K.A.; Lee, Y.E.; Kim, J.H.; Kim, S.H.; Kim, J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-kappaB and ERK1/2 pathways in colon cancer. J. Ginseng Res. 2018, 42, 288–297. [Google Scholar] [CrossRef]
- Li, T.; Sun, W.; Dong, X.; Yu, W.; Cai, J.; Yuan, Q.; Shan, L.; Efferth, T. Total ginsenosides of Chinese ginseng induces cell cycle arrest and apoptosis in colorectal carcinoma HT-29 cells. Oncol. Lett. 2018, 16, 4640–4648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, J.Y.; Han, Y.H.; Mun, J.G.; Um, J.Y.; Hong, S.H. Pharmacological effect of prohibited combination pair Panax ginseng and Veratrum nigrum on colorectal metastasis in vitro and in vivo. J. Ethnopharmacol. 2018, 220, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wan, J.Y.; Zeng, J.; Huang, W.H.; Sava-Segal, C.; Li, L.; Niu, X.; Wang, Q.; Wang, C.Z.; Yuan, C.S. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018, 15, 8339–8348. [Google Scholar] [CrossRef]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.C.; Wolff, R.K.; Samowitz, W.S.; Herrick, J.S. Dysregulated genes and miRNAs in the apoptosis pathway in colorectal cancer patients. Apoptosis 2018, 23, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischlerova, V.; Kello, M.; Budovska, M.; Mojzis, J. Indole phytoalexin derivatives induce mitochondrial-mediated apoptosis in human colorectal carcinoma cells. World J. Gastroenterol. 2017, 23, 4341–4353. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, Y.; Sun, Q.; Zhang, Z.; Guo, X.; Sheng, X.; Tai, G.; Cheng, H.; Zhou, Y. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and -independent DR5 upregulation. Cell Death Dis. 2016, 7, e2334. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, D.; Xing, T.; Su, H.; Zhang, S.; Wen, J.; Bai, Q.; Dang, D. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinase. J. Ginseng Res. 2016, 40, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Liu, F.; Qian, W.; Zhang, T.; Li, F. Combined Effect of Sodium Selenite and Ginsenoside Rh2 on HCT116 Human Colorectal Carcinoma Cells. Arch. Iran. Med. 2016, 19, 23–29. [Google Scholar]
- Han, S.; Jeong, A.J.; Yang, H.; Bin Kang, K.; Lee, H.; Yi, E.H.; Kim, B.H.; Cho, C.H.; Chung, J.W.; Sung, S.H.; et al. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through targeting IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. J. Ethnopharmacol. 2016, 194, 83–90. [Google Scholar] [CrossRef]
- Liu, G.W.; Liu, Y.H.; Jiang, G.S.; Ren, W.D. The reversal effect of Ginsenoside Rh2 on drug resistance in human colorectal carcinoma cells and its mechanism. Hum. Cell 2018, 31, 189–198. [Google Scholar] [CrossRef]
- Ma, J.; Gao, G.; Lu, H.; Fang, D.; Li, L.; Wei, G.; Chen, A.; Yang, Y.; Zhang, H.; Huo, J. Reversal effect of ginsenoside Rh2 on oxaliplatin-resistant colon cancer cells and its mechanism. Exp. Ther. Med. 2019, 18, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Zheng, J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018, 51, e12438. [Google Scholar] [CrossRef] [Green Version]
- Tong-Lin Wu, T.; Tong, Y.C.; Chen, I.H.; Niu, H.S.; Li, Y.; Cheng, J.T. Induction of apoptosis in prostate cancer by ginsenoside Rh2. Oncotarget 2018, 9, 11109–11118. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Huang, H.; Han, Z.; Li, W.; Mai, Z.; Yuan, R. Ginsenoside Rh2 Inhibits Angiogenesis in Prostate Cancer by Targeting CNNM1. J. Nanosci. Nanotechnol. 2019, 19, 1942–1950. [Google Scholar] [CrossRef]
- Zhang, Q.; Hong, B.; Wu, S.; Niu, T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumor Biol. 2015, 36, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Baek, Y.M.; Jang, I.S.; Yang, K.E.; Lee, D.G.; Yoon, S.J.; Rho, J.; Cho, C.K.; Lee, Y.W.; Kwon, K.R.; et al. An enzymatically fortified ginseng extract inhibits proliferation and induces apoptosis of KATO3 human gastric cancer cells via modulation of Bax, mTOR, PKB and IkappaBalpha. Mol. Med. Rep. 2015, 11, 670–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Li, J.; Jia, J.G.; Jin, X.; Yu, D.J.; Guo, C.X.; Xie, B.; Qian, L.Y. Ginsenoside-Rh2 Inhibits Proliferation and Induces Apoptosis of Human Gastric Cancer SGC-7901 Side Population Cells. Asian Pac. J. Cancer Prev. 2016, 17, 1817–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention. World Cancer Reports. Lyon Int. Agency Res. Cancer; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Shin, H.R.; Kim, J.Y.; Yun, T.K.; Morgan, G.; Vainio, H. The cancer-preventive potential of Panax ginseng: A review of human and experimental evidence. Cancer Causes Control. 2000, 11, 565–576. [Google Scholar] [CrossRef]
- Yu, K.; Chen, F.; Li, C. Absorption, disposition, and pharmacokinetics of saponins from Chinese medicinal herbs: What do we know and what do we need to know more? Curr. Drug Metab. 2012, 13, 577–598. [Google Scholar] [CrossRef]
- Chaabane, W.; User, S.D.; El-Gazzah, M.; Jaksik, R.; Sajjadi, E.; Rzeszowska-Wolny, J.; Łos, M.J. Autophagy, apoptosis, mitoptosis and necrosis: Interdependence between those pathways and effects on cancer. Arch. Immunol. Ther. Exp. 2013, 61, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgi, N.; Bell, K.; Ananthakrishnan, A.N.; Atallah, E. Imatinib and Panax ginseng: A potential interaction resulting in liver toxicity. Ann. Pharmacother. 2010, 44, 926–928. [Google Scholar] [CrossRef]
- Myers, A.P.; Watson, T.A.; Strock, S.B. Drug reaction with eosinophilia and systemic symptoms syndrome probably induced by a lamotrigine–ginseng drug interaction. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, e9–e12. [Google Scholar] [CrossRef]
- Yan, Z.; Lai, Z.; Lin, J. Anticancer properties of traditional Chinese medicine. Comb. Chem. High Throughput Screen. 2017, 20, 423–429. [Google Scholar] [CrossRef]
- Smith, B.N.; Bhowmick, N.A. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 2016, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Cort, A.; Ozben, T. Natural product modulators to overcome multidrug resistance in cancer. Nutr. Cancer 2015, 67, 411–423. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Lin, M.; Gao, Y.; Liu, Y.; Yang, S.; Zhou, X.; Zhang, Y.; Hu, Y.; Wang, H.; et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020, 203, 112627. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Yu, D.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 2007, 117, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Amaravadi, R.K.; Thompson, C.B. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 2007, 13, 7271–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, H.; Suzuki, R.; Nagaoka, T.; Tezuka, Y.; Kadota, S.; Saiki, I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol. Pharm. Bull. 2002, 25, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, H.; Lee, K.-S.; Nagaoka, T.; TEZUKA, Y.; UCHIYAMA, M.; KADOTA, S.; SAIKI, I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol. Pharm. Bull. 2000, 23, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Compound/Extract | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Korean Red Ginseng Extract | A549, H1264, H1299, Calu-6 | 400 μg/mL; 48 h | Induction of cancer cell apoptosis | ↑ AIF | [47] |
| Modified Red Ginseng Extract | A549 | 100 μg/mL; 25, 50, 72 h | Inhibition of cancer cell growth Induction of autophagy | ↑ LC3, Beclin-1, ATG5, p-Akt ↓ mTOR, 4EBP1 | [48] |
| Modified Red Ginseng Extract | A549 | 25, 50 μg/mL; 24 h | Induction of cancer cell apoptosis | ↑ Bax, Caspase-3 ↓ Bcl-2, PARP, p-ERK, Caveolin-1, FAK1, FN | [49] |
| Butanol-extracted Mountain Ginseng | A549, NCI-H358, NCI-H596 | 0.08, 0.4, 2, 10 mg/mL; 48 h | Induction of cancer cell apoptosis Inhibition of cancer cell proliferation | ↑ Caspase-3, Caspase-8 | [50] |
| CK | H1299, H460, A549 | 20 µM; 18, 24, 72 h | Sensitization of cisplatin | ↑ p53, p21 | [52] |
| CK, CK-M | A549 | 12.15 µg/mL; 24 h | Induction of apoptosis Inhibition of tumor growth | ↑ Caspase-9, Caspase-3, PARP | [53] |
| CK, CK-M | A549, PC-9 | 20 µg/mL; 24 h | Induction of apoptosis Inhibition of tumor cell invasion, metastasis, and efflux | ↑ Bax, Bcl-2, MMP-2, Caspase-3, p-glycoprotein | [54] |
| G-Rh2 | A549, H1299 | 60, 100 μM; 24 h | Induction of macrophage differentiation Prevention of cancer cell migration | ↓ VEFG-C, MMP2, MMP9 | [55] |
| G-Rh2 | H1229 | 10, 20, 30, 40, 50 μM/L; 24 h | Inhibition of cancer proliferation Induction of ROS meditated ER stress dependent apoptosis | ↑ ATF4, CHOP, Caspase-4 | [56] |
| pseudo-G-Rh2 | A549 | 24, 48, 96 μM; 24 h | Inhibition of cell proliferation Induction of mitochondria-associated apoptosis Induction of ROS production | ↑ mitochondrial cytochrome c, Caspase-9, Bax, PARP, p-Raf, Ras, p53 ↓ Bcl-2 | [57] |
| In vivo | |||||
| G-Rh2 | C57BL/6 mice (n = 14) | 40 mg/kg/day; 21 days | Induction of macrophage differentiation Prevention of cancer cell migration Reduction of tumor size | ↓ VEGF-C, CD206 | [55] |
| G-Rh2 | BALB/c nu/nu nude mice (n = 10) | 20 mg/kg/day, 40 days | Reduction of tumor volume | ↑ ATF4, CHOP, Caspase-4 | [56] |
| Clinical trial | |||||
| Fermented Red Ginseng Extract | Non-small cell lung cancer patients | 3000 mg; 60 days | Improvement of FSI score, CM symptom score, psychological and physical conditions, QOL Inhibition of chemotherapy toxicity | [51] |
| Compound/Extract | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| G-Rh2-MIX | MDA-MB-231 | 12.5, 25, 50, 100 µM; 24 h | Inhibition of growth of cancer cells | [58] | |
| Protopanaxadiol | MDA-MB-231 | 2.5 µM; 24 h | Induction of apoptosis | ↑ c-Caspase-8, -3, c-PARP ↓ BID | [59] |
| Fermented-red ginseng water extract | MCF-7 | 0.5, 1, 2, 5 mg/mL; 48 h | Inhibition of proliferation and viability | [61] | |
| Red ginseng extract, G-Rg3 | MCF-7, MDA-MB-231, | 1 mg/mL; 5 days 25 µM; 5 days | Suppression of manifestation Inhibition of self-renewal | ↓ HIF-1 α, p-Akt, Sox-2, Bmi-1, | [62] |
| CK | MCF-7 | 10, 30, 50, 70 µM; 24, 48 h | Induction of programmed necrosis | ↑ GSK3β ↓ β–catenin, cyclin D1 | [63] |
| CK | MCF-7 | 50 µM; 24, 48, 72, 96 h | Inhibition of proliferation and EMT Induction of apoptosis | ↑ C-cadherin ↓ N-cadherin, vimentin, FN, p-Akt | [64] |
| G-Rh2 | MCF-7 | 20, 50 µM; 24 h | Induction of apoptosis | ↑ CLINT, ST3GAL4, C1orf198 ↓ Caspase-1, INSL5, OR52A1 | [65] |
| G-Rh2 | MDA-MB-231 | 20 µg/mL; 48 h | Amelioration of doxorubicin-induced SASP | ↓ p-MEK1, p-p38MAPK, p-Stat3, p-NF-κB p65, IL-6, -8, MCP-1, CXCL1 | [66] |
| G-Rh2 | MCF-7 MCF-7/ADM | (1) 0.625, 1.25, 5, 10, 20, 40, 80 μmol/L; 24, 48, 72 h (2) 5, 10, 20, 40 μmol/L; 72 h | (1) Induction of apoptosis (2) Reversal of MDR | (1) ↑ c-Caspase-3 | [67] |
| G-Rh2 | MCF-7 MDA-MB-231 | 30, 35, 40, 45, 50, 55, 60, 70 µM; 72 h | Inhibition of proliferation, invasion | ↑ p-p53, p-p38, p-ASK1 ↓ TRAF2 | [68] |
| G-Rh2 | MCF-7, MCF-7/Doc MCF-7/Adr | 10, 20, 40, 60, 80, 100 μM; 24 h | Reduction of drug resistance | ↑ miR-29a, -222, Bax ↓ miR-34a | [69] |
| Compound/Extract | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Panax Ginseng root water extract | HCT116, HT29 | 1, 2 mg/mL; 24, 72 h | Repression of hypoxia-induced EMT | ↓ NF-κB, ERK1/2, Snail, Slug, Twist, αVβ6 integrin, E-cadherin | [71] |
| Panax Ginseng root water extract | HT29 | 50, 100, 200 µg/mL; 24, 48, 72 h | Inhibition of cell proliferation, Induction of cell cycle arrest and cell apoptosis | ↑ BAX, CDNK2B, Caspase-8, -3, TP53, Bax, p21WAF1, p27Kip1, c-Myc, p15INK4b, p53 ↓ CDK2, CDK4, CDK6, TOP1, MYC, MDM2, CCND1, Cdk2, CDK4 | [72] |
| Panax Ginseng root ethanol extract | CT26 | 0.5, 1 mg/mL; 24 h | Inhibition of EMT process Induction of apoptosis and cell cycle arrest | ↓ E-cadherin, N-cadherin, Vimentin, Snail, MMP-2, MMP-9 | [73] |
| CK | HCT116, HT29 | (1) 40, 50, 60, 70 µM; 48 h (2) 40, 60, 80 µM; 6, 12 h | (1) Arrest in the G1 phase, Induction of cell apoptosis, (2) Inhibition of inflammation | (1) ↑ p21, p53, Bax ↓ CDK6, cyclin E, Bcl2 (2) ↓ IL-8 | [74] |
| CK | HCT116 | 40 μM; 24, 48, 72 h | Proteolytic cleavage of PARP Release of cytochrome c Induction of Caspase-dependent apoptosis | ↑ Caspase-3, -9, Bcl-2, p-p38 MAPK ↓ p-ERK 1/2, p-Akt, NF-κB p50, NF-κB p65, p-BAD | [76] |
| CK | HCT116, HT29 | 25, 50 μM; 48 h | Induction of TRAIL-induced apoptosis | ↑ c-Caspase-8, -9, -3, Bax, tBid, cytochrome c, DR5, CHOP ↓ Mcl-1, Bcl-2, XIAP, cFLIP | [77] |
| G-Rh2 | LoVo, LoVo/L-OHP | 250 µg/mL; 24 h | Inhibition of cell proliferation. Promotion of apoptosis and changes in drug resistance genes | ↑ Smad4, Bax, Caspase-3. ↓ p-gp, Bcl-2 | [82] |
| G-Rh2 | HCT8, LoVo | 5, 10, 20 μM; 24 h | Inhibition of migration and EMT process, proliferation. promotion of apoptosis | ↑ c-Caspase-3, p-IκB, MRP1, MDR1, LRP, GST ↓ cyclin D1, CDK2, p-Rb, Bcl-2 | [81] |
| G-Rh2 | HCT116 | 5, 15, 25, 50 μM; 3,6,12 h | Inhibition of cancer cell proliferation | ↑ p-EKR2, p-Histone H3, p- ERK1/2, p-p90RSK | [78] |
| 20(S)- G- Rh2 | HCT116, SW620 | 1, 3, 5, 7, 10 μM; 0.5, 1, 3 h | Suppression of cancer invasion and tumor metastasis | ↓ pY-STAT3, p-JAK2, MMP-1, MMP-2, MMP-9 | [80] |
| G- Rh2 | HCT116 | 12.5 μM; 24 h | Arrest in the G1 phase Promotion of apoptosis Induction of ROS depletion and autophagy | ↑ Bax/Bcl2, Caspase-3 | [79] |
| Compound/Extract | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| G-Rh2 | PC3, DU145 | 0.01, 0.1, 1 mg/mL; 24, 48, 96 h | Inhibition of proliferation | ↓ CDKN1A | [83] |
| G-Rh2 | PC3, DU145 | 0.05, 0.075, 0.1 mM; 24 h | Induction of apoptosis | ↑ PPAR- δ ↓ p-STAT3 | [84] |
| In vivo | |||||
| G-Rh2 | LNCaP, PC3, DU145/nude mice | 0, 100, 500, 1000, 5000, 10,000 mg/kg; 32 days | Inhibition of angiogenesis | ↓ CNNM1 | [85] |
| G-Rh2 | (1) nude mice (2) PC3 | (1) 1 mg/kg; 4 weeks (2) 0.1 mg/mL; 2, 4, 7 days | Inhibition of proliferation and invasion | ↑ TGFβ receptor, p-SMAD2, CyclinD1, CyclinB1, MMP2, MMP9 ↓ p27 | [86] |
| Compound/Extract | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|
| In vitro | |||||
| Enzymatically fortified ginseng extract (FGX) | KATO3 | 31.25, 62.5, 125 μg/mL; 24 h | Inhibition of proliferation Induction of apoptosis | ↑ Bax, IκBα ↓ p-mTOR, p-PKB | [87] |
| G-Rh2 | SGC-7901 | 5, 10, 20 μg/mL; 24, 48 h | Inhibition of proliferation Induction of cell cycle arrest and apoptosis | ↑ Bax ↓ Bcl-2 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, N.; Jeong, J.; Lee, S.; Kim, W.; Ko, S.-G.; Kim, B. Anti-Cancer Effect of Panax Ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery. Processes 2021, 9, 1344. https://doi.org/10.3390/pr9081344
Kim S, Kim N, Jeong J, Lee S, Kim W, Ko S-G, Kim B. Anti-Cancer Effect of Panax Ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery. Processes. 2021; 9(8):1344. https://doi.org/10.3390/pr9081344
Chicago/Turabian StyleKim, Sejin, Nayeon Kim, JaYeon Jeong, Soojin Lee, Woojin Kim, Seong-Gyu Ko, and Bonglee Kim. 2021. "Anti-Cancer Effect of Panax Ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery" Processes 9, no. 8: 1344. https://doi.org/10.3390/pr9081344
APA StyleKim, S., Kim, N., Jeong, J., Lee, S., Kim, W., Ko, S.-G., & Kim, B. (2021). Anti-Cancer Effect of Panax Ginseng and Its Metabolites: From Traditional Medicine to Modern Drug Discovery. Processes, 9(8), 1344. https://doi.org/10.3390/pr9081344








